Characterization of Specific Spoilage Bacteria and Volatile Flavor Compounds of Flavored Crayfish
2022-01-12YUMeijuanTANHuanHEShuangYANGHui
YU Mei-juan, TAN Huan, HE Shuang, YANG Hui
Hunan Institute of Agricultural Product Processing, Changsha 410125, PRC
Abstract It is a common issue in the processing industry of crayfish that flavored crayfish stored at room temperature is perishable. In order to establish an effective putrid prediction mechanism, high-throughput sequencing and solid phase microextraction gas chromatography-mass spectrometry (SPME-GCMS) were used to analyse the microbial community structure and volatile flavor compounds of normal and putrid crayfish. The results showed that Aeromonas (57%),Macrococcus (7.7%), Vibrio sp. (6.6%), Acinetobacter (5%), Citrobacter (4.9%)and Enterobacter (1.49%) were the main bacterial genus in the refrigerated fresh crayfish (HNA). And Staphylococcus (17.04%), Aeromonas (4.46%), Xanthomonas(4.16%), Streptococcus (4.62%) and Enterococcus (2.77%) were the main bacterial genus in the marinated and refrigerated crayfish (HND). With the spoilage of samples (HNE and HNC), the diversity of bacteria decreased, and the specific spoilage bacteria grew rapidly, mainly Enterococcus, Bacillus, Lactobacillus,Leuconostoc, Weissella. Meanwhile, the volatile compounds in non-spoilage sample (HNA and HND) were mainly alkane compounds, aldehydes compound and esters compounds; and the volatile compounds in spoilage samples were mainly alcohols, acids, benzene compounds, terpenoids, N-containing compounds,S-containing compounds and ethers. This indicated that the contents and types of volatile compounds changed with the sample spoilage and deterioration. Correlation analysis results showed that Enterococcus, Lactobacillus and Bacillus were significantly positively correlated with alcohols, acids, benzene, terpenoids, N-containing compounds, S-containing compounds and ether compounds, while Aeromonas,Megasphaera, Acinetobacter, Citrobacter and Vibrio were significantly positively correlated with alkane compounds and esters compounds, and Leuconostoc were significantly positively correlated with alcohol compounds. These results can provide a theoretical guidance for the storage of cooked flavor crayfish at room temperature.
Key words Crayfish; High-throughput sequencing; SPME-GC-MS; Microbial community structure ; Volatile compounds
1. Introduction
Crayfish, orProcambarus clarkia
(scientific n ame), is very popular for its delicious taste and unique flavor. Traditional crayfish products mainly include quick-frozen crayfish, crayfish tail, crayfish meatetc
.Nowadays many suppliers have developed a series of cooked flavored crayfish to satisfy the demands of consumers.Cooked flavored crayfish products usually have the problem of short shelf life when stored at room temperature. Because microorganisms grow easily when flavored crayfish are under processing or storage. Of these microorganisms, specific spoilage bacteria is the major cause to the aquatic spoilage.Specific spoilage bacteria would reproduce and then decompose nutrients, producing metabolites such as amines, sulfides, alcohols, aldehydes, ketones, organic acidsetc
. These metabolites would cause inflation of the package, rapid deterioration of the product and produce unpleasant smell. The species of specific spoilage bacteria in aquatic products processed by different ways are also different. Researches have shown that the main microorganisms species in fresh crayfish includeFlavobacteria
,Aeromonas
,Shivadella
,Microbacter
andBacillus
; the main microorganisms species in low-temperature stored spoilage crayfish includeSheva
,Carnivorous bacteria
,Cyclofilaria
,Thermophilic bacteria
,Roaming Coccus
,Acinetobacter
,Aeromonas
andFascicococcus
,etc
.;while the main species in cooked flavored crayfish wasBacillus
. Different spoilage bacteria have different metabolic pathways, therefore the types and contents of the produced volatile compounds are also different and have species specificity. Therefore,volatile compounds can be used to label the specific spoilage bacteria.This study applied high throughput sequencing and solid phase microextraction gas chromatographymass spectrometry (SPME-GC-MS) to determine the spoilage microorganisms and the volatile flavored compounds of flavored crayfish with different treatments,which can provide theoretical guidance for the storage of cooked flavor crayfish at room temperature.
2. Materials and Methods
2.1. Materials
Fresh crayfish were provided by the processing company; spicery and seasonings were purchased from hypermarkets.
