Effects of high temperature and short time heat shock treatment on the callus formation of sweet potato tuber
2022-02-07XinQiZhangMinLiuBangdiSunJingLiLanxinHaoGuangfeiZhouXinqunChengQinyang
Xin Qi, Zhang Min, Liu Bangdi, Sun Jing, Li Lanxin, Hao Guangfei, Zhou Xinqun, Cheng Qinyang
Effects of high temperature and short time heat shock treatment on the callus formation of sweet potato tuber
Xin Qi1,2,3, Zhang Min1,2, Liu Bangdi1,2※, Sun Jing1,2, Li Lanxin1,2, Hao Guangfei2,3, Zhou Xinqun1,2, Cheng Qinyang1,2
(1.,,100125,; 2.,100121,; 3.,,056038,)
Sweet potato is a kind of vegetable and grain crop which is grown world-wide, and it is also the second largest potato crop in China. During the harvest and storage process, sweet potato is damaged by machinery and susceptible to pathogenic microorganisms, resulting in great economic losses caused by the deterioration of sweet potato. Therefore, callus pretreatment of potato crops before storage has become the main methods to reduce the storage loss of potato crops. To improve the existing problem of traditional callus treatment (35 ℃ heated in storage condition for more than 2 d), such as high energy consumption, low efficiency, instability, in sweet potato callus formation technology, the purpose of this research was to study the effects of different heat shock treatments on the formation of sweet potato root callus healing, the sweet potatoes were treated with High Temperature Short Time Callus (HTSTC) at 55-70 ℃ lasting 10-20 min with hot air by self-designed high-temperature-short-time healing equipment. After the callus healing was completed at (13±1)℃ for 7 d, the appearance color, the thickness of the phelloderm layer, the content of lignin and the mass loss rate of sweet potato were measured, and the suberin deposition was observed under a microscope. The results showed that after callus heat shock treatment at 65 ℃ for 15 min, the lignin accumulation and suberin deposition were the best among HTSTC groups. The HTSTC treatment under 65 ℃ for 15 min groups showed a stronger blue fluorescence intensity and lager blue fluorescence region than other HTSTC groups, which was similar to the fluorescence intensity of traditional callus treatment. On the other hand, the lignin content and cork layer thickness of 65 ℃ for 15 min HTSTC groups were 12.27% and 19.41% higher than those sweet potatoes under traditional 35 ℃ healing for 2 d callus treatment. The traditional callus method would lead to a serious mass loss problem by dehydration and respiration acceleration. Compared with traditional callus method, the mass loss of 65 ℃ for 15 min HTSTC treatment group was only (3.78 ± 0.34)%, which could effectively reduce the mass loss of damaged sweet potatoes. In addition, the optimal HTSTC treated sweet potato could increase the Landreduce partial browning better compared with traditional callus treatment on sweet potatoes. It was found that the callus color of the 65 ℃ heat for 15 min was gray green without obvious browning, which was an acceptable appearance for consumers. In order to verify the experimental results, the HTSTC treatment temperature and time were optimized and calculated by response surface design method. Through response surface simulation optimization, the optimal heat shock treatment condition is at 67.01 ℃ for 12.69 min, which was very close to the actual experimental parameter of 65 ℃ for 15 min. The2of cork layer thickness, mass loss rate, and lignin content in response surface were 0.999, 0.998, and 0.997 respectively. In conclusion, the experimental results are very close to the response surface optimization results. The 65 ℃ heat for 15 min HTSTC treatment could not only achieve better result of calls formation than traditional callus treatment, but also ensure the lowest mass loss rate and maintain the best appearance quality, which could become a new scientific method for rapid, efficient, energy saving, and quality assurance callus treatment of postharvest sweet potato and other root crops.
optimization; postharvest; storage; heat shock pretreatment; callus healing; sweet potato
0 Introduction
Sweet potato () is one of the most important staple food grains and vegetables in the world due to its characteristics of easy-to-grow and high yield[1]. According to the statistics of the Ministry of Agriculture and Rural Affairs of China in 2017, the total planting area of sweet potato in China was 8 937 300 hm2, and the total output was 3.42 × 107t, which both ranked first in the world. However, sweet potato is a kind of root crop which had the common characteristic of thin-skin, high water content and vulnerability during harvest. These biological characteristics are easy to lead to the increase of respiration, nutrient consumption and the possibility of infection by exogenous microorganisms in the postharvest of sweet potatoes, which lead to the rapid rot and the decrease of economic value[2]. According to the statistics data of Ministry of Agriculture and Rural Affairs, the comprehensive loss rate of sweet potato root tubers caused by improper storage and transportation in China exceeded 30% in 2019. Therefore, there is of great industrial value and social significance to solve the loss of sweet potato during storage and transportation.
Callus healing is an effective method to solve the loss of root crops during postharvest, and callus is also a plant self-defense system after being damaged. Callus healing mainly stimulates the metabolic pathways of reactive oxygen species, phenylpropane and fatty acids through the generation of wound transduction signals, and promotes oxidative cross-linking of related metabolites to gradually form healing callus in the wound[3]. Callus plays an important role in preventing water loss and resisting to microbial infection. There were many researches on postharvest fruit callus of root crops, especially on potatoes. In the study of potato callus, the formation mechanism of potatoes had been proved[4-5], and it was pointed out that the factors affecting fruits and vegetables’ healing callus mainly included variety[4], harvest maturity[6], temperature[7], relative humidity, exogenous lights[8], gas composition[9], and chemical stimulation[10-11]. At present, the formation of callus stimulated by high temperature has been widely explored on many fruits and vegetables, and the research range of callus temperature is generally between 30-80 ℃. Some studies proposed a method of refrigeration or cellaring at relatively low heating temperatures (30-35)℃ for 3-7 d. Xue et al.[12]found that sweet potatoes with continuous callus healing treatment at 32 ℃ and 90% relative humidity for 4 d were more durable than those without healing treatment. Amand found that samples heated under 30 ℃ for 7 d were more conducive formation callus on wound than untreated samples in 18 different varieties of sweet potatoes[13]. However, due to sweet potatoes’ seasonal harvesting resulting in the increasing demand for fresh sweet potato market, this method of long-term healing callus could not fully meet the needs of the market and production process due to its shortcoming of long-time, high-cost, non-environmental friendly and complex equipment[14]. It was also point out that sweet potatoes would suffer from serious mass loss because of the uneven heating during callus in cold storage[15]. Therefore, shortening callus healing treatment time, homogenizing healing treatment, and improving healing efficiency are practical and technical problems to be solved in the field of sweet potatoes postharvest research.
Heat shock treatment is a pre-processing method for postharvest fruits and vegetables, which exposed fruits and vegetables to non-lethal high-temperature conditions for short time. Its main purpose was on one hand sterilization and inactivating of microorganisms on fruits and vegetables surface[15], on the other hand promoting the metabolism and accumulation of bioactive substances by activating enzymes in fruits and vegetables[16]. As a safe and efficient new physical pretreatment method, heat shock treatment has been widely used in post-harvest storage of fruits and vegetables like sweet orange[3], tomato[17]and zucchini[18]. Studies have shown that heat shock treatment can stimulate fruits and vegetables respiratory intensity in a short time, supplying sufficient energy for stress-resistance and self-healing[14]. In postharvest studies of cherries[19], it was found that heat shock treatment can also effectively stimulate phenylpropanoid metabolism and reactive oxygen species metabolism pathways, promote the generation and accumulation of resistant substances, and participate in oxidative crosslinking of phenols and aromatic substances during callus healing as signal molecules[20]. At present, although there have been a lot of researches about heat shock treatment delaying fruit senescence, improving fruit resistance, improving fruit quality, and inhibiting fruit diseases, there is still a lack of in-depth research about heat shock treatment on formation of sweet potato callus healing and diversified techniques.
In order to meet the demands of continuous mass operation, uniform treatment effect, large processing capacity, low carbon emission and environmental protection in the sweet potato industry. The purpose of this study was to verify whether High Temperature Short Time Callus (HTSTC) treatment could effectively promote the sweet potato callus, comparing with traditional callus method. The effects on the formation of sweet potato callus healing after heat shock treatment at 55-70 ℃ for 10-20 min were determined and discussed. The study could provide a new environmentally friendly and fast healing method for sweet potato research and production.
1 Materials and methods
1.1 Purchase and preparation of sweet potato samples
Sweet potato (‘Xiguahong’) in this study were purchased and transported from Fujian Provence, China. Each sweet potato was bagged in bubble bag to prevent collision and transported from Fujian to Beijing in refrigerated truck under (13±2) ℃ in 1d. After arriving at the lab, the sweet potatoes with neat appearance, uniform size, no epidermal damage, no disease and insect pest, and a single mass of (250±50) g were selected as the experimental materials.
1.2 Artificial wounds and heat shock treatment of sweet potato samples
The selected sweet potato roots were washed with tap water and distilled water for twice and then dried at the room temperature for an artificial wound experiment. The knives and hole punches (15 mm diameter) for artificial wound were disinfected with 95% (v/v) ethanol. Firstly, each sweet potato was marked by hole punches, then cut the sweet potato skin with a diameter of 15 mm and a depth of 3 mm by knives along the mark. Six artificial wounds were performed on each sweet potato root. The six wounds were divided into two groups and distributed on opposite sides of the sweet potato. The spacing between each wound on a single surface should not be less than 1 cm.
Based on the results of preliminary experiments and research results[21], the heat shock treatment between 50 ℃ and 70 ℃ within 30min has the best stimulation effect on sweet potato callus. In this study, the artificial wounded sweet potatoes were placed in the self-designed sweet potato HTSTC machine (Fig. 1). A total of 12 HTSTC treatment groups were set based on the treatment temperature and treatment time gradient. The sweet potato samples were heated at 55, 60, 65, and 70 ℃ for 10, 15, and 20 min. After HTSTC treatment, the sweet potatoes were cooled at room temperature (25±2)℃ and waited for the following experiment steps.
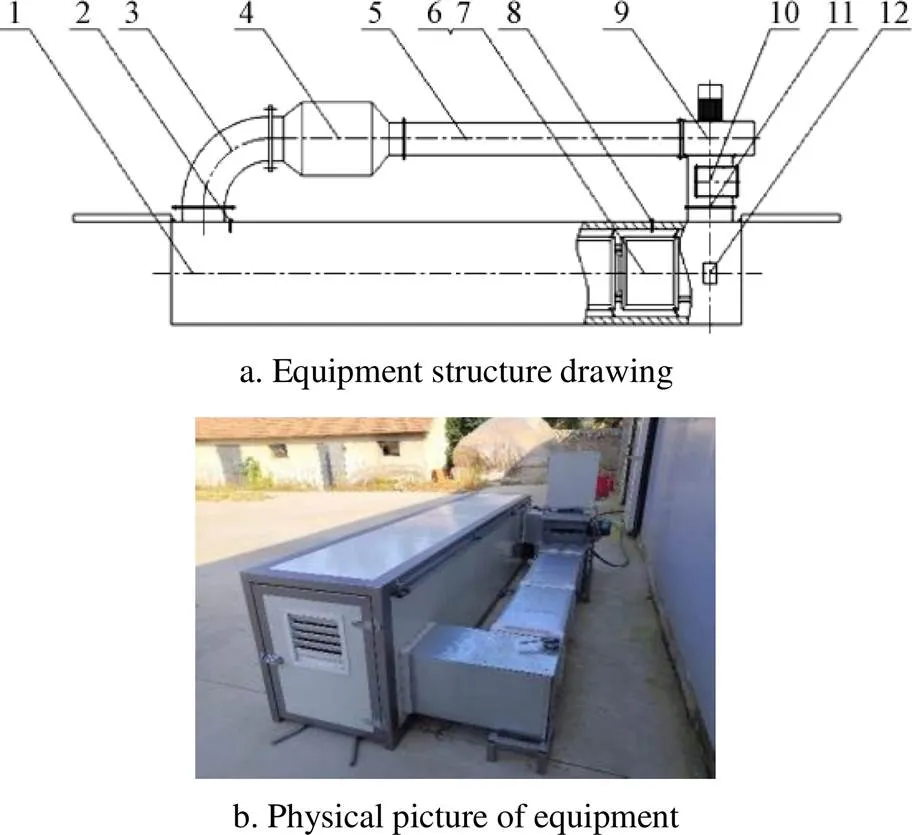
Note: 1.Main tunnel body 2.The No.1 temperature and humidity sensor 3.The No.1 connection line 4.Heater 5.The No.2 connection line 6.Loading cart 7.Loading basket 8.The No.2 temperature and humidity sensor 9.Centrifugal blower 10.The No.3 connection line 11.Valve 12.Dehumidification fan
In this experiment, the positive and negative control groups were designed. Continuous healing treatment in conventional cold storage or store cellar was set as positive control group (CK+), and samples without any heat shock treatment were set as negative control group (CK-). The CK+ heat shock treatment method was to place sweet potatoes in the artificial climate box, and heated under (35±0.5)℃ and humidity (80±5)% for 2 d.
In this study, the complete callus formation period of sweet potato was 7 d.After HTST treatment groups finished processing and cooling steps, samples were transferred to the laboratory in small cold storage for 7 d, which was in order to complete the healing. To ensure the same callus period in all groups, sweet potatoes in CK+ treatment were treated at (35±0.5)℃ for 2 d and stored in small cold storage for 5 d, which was 7 d totally. Sweet potatoes in CK- group were directly stored in small cold storage for 7 d without any heating callus treatment.
The cold storage of CK+, CK-, and HTSTC groups was set at (13±1)℃ and relative humidity at (80±5)%, which was the optimal storage condition for sweet potato storage. After the sweet potato wound cured completely, the relevant indexes were determined with 150 sweet potato samples in each treatment, and this sample healing preparation process was repeated for 3 times.
1.3 Determination of physiological indexes on sweet potato wound after healing
1.3.1 Determination of color
The color index of sweet potato callus after heat shock treatment and healing were determined by spectrophotometric colorimeter (3NH, Shanghai Yuezi Electronic Technology Co., Ltd., China).,, andvalues were measured perpendicular to the callus of sweet potatoes. Where,* value represents the change of red and green, positive value represents red, negative value represents green* value represents the change of yellow and blue, positive value represents yellowish, negative value represents bluish; the* value represents brightness, 100 represents absolute white and 0 represents absolute black. Six sweet potatoes from each treatment were taken to determine and data were averaged. According to the lab average value of callus measured by spectrophotometric colorimeter, the three values was input into Photoshop to obtain the standard color card of each treatment group.
1.3.2 Determination of cork layer thickness
The tissues of sweet potatoes at (1±1) mm below the callus were cut to measure the cork layer thickness with digital caliper (SL01-22, Shanghai Niuhui industrial limited company, China). The position of outer cortex of sweet potato callus was taken as the measuring position of vernier caliper. Six different sweet potato callus layers were measured in each group and the average value was taken.
1.3.3 Lignin and suberin accumulation in wounded tissue
Sweet potato lignin staining was observed and modified according to the method of Jiang et al[10]. Tissue blocks with the injured surface were hand-sliced (0.4-0.5 mm depth) vertically with a blade. The prepared slices were immediately rinsed with distilled water to remove starch granules and then immersed in 1% (w/v) phloroglucinol solution for 2 h staining on a glass slide with few drops of concentrated hydrochloric acid. After 5 min, the images of red-stained deposited lignin were captured with a microscope (DM500, Leica Shanghai limited company, China) under 10 × magnification. Suberin deposition was microscopically detected by its autofluorescence according to Fugate et al[22]. The autofluorescence of suberin was analyzed using a microscope (DM500, Leica Shanghai limited company, China) with fluorescence excitation filter at 340-390 nm and emission filter at 420 nm. The prepared sections (0.3-0.4 mm depth) were rinsed with distilled water 2-3 times before capturing images under 10 × magnification. Six potato tubers for each group were used to observe staining and autofluorescence.