2.2. Major instruments
Nanodrop2000 ultramicro spectrophotometer(Thermo Fisher company from the United States);GeneAmp®9700 PCR system (ABI company from the United States); Illumina Miseq sequenator (Illumina company from the United States); solid phase mioroextraction (75 μm CAR/PDMS extraction head);7890—5975 GC-MS (Agilent Technologies from the United States).
2.3. Methods
2.3.1. Sample preparation
The crayfish were cleaned, the head and sand vein were removed, the tail were rinsed and drained. Some drained crayfish tails were put in -20℃ refrigerator and marked as HNA; some were deep-fried, seasoned with traditional marinating sauce, cooled and divided into two packages: one package was preserved in -20 ℃refrigerator and marked as HND and the other package was preserved at room temperature for bulging spoilage and marked as HNE; some drained crayfish tails were deep-fried, seasoned with quantitative marinating sauce, cooled and packed under room temperature for bulging spoilage and marked as HNC.
2.3.2. Identification of dominant spoilage bacteria through high throughput sequencing method
10 g of each sample was taken under sterile condition, the sample was fully ground, and the total RNA was extracted. cDNA synthesis was carried out based on the RNA stripe, and Shanghai Majorbio Biopharm Technology Co., Ltd was entrusted to perform sequencing analysis using Illumina Hiseq 2500 platform.
Fastpsoftware was used for the quality control of the original sequence and FLASHsoftware was used for the joint of the sequences; then UPARSE software was applied for the OTU aggregation of the sequences based on the 97% similarity; RDP classifierwas used for the species classification notes for each sequence and the confidence threshold was set as 0.7.
2.3.3. GC-MS measurement of volatile flavor compounds
Headspace solid phase microextraction conditions:put 8 g samples into HS-SPME sample flask, add 4~5 mL 25 g/100 mL of sodium chloride solution, water bath for 10 min at 65℃, insert a 75 μm CAR/PDMS extraction head (after aging treatment) into the headspace flask, extract for 40 min at 65 ℃ and then quickly place the extraction head into the 250 ℃ injection port to desorb for 3 min.
Chromatographic conditions: DB-5MS capillary column (30 m×25 mm, 0.25 μm); carrier gas:high purity helium; helium gas flow rate: 1.0 mL/min; injection volume: 1 μL; splitless sampling; inlet temperature: 250 ℃; temperature programming: column temperature of 40 ℃ for 2 min, increase the temperature to 60 ℃ at a speed of 3 ℃/min and keep for 5 min,then increase the temperature to 100 ℃ at a speed of 4℃/min and then to 240℃ at a speed of 8℃/min and keep for 10 min. Mass spectrometry conditions: ion source temperature: 230℃; electron energy: 70 eV; ionization mode: electron bombardment of ion source;mass scan range: m/z 40~400.
Qualitative and quantitative analysis: NIST 2005 and Willey 7 standard library automatic retrieval to determine the composition of compounds; relative amount was calculated according to the peak area normalization.
2.4. Data processing
SPSS Statistics 20.0 was used for data statistical analysis; the Alpha-diversity and one-way ANOVA were calculated and LEfSe variation analysis was performed through QIIME; the correlation heatmap and two-factor network diagram of the microorganisms and volatile flavor substances were analyzed based on correlation coefficient of Spearman grade number.