1.3.4 Determination of lignin content
Lignin determination samples were taken after callus completion, the callus and tissue with a depth of 1 mm below callus of sweet potato were cut. The determination of lignin in sweet potato callus was referenced from Chapple method and modified slightly[23]. Sweet potato samples were taken from the roots of sweet potato in each treatment group with a stainless steel knife. During the determination, 1 g of frozen callus was taken, 4 mL 95% ethanol precooled at 4 ℃ was added, and beaten evenly with a beater. The callus was transferred to a 10 mL centrifuge tube and centrifuged at 4 ℃ at 10 000 r/min for 20 min. After the supernatant was discarded, add 2 mL 95% (v/v) ethanol, mix, centrifuge (4 ℃ 10 000 r/min 10 min), repeat for 3 times, then use ethanol. After the precipitation is collected and dried to a constant mass, the dry matter is moved to a small test tube. Before the measurement, the water bath should be placed in a fume hood in advance and the temperature should be adjusted to 70 ℃ for preheating. First, add 1 mL 25% acetyl bromide solution into the test tube and mix well. Immediately placed in a water bath for reaction for 30 min, 1 mL sodium hydroxide (2 mol/L), 0.1 mL hydroxylamine hydrochloride (7.5 mol/L), and 2 mL glacial acetic acid were added successively, centrifuged at 4 ℃ at 10 000 r/min for 20 min, and 0.5 mL supernatant was absorbed. The absorbance was measured at 280 nm with glacial acetic acid at a constant volume of 5 mL, and repeated for 3 times. Lignin content was characterized by A280 nm and measured by fresh mass. The unit of lignin content was U/g, U represented the absorbance change of the sweet potato at A280 nm per gram.
1.3.5 Determination of mass loss during heat shock and healing treatment
For determination of mass loss, the sweet potatoes were weighed after 7 d of healing treatment to analyze mass loss by calculating quotient of fresh mass decrease of tubers at each time to their corresponding initial mass[23]. The mass loss was expressed as a percentage of lost mass, and 3 replicates per treatment were made.
1.4 Response surface test factors and horizontal design
In order to further optimize HTST healing conditions, selected Design-expert 10 was used to conduct two-factor and three-level optimization test on the healing parameters range, and Central Combined Design (CCD) test was selected.
1.5 Data statistics and analysis
The above experiments were repeated three times. Excel 2010 software was used to make statistics on all data, calculate mean value and standard deviation and plot. SPSS 26.0 software was used to analyze variance and multiple difference significance analysis of experimental data,< 0.05 indicated significant difference. Design-Expert 10 was used to carry out the optimization experiment of heat shock treatment.
2 Results
2.1 Effects of heat healing treatment on appearance and color of sweet potato callus
The effects of different heat shock treatments on the appearance of the sweet potato callus were shown in Fig. 2. Although the sweet potatoes in CK- group were not pretreated with heat shock healing, callus still appeared at 7th day, and a serious browning phenomenon was found in the callus of the sweet potatoes in the CK- group. The sweet potatoes in CK+ group pre-heated at low temperature for a long time, and the callus showed partial browning. In the HTSTC treatment group, 60 ℃ heat shock for 20 min, 65 ℃ heat shock for 15 min and 65 ℃heat shock for 20 min showed the best appearance, while the groups of heat shock under 55 ℃, and groups of heat shock for 10 min showed different degrees of browning phenomenon. This browning of callus directly affected the subsequent sales ability of sweet potato. Table 1 was the color value and Fig. 3 was the standard color card of sweet potato callus. The color card was input according to*,*, and b* of sweet potato callus, so as to intuitively reflect the mean color change of sweet potato callus. As shown in Table 1, theof sweet potato callus at 65 ℃ heat for 15 min, 65 ℃ heat for 10 min, and 60 ℃ heat for 20 min reached a significant level compared with other groups (< 0.05), indicating that these three HTSTC groups were no more likely to be callus browning. Combined with the standard color card in Fig. 3, it was found that the callus color of the 65 ℃ heat for 15 min and 60 ℃ heat for 20 min groupsshowed no obvious browning, which was an acceptable appearance for consumers.

Fig.2 Effects of different HTST heat shock treatments on the appearance of sweet potato

Table 1 Effects of different heat shock treatments on the callus color parameters
Note: Different lowercase letters in the same column indicate significant differences among different treatments (< 0.05). Same below.* value represents the change of red and green, with positive value represents red and negative value represents green;* value represents the change of yellow and blue, with positive value represents yellowish and negative value represents bluish;* value represents brightness, with 100 represents absolute white and 0 represents absolute black.
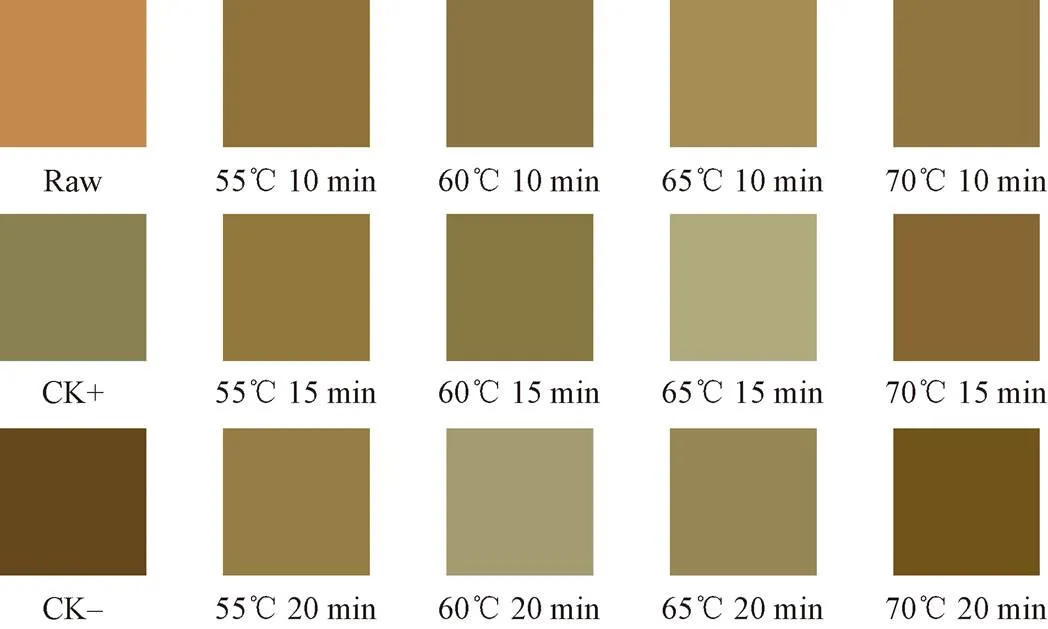
Fig.3 Standard card of different heat shock treatments on the callus during sweet potato healing
2.2 Effects of heat healing treatment on lignin content and cork layer thickness of sweet potato callus
The callus of sweet potatoes is called cork layer. The cork layer is independent of sweet potato epidermis and pulps, and it usually takes 2-3 weeks or more to completely form. Therefore, the thickness of cork layer after callus is often used as the most direct index to measure the effect of sweet potato callus. Table 2 showed the cork layer thickness measured on the 7th day of sweet potato callus. It was found that the cork layer thickness in 65 ℃ heat for 10 min and 60 ℃ heat for 15 min was 0.42 and 0.49 mm respectively, which was significantly higher than other groups (< 0.05) except CK+(> 0.05). In particular, the thickness of cork layer in HTST group treated at 65 ℃ for 15 min was 19.41% higher than that of sweet potato treated at 35 ℃ for 2 d. This was similar to the results of Yang’s study on potato callus stimulation with 45 ℃ hot water for 10 min, indicating that heat shock treatment for a short time could effectively promote the accumulation of cork layer during sweet potato callus. And the results of 45 ℃ hot water for 10 min treatment was similar to traditional heat treatment at 30 ℃ under the store condition[2].