3. Results and analysis
3.1. Bacteria diversity analysis of flavored crayfish in the different treatments
803 752 effective sequences were obtained from 15 samples through Illumina Miseq platform(amplified region 338F_806 R), an average of 53 583.47 sequences were generated per sample.Flavored crayfish with different treatments showed significant differences in terms of OTU number,Chao1 and Shannon (P
<0.05), as shown in Table 1.The diversity, abundance and complexity of species of the non-spoilage samples of HNA and HND were obviously higher than the spoilage samples of HNE and HNC. However, the Simpson index results of the four group of samples were not much different, which indicated there were some changes in species, but the relative proportional changes between the species were not obvious on overall. It was confirmed that the growth and reproduction of certain spoilage bacteria could inhibit the growth of other microbial flora.The Coverage indexes of all the samples were above 0.998, which indicated that the sequencing depth has basically covered the bacteria diversity of the samples and the data can be used for subsequent statistical analysis.3.2. Bacterial community structure and difference analysis of flavored crayfish in the different treatments
The sequencing data were statistically analyzed at phylum and genus level through RDP database homologous sequence alignment classification method. At phylum level (Fig. 1A), the Firmicutes,Proteobacteria, Cyanobacteria, Bacteroidetes, and Actinobacteria were identified in the all samples; the dominant bacteria phylum of the non-spoilage group HNA and HND were Proteobacteria and Firmicutes respectively, while the dominant bacteria phylum of the spoilage group HNE and HNC was Firmicutes.At genus level (Fig. 1B), the dominant bacteria genus of fresh crayfish HNA group wereAeromonas hydrophila
,Megalococcus
,Vibrio
,Acinetobacter
,Citrate bacteria
andXanthomonas
. This was consistent with the research of TANG Cet al
.. Compared with fresh crayfish HNA group, the relative abundance ofAeromonas hydrophila
in the HND group decreased significantly, which was 22.6%; while the relative abundance ofStaphylococcus
,Aerococcus
,Xanthomonas
,Leuconostoc
andEnterococcus
increased significantly, which were 17.04%, 4.46%, 4.16%,4.62% and 2.77%, respectively. This indicated that the initial microorganisms were decreased and other microorganisms were increased in the HNA group after sample was fried.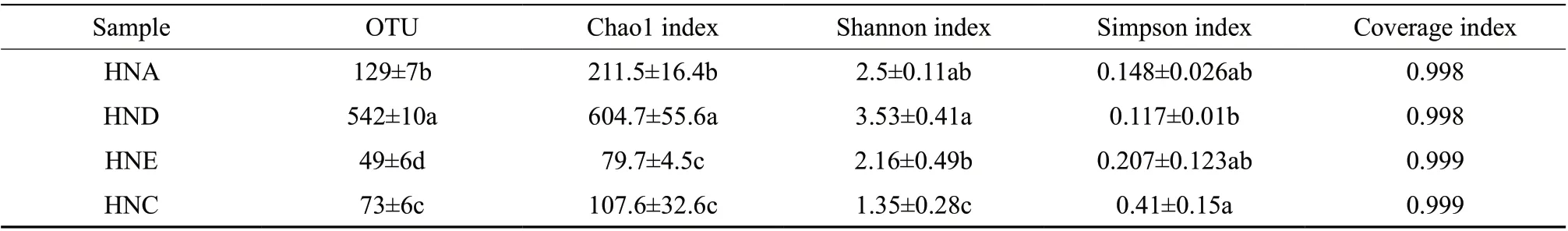