Lignin is a glycerol-phenol-lipid polymer formed in the wound healing process of fruit and vegetable, mainly locates between the cell wall and plasma membrane. The lignin composition is similar to wax structure, which could prevent water and nutrient loss, resist to microbial and parasitic infection according to Fugate et al[22]. Therefore, the lignin content of callus also reflects the effect of healing progress. As shown in Table 2, heat shock treatment could significantly affect the lignin content (< 0.05) during sweet potato healing, except for the 70 ℃ heat shock for 20 min group. The lignin content and cork layer thickness of 65 ℃ for 15 min HTST groups were 12.27% higher than those sweet potatoes under traditional 35 ℃ healing for 2 d callus treatment. The lignin content of CK+ traditional low-temperature-long-term treatment was 1.2 times that of CK-, while the lignin content of 65 ℃ for 15 min and 60 ℃ for 20 min HTSTC treatment group was 24.40% and 16.60% higher than CK- respectively, which was similar to CK+. However, the lignin content of sweet potato callus heat shock treated at 65 ℃ for 15 min was the highest, which was the only group higher than CK+.
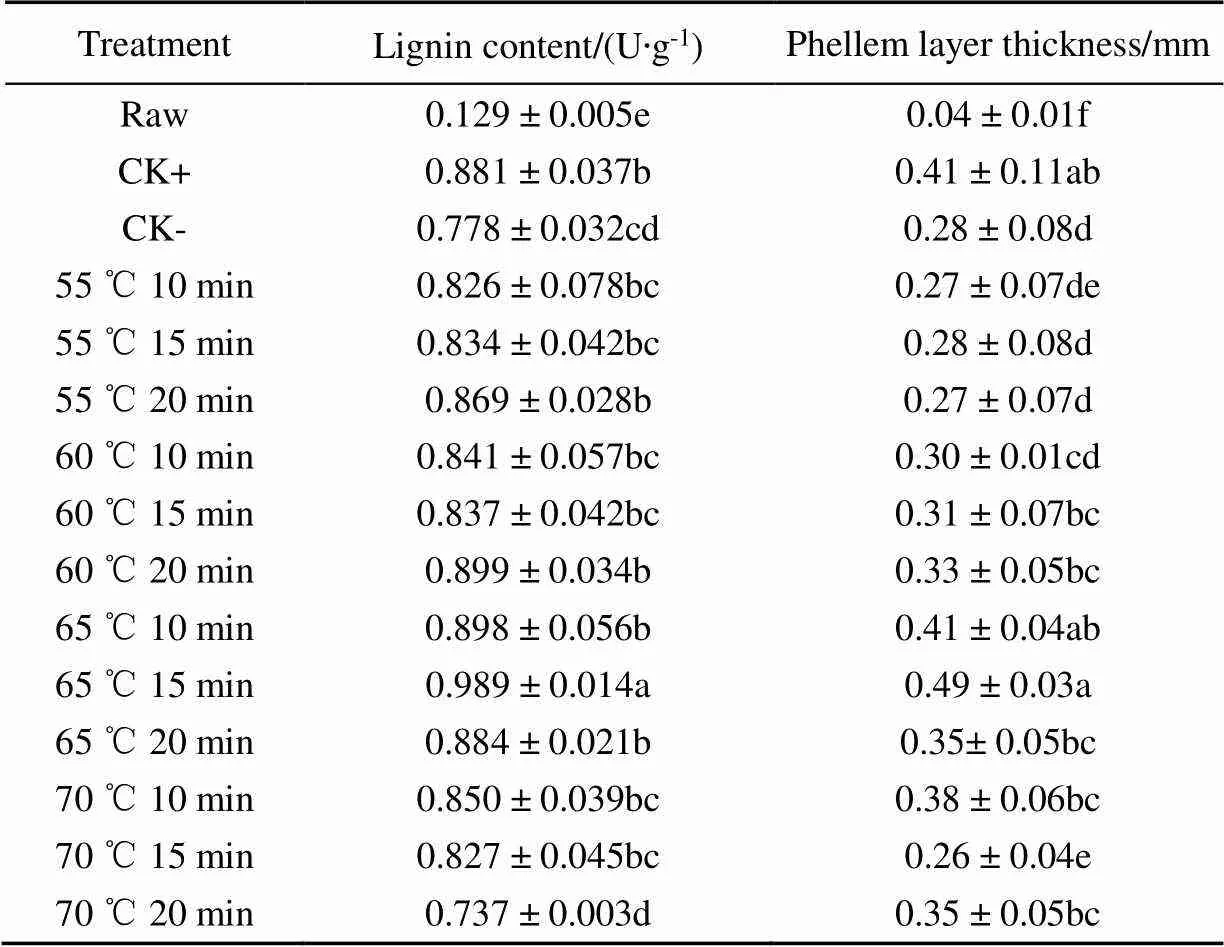
Table 2 Effects of different heat shock treatments on the lignin content and phellem layer thickness during sweet potato callus
2.3 Effects of heat healing treatment on suberin accumulation of sweet potato callus
Suberin is also one of the important components of sweet potato callus. It is formed by the polymerization of a large number of,-diacids,-hydroxy acids, and monomer fatty acid produced by fatty acid metabolism pathway. These monomer substances could be dyed purplish blue by cations in toluidine blue[24]. Therefore, it is intuitive to identify the formation of callus cork layer. As seen from Fig. 4, blue fluorescence hardly appeared in the wound tissue of the initial sweet potato samples, while fluorescence of different intensities appeared in other callus treatment groups. Among them, CK+, all three 65 ℃ heat shock groups, 60 ℃ heat shock for 15 and 20 min groups showed a stronger blue fluorescence intensity and lager blue fluorescence region. However, after heat shock treatment at 70 and 55 ℃, the fluorescence staining reaction of suberin in the sweet potato callus was visually dim[25]. The fluorescence reaction results of 55 ℃ heat for 10min and 70 ℃ heat for 10-20 min were even similar to those of CK- group which was without heat treatment at all. Fugate et al[22, 26]also pointed out that improper heat treatment temperature could not stimulate the fatty acid metabolic pathway of roots to accelerate the generation of suberin.
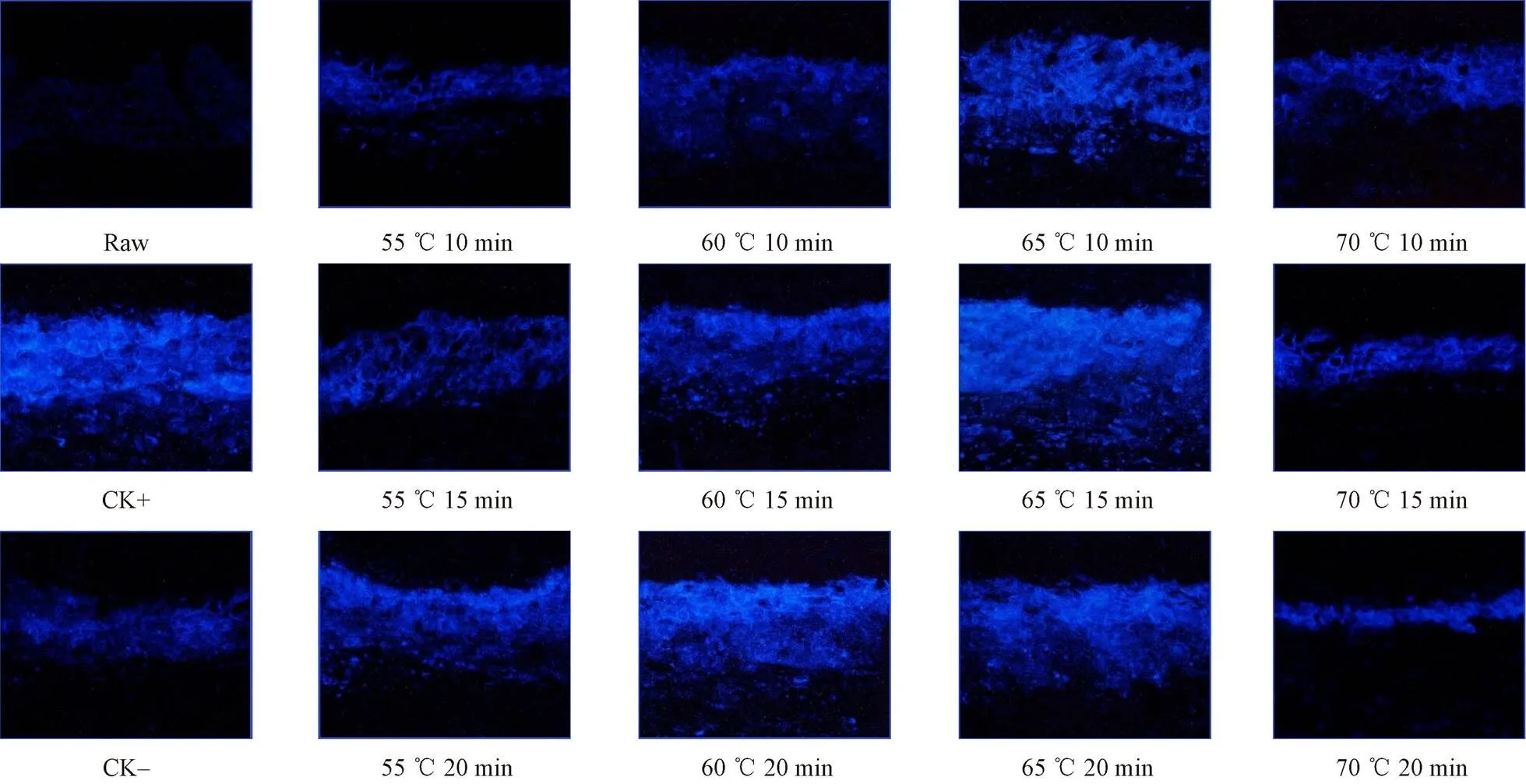
Fig.4 Effects of different heat shock treatments on the deposition of cork resin at the root tuber wound of sweet potatoes
2.4 Effects of heat healing treatment on mass loss during sweet potato healing
Mass loss seriously affects the edible and economic value of fruits and vegetables[27]. It is important for the practical application to reduce mass loss of fruits and vegetables in storage and transportation. During callus healing and storage of root crops, there are two factors leading to mass loss, including dehydration and respiration during storage, and accelerates evaporation caused by improper callus healing pretreatment[22]. As shown in Fig. 5, the mass loss rate in CK+ group was the highest, reaching (13.40±0.24)%, after long-term callus healing heat treatment at low temperature. However, the mass loss of CK- group without any heat treatment was significantly lower than CK+ group after 7 d (< 0.05). It is showed that it is inappropriate to reduce mass loss by callus healing treatment at low temperature for a long time. The mass loss rate of all HTST healing treatment groups was lower than that of CK-, and the mass loss rate of 65 ℃ heat shock for 15 min group and 65 ℃ heat shock for 20 min group were the lowest mass loss rate group. After high-temperature short-time healing and 7 d storage, the mass loss rate of 65 ℃ 15 min and 65 ℃ 20 min HTST group was only (3.78 ± 0.34)% and (4.12 ± 0.08)%, which were significantly lower than that of all other groups (< 0.05). In addition, although a large amount of lignin could be accumulated to form cork layer within the 60 ℃ 20 min HTST treatment group, the mass loss rate was (5.87±0.34)%, which was significantly higher than the 65 ℃ 15 min treatment group (< 0.05). The study of Fugate et al[22]pointed out that the traditional callus healing would result in more than 10% water loss due to the long time heat stress at 35 ℃, which greatly affected the economic value of sweet potato storage. Yang et al[25]pointed out that improper lower temperature callus treatment and poor callus effect would also affect the mass loss of yam in storage, making the mass loss rate more than 10%.
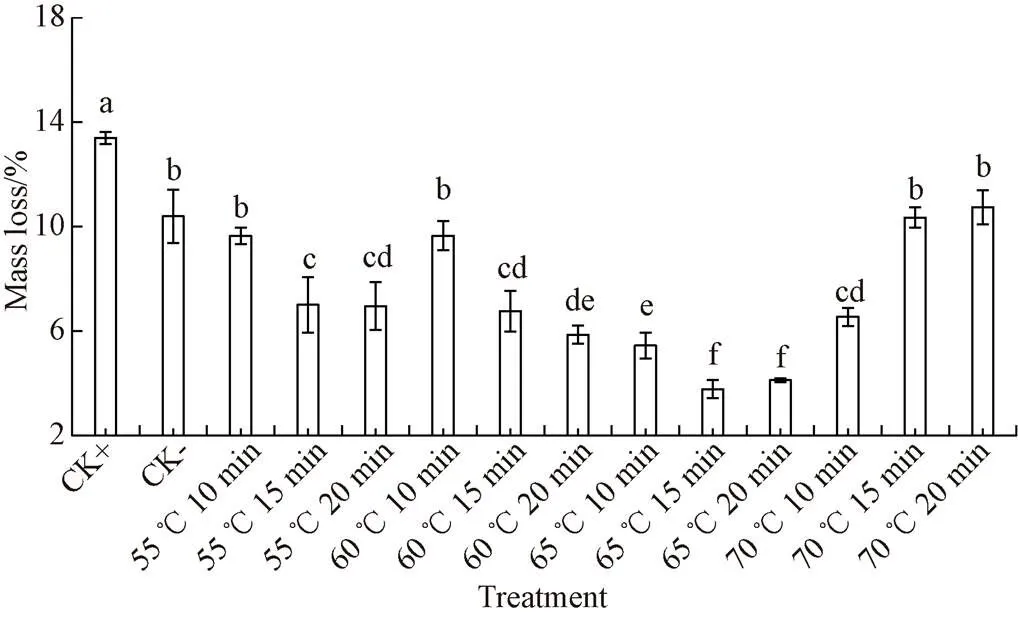
Fig.5 Effects of different heat shock treatments on mass loss rate of sweet potatoes
2.5 Response surface optimization design for the optimal HTST healing parameters of sweet potatoes
Different time and temperature were used to treat sweet potato healing, and 13 groups of experiments were compared with cork layer thickness, lignin content and mass loss rate as response values. The results of response values under various conditions were shown in Table 3. By multiple regression fitting, the following two quadratic regression equations were obtained.