Table 1 Alpha-diversity analysis of flavored crayfish with different treatments (n=3)
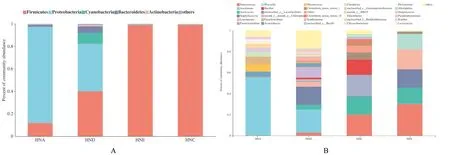
Fig. 1 Bacterial community composition at phylum level (A) and genus level (B) of flavored crayfish in the different treatments
TheEnterococcus
in the HNC and HND groups reproduced rapidly, which reached 20.06%and 30.16%. Meanwhile,Bacillus
,Lactobacillus
,Streptococcus
andWeisseria
, which were commonly seen in food, also increased. It indicated that as samples went bad when spoilage occurred, the bacterial diversity was reduced, the dominant spoilage bacteria grew rapidly.Difference significance analysis between community groups of the top 20 dominant microorganisms in abundance among the four groups of samples (Fig.2A) showed that extremely significant difference existed in the communities ofAeromonas hydrophila
,Enterococcus
,Staphylococcus
,Paeniclostridium
,Bacillus
,Paraclostridium
,Citrobacter
,Xanthomonas
,Clostridium_sensu_stricto_1, and Clostridium_sensu_stricto_18. Based on the LEfSe difference analysis,microorganisms with significant differences were clearly screened out from the four groups of crayfish(threshold value: 2). For non-spoilage groups, HNA had 15 communities, and HND had 19 communities;for spoilage groups, HNE had 9 communities and HNC had 3 communities. The results were consistent with the previous conclusions that processing could affect the number of bacterial communities and species.3.3. Difference analysis of volatile flavor compounds in flavored crayfish with different treatments
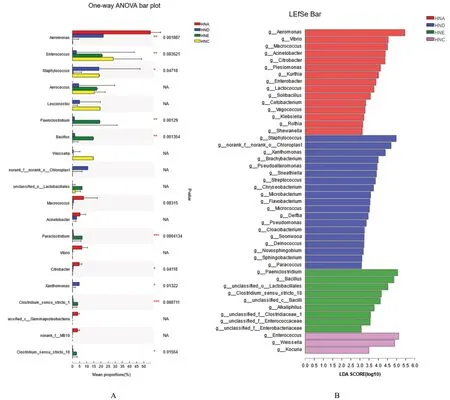
Fig. 2 Significance test of the difference between the dominant bacteria of flavored crayfish in the different treatments
As shown in Table 2, totally 81 types of volatile compounds were detected from the four groups of crayfish, which were alkane (4 types), alcohols (5 types), acids (5 types), aldehydes (2 types), esters (9 types), benzene compound (17 types), terpenoid (27 types), N-containing compound (7 types), S-containing compound (3 types) and APEOs (2 types). In HNA group, 19 volatile substances were detected; in HND,27 volatile substances were detected; in spoilage group HNE, 40 volatile substances were observed; in spoilage group HNC, 45 volatile substances were observed.This indicated that the variety of volatile compounds in samples increased with the occurrence of spoilage.In the non-spoilage group HNA and HND, the major volatile