The quadratic regression equation of cork layer thickness was:
Cork layer thickness:
1= 59.35+1.71+0.66-4.10×10-3-0.012-0.012(1)
Lignin content:
2= 0.99+0.05+0.04-0.05-0.162-0.182(2)
Mass loss rate:
3=3.57+0.92-1.029+2.00+2.652+1.862(3)
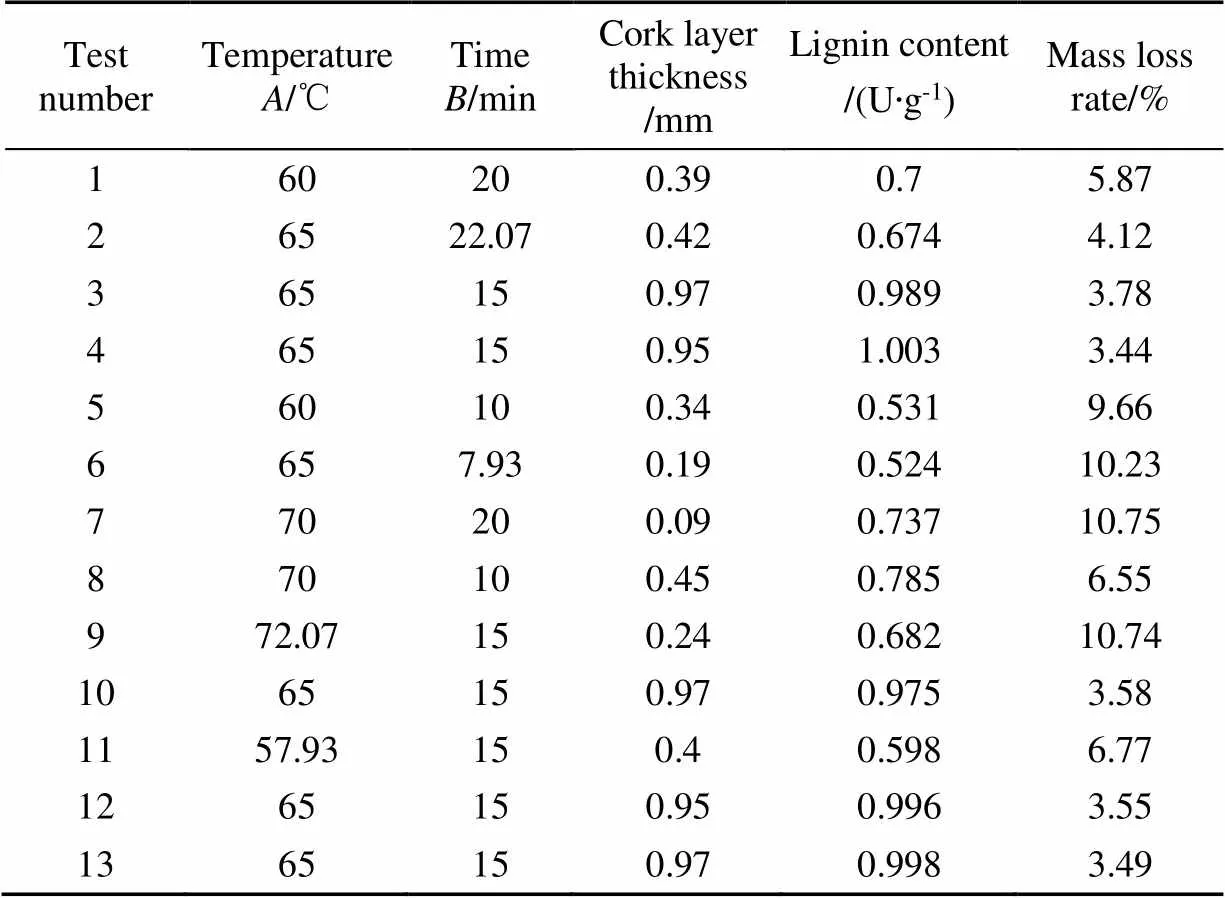
Table 3 Response surface design and experimental result
Design-Expert10 was used to perform variance analysis on the data in Table 3, and the relationship between the variables () and the response values of cork layer thickness (1), lignin content (2), and mass loss rate (3) was constructed. The variance results showed that the three response value models were significant at<0.05; the lack of fit item>0.05 was not significant, indicating that the quadratic regression model was fit. This method can reflect the relationship between factorsandand each response value. The model fitted well and could accurately predict and analyze the actual situation. The determination coefficients of cork layer thickness, mass loss rate, and lignin content were 0.999, 0.998, and 0.997, indicating that the predicted values were highly correlated with the actual values. Therefore, this design model had high reliability, and then the influence of callus temperature and time on the three response values was analyzed by contour lines and response surface.
As shown in Fig. 6, the response surface obtained from multiple quadratic regression model was used to evaluate the healing ability of sweet potatoes under the interaction of temperature and time. Under the interaction of temperature and time, the thickness of cork layer and lignin content showed a hill shape with a significant peak value. And the mass loss rate appears obvious trough. Combined with the actual data in Table 3, the thickness of cork layer, lignin content and mass loss rate all changed significantly under the interaction of temperature and time (<0.05). Thus, Design-Expert10 speculated that the optimal HTST healing pretreatment conditions for sweet potato callus were heat shock temperature 67.01 ℃ and time 12.69 min according to the quadratic regression equation of three factors given. Under these conditions (67.01 ℃ for 12.69 min), the cork thickness and lignin content of sweet potato callus reached 0.97 mm and 1.003 U/g, respectively, but the mass loss rate was only 3.44%. It was found that these callus parameters under 67.01 ℃ for 12.69 min condition was little different from that under 65 ℃ for 15 min.

Fig.6 Optimized response surface results of HTSTC condition of sweet potatoes
3 Discussion
Callus healing was a complex process involving cell proliferation, signal transduction, disease resistance, secondary metabolism and energy production[28]. It was mainly affected by exogenous physical stimulation, hormone content and metabolism level[29]. In the previous studies, the physical callus healing of root crops was mainly stimulated by thermal stimulation at low temperature for a long time. The study of Yang et al[25]pointed out that stimulation of temperature around 35 ℃ for 4 d could promote the formation of phellem layer on the outer epidermis of yam, activate phenylpropanoid metabolism and increase Reactive Oxide Species (ROS) level, effectively prevent the infection of penicillium and reduce rotting during storage. However, this method of continuous stimulation for callus healing in physical heating field also had obvious disadvantages, mainly the phenomenon of mass loss. Emragi et al[30]pointed out that potatoes stored for healing under 20 ℃ for a long time would have a mass loss about 15% higher than 0 ℃. This phenomenon was due to the thin epidermis of root crops. When stored for a long time at a higher temperature it was easier to promote water evaporation, increase respiratory intensity and increase substrate consumption, resulting in mass-loss[24]. The HTSTC technique in our study, which is heat shock at 65 ℃ for 15 min, could effectively inhibit the mass loss rate of sweet potato during callus healing. It might be because 65 ℃ as high temperature stress stimulated the formation of jasmonic acid, which was an important plant hormone, and induced stomatal closure, thus reducing transpiration of water through stomatal[31]. In addition, short-term high temperature activated the active oxygen metabolism and phenylpropane metabolic pathway around the wound, accelerated the accumulation of Suberin polyphenolic (SPP) and Suberin polyaliphatic (SPA)[32], and also effectively inhibited water transpiration and respiration[33].
The formation process of callus healing in root crops was called corkification. The corkification consisted of two parts, namely sealing layer and pericarp wound. The first sealed layer was mainly composed of stacking and oxidative crosslinking of fatty acids generated by fatty acid metabolism and polyphenols generated by phenylpropane metabolism in sweet potato cells[34]. In studies of yam and sugar beet[22], they pointed out that ROS metabolism could be activated due to stress response after damage, resulting in the production of a large number of superoxide anions, such as O2-, -OH and H2O2, which triggered a rapid reaction of intracellular fatty acid metabolism and phenylpropane metabolism. Thus, the suberin polyhumic domain and suberin polyalphatic domain are deposited, and finally formed the enclosure layer. Therefore, by measuring the thickness of cork layer and microscopic examination the cork layer, this experiment confirmed that the damaged sweet potato could significantly accelerate cork layer formation in healing by suitable HTSTC treatment (< 0.05). On the other hand, lignin was the main component to complete formation of callus around wound. In this study, the lignin content was the same as that of the phellem layer, and the HTSTC heat shock treatment produced more lignin than the traditional long time low temperature callus healing method[34]. This might be related to the activation of phenylpropane metabolic under 65 ℃ heat shock to produce a large amount of lignin monomer[35]. It was also found that inappropriate HTSTC treatment temperature and time could not effectively stimulate lignin accumulation.