compounds were alkane, aldehydes and ester compounds. The main component in alkane compound was hexane. Compared with HNA group, the alkane compounds contents in the HND group were low,this was because the high threshold value of alkane compound had small contribution to the overall flavor of aquatic products. While, aldehydes compounds contents in the HND group were high compared to HNA group. For ester compound, HNA mainly contained ethyl caprylate and ethyl caprate; HND mainly contained ethyl mandelate. This indicated that processing changed the varieties of ester compounds in crayfish. Compared with the non-spoilage group HNA and HND, the alcohols, acids, benzene, terpenoid,N-containing, S-containing, APEOs compounds in HNE and HNC also increased. The relative contents of alcohol compounds in HNE and HNC groups had reached 1.024% and 8.01%, respectively. This was consistent with NOSEDA B 's findings that ethanol and 2,3-butanediol had close relationship with the spoilage. The major acid compounds in the HNC and HNE group were 2-methylpropionic acid, 3-methylbutyric acid and 4-methylpentanoic, of which, 3-methylbutyric acid had great relation with aquatic spoilage. The relative contents of benzene compounds in HNE and HNC were high, up to 38.07% and 21.37%, respectively. This indicated that benzene compound was the dominant flavor component in spoilage crayfish. Studies showed that benzene compound was usually produced from fat oxidation or PHE catabolism or from the environment, which could make the crayfish generate unpleasant flavor.Compared with non-spoilage group HNA and HND,the relative contents of terpenoid compound were significantly increased, which indicated that terpenoid was also a major characteristic flavor componentof spoilage crayfish. With the spoilage of samples, the contents and types of N-containing and S-containing compounds in the HNE and HNC group also increased.This was because the threshold and concentration of N-containing compound and S-containing compound presented significant impact on the flavor of aquatic products. In storage of aquatic products, the composition and content of N-containing compound and volatile sulfide were usually used to determine whether the products were spoilage or not.
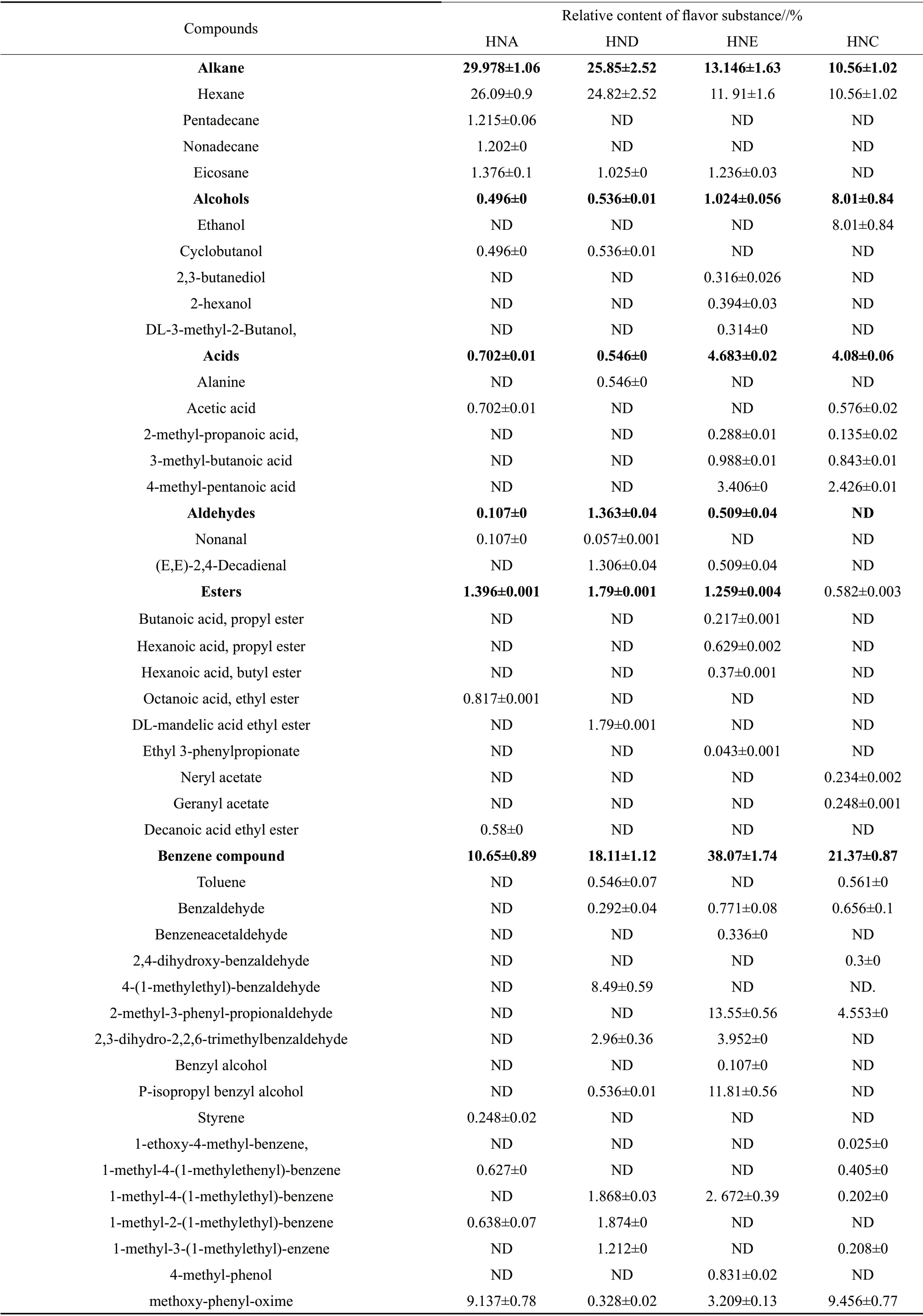
Table 2 Relative contents of volatile flavor compounds in flavored crayfish with different treatments
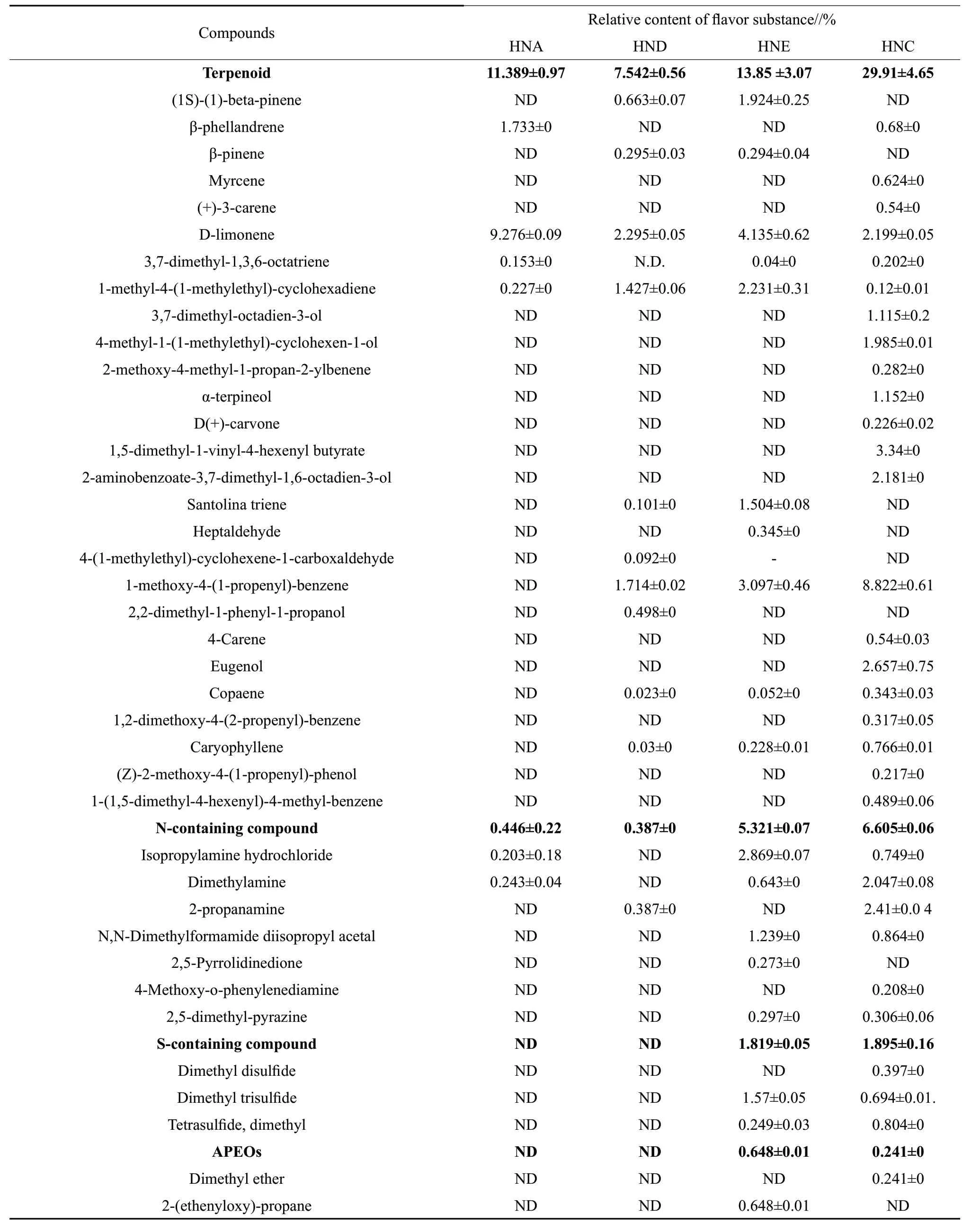
ND indicated not detected.