Therefore, combined with the theory and our research results, it is believed that 55 ℃ treatment may not be able to effectively stimulate corkification due to insufficient temperature. Usually, high temperature treatment on fruits and vegetables to stimulate some metabolic pathways or improve plant quality was required to achieve certain temperature or heat accumulation conditions. The study of Liu et al[36]on sweet potato found that the samples was supposed to be treated at 49 ℃ for 137 min to accumulation of heat, then the lignin synthesis of sweet potato could be effectively promoted. Yang’s study also pointed out that potatoes dipped in hot water at 45 ℃ needed 10 min to effectively stimulate the metabolism of reactive oxygen species and phenylpropane at the wound site, thus promoting potato callus[37]. And our result also showed the 70 ℃ might inhibit the corkification due to the excessive temperature. Many studies on postharvest heat treatment have pointed out that improper temperature treatment of plant tissues not only fails to achieve the target effect, but may also produce side effects represented by reducing the quality of fruits and vegetables[38]. In study of Sun et al[39], when comparing the inactivation of lipoxygenase in sweet corn with boiling water blanching and radiofrequency blanching, It was found that boiling water blanching at 80 ℃could not only effectively inactivate the enzymes, but also destroy cell walls and membranes. The HTSTC heat shock at 65 ℃ for 15 min and 60 ℃ for 20 min had the best effect on stimulating lignin accumulation and corkification. Considering data of mass loss, it is showed that at 65 ℃ for 15 min heat shock treatment can have the best effect on sweet potato callus healing keeping lower mass loss rate. On this basis, response surface simulation optimization was carried out for the processing temperature and time parameters, and the optimal HTSTC treatment condition was obtained at 67.01 ℃ for 12.69 min heat shock treatment in theory. This simulation results were very close to the actual experimental results. At last, there are still some parts to improve in this study. Firstly, based on the response surface results, follow-up experiments could be conducted to verify the optimal simulated HTSTC conditions. Secondly, the expressions of related hormones and enzymes in the callus process could be further explored, in order to provide theoretical and methodological basis for the rapid callus of postharvest sweet potato roots.
4 Conclusion
In this study, the High Temperature Short Time Callus treatment (HTSTC) technique was optimized by self-designed equipment to effectively promote the formation of sweet potato callus. Through the optimization of the treatment time and temperature factors, the optimal HTSTC technical conditions were obtained as 65 ℃ treatment for 15 min. The results showed that HTSTC treatment could effectively increase the lignin content, promote the suberin accumulation, and increase cork layer thickness of sweet potato. The sweet potatoes callus treated at 65 ℃ for 15 min had better appearance and color, and it showed a lower mass loss rate during storage. In addition, the optimal technical condition of HTSTC was 67.01 ℃ for 12.69 min, which was calculated and optimized by response surface method and was very close to the actual result. This study provides a new scientific method and theoretical basis for the rapid wound healing of postharvest sweet potato, and provides an energy saving, efficient and quality assurance technology choice for the actual production and storage of fresh sweet potato.
[1] Jazmín V P P, Amparo R, Esteban B E, et al. Influence of morpho-physiological traits on root yield in sweet potato (Lam. ) genotypes and its adaptation in a sub-humid environment[J]. Scientia Horticulturae, 2020, 275: 109703.
[2] Yang W L, Pollayd M, Li-Beisson Y H. Quantitative analysis of glycerol in dicarboxylic acid-rich cutins provides insights into Arabidopsis cutin structure[J]. Phytochemistry, 2016, 130: 159-169.
[3] Hu M J, Zhu Y Y, Liu G S, et al. Inhibition on anthracnose and induction of defense response by nitric oxide in pitaya fruit[J]. Scientia Horticulturae, 2019, 245: 244-230.
[4] 贾朝爽,孙世民,包敖民,等. 四种特色品种小苹果采后生理及耐贮性比较[J]. 农业工程学报,2022,38(12):308-316.
Jia Chaoshuang, Sun Shimin, Bao Aomin, et al. Comparison of postharvest physiology and storage tolerance of four characteristic small apples[J]. Transactions of the Chinese Society of Agriculture Engineering (Transactions of the CSAE), 2022, 38(12): 308-316. (in Chinese with English abstract)
[5] Woolfson K N, Bjelica A, Haggitt M L, et al. Differential induction of polar and non-polar metabolism during wound-induced corkification in potato (L. ) tubers[J]. The Plant Journal, 2018, 93(5): 931-942.
[6] Kumar G N M, Lulai E C, Suttle J C, et al. Age-induced loss of wound-healing ability in potato tubers is partly egulated by ABA[J]. Planta, 2010, 232(6): 1433.
[7] Ji C Y, Kim H S, Lee C J, et al. Comparative transcriptome profiling of tuberous roots of two sweetpotato lines with contrasting low temperature tolerance during storage[J]. Gene 2019, 727: 144244.
[8] 刘帮迪,张雅丽,柯泽华,等. LED光照对青熟香蕉贮运中后熟调控的影响[J]. 农业工程学报,2021,37(20):295-302.
Liu Bangdi, Zhang Yali, Ke Zehua, et al. Effects of LED light on the ripening regulation of green mature banana during storage and transportation[J]. Transactions of the Chinese Society of Agriculture Engineering (Transactions of the CSAE), 2021, 37(20): 295-302. (in Chinese with English abstract)
[9] Lulai E C, Suttle J C, Pederson S M. Regulatory involvement of abscisic acid in potato tuber wound healing[J]. Journal of Experimental Botany, 2008: 59(6):1175-1186.
[10] Jiang H, Wang B, Ma L, et al. Benzo-(1, 2, 3)-thiadiazole-7-carbothioic acid smethyl ester (BTH) promotes tuber wound healing of potato by elevation of phenylpropanoid metabolism[J]. Postharvest Biology and Technology, 2019, 3(153): 125-132.
[11] Lobato M C, Andreu A B, Daleo G R, et al. Cell wall reinforcement in the potato tuber periderm after crop treatment with potassium phosphite[J]. Potato Research, 2017, 61(1): 19-29.
[12] Xue W, Liu N, Zhang T, et al. Substance metabolism, IAA and CTK signaling pathways regulating the origin of embryogenic callus during dedifferentiation and redifferentiation of cucumber cotyledon nodes[J]. Scientia Horticulturae, 2022, 293: 110680.
[13] St Amand P C, Randle W M. Ethylene production as a possible indicator of wound healing in roots of several sweet potato cultivars[J]. Euphytica, 1991, 53: 97-102.
[14] Vicente A R, Gustavo A, Martinez. Effect of heat treatment on strawberry fruit damage and oxidative metabolism during storage[J]. Postharvest Biology and Technology, 2006, 40(2): 116-122.
[15] Wang J, Miao F, Zhou R. Effect of different processing methods on storage quality of strawberries[J]. Asian Agricultural Research, 2020, 12(12): 47-50.
[16] Juan F G, Olmo M, Jose M. Decay incidence and quality of different citrus varieties after postharvest heat treatment at laboratory and industrial scale[J]. Postharvest Biology and Technology, 2016, 118: 96-102.
[17] Bai C, Fang M, Fu A, et al. Regulation of of mA methylation on tomato fruit chilling injury[J]. Horticultural Plant Journal, 2021, 7(5): 434-442.
[18] Zhang M, Liu W, Li C, et al. Postharvest hot water dipping and hot water forced convection treatments alleviate chilling injury for zucchini fruit during cold storage[J]. Scientia Horticulturae, 2019, 249: 219-227.
[19] Wang L, Jin P, Wang J, et al. Hot air treatment induces resistance against blue mold decay caused by Penicillium expansum in sweet cherry (L.) fruit[J]. Scientia Horticulturae, 2015, 189(25): 74-80.
[20] Ge X, Bi Y, Li Z, et al. Preharvest multiple fungicide stroby sprays promote wound healing of harvested potato tubers by activating phenylpropanoid metabolism[J]. Postharvest Biology and Technology, 2021, 171: 111328.
[21] Xin Q, Liu B, Sun J, et al. Heat Shock Treatment Promoted Callus Formation on Postharvest Sweet Potato by Adjusting Active Oxygen and Phenylpropanoid Metabolism[J]. Agriculture-Basel, 2022, 12(9): 1351.
[22] Fugate K K, Ribeiro W S, Lulai E C, et al. Cold temperature delays wound healing in postharvest sugarbeet roots[J]. Frontiers in Plant Science, 2016, 7: 499.
[23] Van Oirschot Q E A, Rees D, Aked J, et al. Sweetpotato cultivars differ in efficiency of wound healing[J]. Postharvest Biology and Technology, 2016, 42(1): 65-74.
[24] Boivin M, Bourdeau N, Barnabé S, et al. Black spruce extracts reveal antimicrobial and sprout suppressive potentials to prevent potato (L. ) losses during storage[J]. Journal of Agriculture and Food Research, 2021, 5: e100187.
[25] Yang R, Han Y, Han Z, et al. Hot water dipping stimulated wound healing of potato tubers[J]. Postharvest Biology and Technology, 2020, 167: e111245.
[26] 杨书珍,刘灿,苏小军,等. 高温处理对采后山药块茎愈伤和抗病性的影响[J]. 华中农业大学学报,2019,38(4):113-119.
Yang Shuzhen, Liu Can, Su Xiaojun, et al. Effects of high temperature treatment on callus and disease resistance of postharvest yam tubers[J]. Journal of Huazhong Agriculture University, 2019, 38(4): 113-119. (in Chinese with English abstract).
[27] Liu B, Zhao H, Fan X, et al. Near freezing point temperature storage inhibits chilling injury and enhances the shelf life quality of apricots following long‐time cold storage[J]. Journal of Food Processing and Preservation, 2019, 43: e13958.
[28] Lulai E C, Suttle J C, Pederson S M. Regulatory involvement of abscisic acid in potato tuber wound healing[J]. Journal of experimental botany, 2008; 59: 1175-1186.
[29] Zhu Y, Zong Y, Liang W, et al.-Aminobutyric acid treatment accelerates the deposition of suberin polyphenolic and lignin at wound sites of potato tubers during healing[J]. Postharvest Biology and Technology, 2021, 179: 111566.
[30] Emragi E, Kalita D, Jayanty S S. Effect of edible coating on physical and chemical properties of potato tubers under different storage conditions[J]. LWT-Food Science and Technology, 2022, 153: 112580.
[31] Förster S, Schmidt L K, Kopic E, et al. Wounding-Induced Stomatal Closure Requires Jasmonate-Mediated Activation of GORK K+Channels by a Ca2+Sensor-Kinase CBL1-CIPK5 Complex[J]. Developmental cell, 2019, 48(1): 87-99.
[32] Lulai E C, Olson L L, Fugate K K, et al. Inhibitors of tri- and tetra- polyamine oxidation, but not diamine oxidation, impair the initial stages of wound-induced corkification[J]. Journal of Plant Physiology, 2020, 246/247: e153092.
[33] Islam M T, Lee B R, Das P R, et al. Characterization of-Coumaric acid-induced soluble and cell wall-bound phenolic metabolites in relation to disease resistance topv. campestris in Chinese cabbage[J]. Plant Physiology and Biochemistry, 2018, 125: 172-177.
[34] Lulai E C, Neubauer J D. Wound-induced corkification genes are differentially expressed, spatially and temporally, during closing layer and wound periderm formation[J]. Postharvest Biology and Technology, 2004, 90: 24-33.
[35] Sun M, Jiang F, Zhou R, et al. Respiratory burst oxidase homologue‐dependent H2O2is essential during heat stress memory in heat sensitive tomato[J]. Scientia Horticulturae, 2019, 258: 108777.
[36] 刘帮迪,吕晓龙,王彩霞,等. 高温短时热空气处理促进甘薯愈伤的工艺优化[J]. 农业工程学报,2020,36(19):313-322.
Liu Bangdi, Lyu Xiaolong, Wang Caixia, et al. Process optimization of high temperature and short time hot air treatment to promote the callus formation of sweet potatoes[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2020, 36(19): 313-322. (in Chinese with English abstract)
[37] Yang R, Han Y, Han Z, et al. Hot water dipping stimulated wound healing of potato tubers[J]. Postharvest Biology and Technology, 2020, 167: 111245.
[38] Su D, Lvu W, Wang Y, et al. Influence of microwave hot-air flow rolling dry-blanching on microstructure, water migration and quality of pleurotus eryngii during hot-air drying[J]. Food Control, 2020, 114: 107228.
[39] Sun Y, Wang K, Dong Y, et al. Effects of radiofrequency blanching on lipoxygenase inactivation, physicochemical properties of sweet corn (L. ), and its correlation with cell morphology[J]. Food Chemistry, 2020, 394: 133498.
高温短时热激处理对甘薯块根愈伤组织形成的影响
辛 奇1,2,3,张 敏1,2,刘帮迪1,2※,孙 静1,2,李岚欣1,2,郝光飞2,3,周新群1,2,程勤阳1,2
(1. 农业农村部规划设计研究院,北京 100125;2. 农业农村部产地初加工重点实验室,北京 100121;3. 河北工程大学生命科学与食品工程学院,邯郸 056038)
甘薯采后贮藏过程中受机械损伤,极易被病原微生物侵害,使得甘薯腐烂变质造成极大的经济损失。基于采后高温预处理可加快果蔬受损组织的愈伤速度,该研究采用不同热激条件处理对甘薯块根,并探究其对愈伤组织形成的影响。以人工损伤甘薯为材料,采用热空气对甘薯块根进行55~70 ℃温度和10~20 min时间的高温短时愈伤热激处理(High Temperature Short Time Callus,HTSTC)。在13 ℃条件下贮藏7 d后,对甘薯块根的外观色泽、木栓层厚度、木质素含量和失重率进行测定,并观察愈伤后软木脂沉积情况。结果表明,65 ℃热激15 min处理后甘薯伤口部位木质素积累和软木脂沉积现象良好,木质素含量、木栓层厚度比冷库内(35±0.5) ℃进行愈伤2 d的传统愈伤方式分别高出12.27%和19.41%。并且和传统愈伤相比,由于愈伤速度快,愈伤层厚度较高,使得热激处理组能有效降低损伤甘薯块根的失重率。通过响应面优化探究热激愈伤甘薯的最佳条件,计算得出最佳热激处理条件为67.01 ℃热激12.69 min,且此条件下愈伤效果与65 ℃热激15 min十分接近。综上,HTSTC处理不仅可以达到比传统愈伤更好的效果,还可以保证较低的失重率,保持外观品质,能够成为一种新型的块根类作物愈伤处理方式。
优化;采后;贮藏;热激处理;愈伤组织;甘薯
10.11975/j.issn.1002-6819.2022.18.035
S531
A
1002-6819(2022)-18-0316-10
Xin Qi, Zhang Min, Liu Bangdi. et al. Effects of high temperature and short time heat shock treatment on the callus formation of sweet potato tuber[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2022, 38(18): 316-325. (in English with Chinese abstract) doi:10.11975/j.issn.1002-6819.2022.18.035 http://www.tcsae.org
辛奇,张敏,刘帮迪,等. 高温短时热激处理对甘薯块根愈伤组织形成的影响[J]. 农业工程学报,2022,38(18):316-325. doi:10.11975/j.issn.1002-6819.2022.18.035 http://www.tcsae.org
2022-05-09
2022-08-14
s:Key R&D Plan Project of the 13th Five-Year Plan (2017YFD0401305); Agricultural Technology Innovation Team Project of the Planning and Design Institute of the Ministry of Agriculture and Rural Affairs (CHXTY-2021-08)
Xin Qi, research interests: food storage and postharvest fruit and vegetable preservation technology. Email: 978093076@qq.com
Liu Bangdi, Ph.D., Senior Engineer, research interests: physiology and technology of postharvest storage of fruits and vegetables. Email: 328442307@qq.com