Fig. 4 Correlation network of dominant bacteria and volatile flavor compounds
3.4. Correlation analysis between dominant bacteria and volatile flavor compounds
As shown in Fig. 4, the alcohols, acids, benzene,terpenoid, N-containing, S-containing, APEOs compounds were significantly negatively correlated withAeromonas
,Megalococcus
,Acinetobacter
,Citrobacter
andVibrio
, and were significantly positively correlated withEnterococcus
,Lactobacillus
andBacillus
. On the contrary, alkane and ester compounds were significantly positively correlated withAeromonas
,Megalococcus
,Acinetobacter
,Citrobacter
andVibrio
, and were significantly negatively correlated withEnterococcus
,Lactobacillus
andBacillus
. Aldehydes compounds were positively correlated withParaclostridium
,Paeniclostridium
,Bacillus
andClostridium
. In the spoilage groups of HNC and HNE, the main volatile compounds were alcohols, acids, benzene, terpenoid, N-containing,S-containing, APEOs compounds, the dominant bacteria wereEnterococcus
,Bacillus
,Lactobacillus
,Leuconostoc
,Weissella
, there was significant positive correlation between their main volatile compounds and dominant bacteria, the results further indicated that these volatile compounds and dominant bacteria were closely related to the spoilage of aquatic products.4. Conclusion
This study compared the differences between normal group and spoilage group of crayfish in the diversity of microbial diversity and volatile flavor substances. It also identified the bacteria and volatile compounds that were highly correlated with spoilage, i.e.Enterococcus
,Bacillus
,Lactobacillus
,Leuconostoc
,Weissella
and alcohols, acids, benzene,terpenoid, N-containing, S-containing, APEOs compounds. The findings of the study were of great significance in the prevention and reduction of the spoilage of flavored crayfish.杂志排行
Agricultural Science & Technology的其它文章
- Screening of an Antagonistic Strain of Phytophthora nicotianae and Its Application Potential
- Protective Effect of Immaturue Bitter Orange (Citrus aurantium L.) Flavonoids Extracts on PC12 Cell Injury Induced by 6-Hydroxydopamine
- Effects of Boron Application on Growth and Quality of Flue-cured Tobacco NC297 under Different Potassium Supply Levels
- Efficient Extraction of Tea Saponin from Tea Seed Meals by 60Co γ-Irradiation
- Community Characteristics of Cardamine in Brassicaceae
- Effects of 4 Types of Remediation Agents on Reducing Cd Contents in Soil and Rice on Cd-contaminated Farmland
