Protective Effect of Immaturue Bitter Orange (Citrus aurantium L.) Flavonoids Extracts on PC12 Cell Injury Induced by 6-Hydroxydopamine
2022-01-12LIANGZengenniLIZhijianSHANYang
LIANG Zeng-en-ni, LI Zhi-jian, SHAN Yang
Hunan Institute of Agricultural Product Processing, Hunan Academy of Agricultural Sciences, Changsha 410125, PRC
Abstract To study the neuro protective effect of flavonoids extracts from immature bitter orange (Citrus aurantium L.), the PC12 cells treated with 6-hydroxydopamine(6-OHDA) were used as the Parkinson's disease (PD) model. To determine the optimal dose of 6-OHDA for constructing a PD model, PC12 cells were incubated with different concentrations of 6-OHDA for 24 h. After 24 h incubation, PC12 cells of drug groups were added 6-OHDA and different concentrations of flavonoids extracts were measured cell viability by CCK8 for selecting effective concentration of flavonoids extracts; the ROS level was determined using flow cytometry; the levels of MDA, CAT, SOD and GSH-Px were assayed by Colorimetric kit for oxidative stress investigation. Compared with the model group, PC12 cell viability was significantly enhanced (P<0.05), the levels of ROS and MDA were reduced significantly (P<0.05),and the activities of SOD, CAT and GSH-Px were significantly enhanced (P<0.05) in drug groups. In conclusion, immature bitter orange flavonoids extracts could protect PC12 cells against 6-OHDA-induced oxidative stress.
Key words Immature bitter orange (Citrus aurantium L.); Flavonoids;6-hydroxydopamine; PC12 cells
1. Introduction
Parkinson’s disease (PD), also known as tremor paralysis, is characterized by static tremor, bradykinesia, muscle tension spasm and abnormal behavior as main clinical manifestations. It is a progressive neurodegenerative disease that occurs before old age or during old age period. It is related to the degeneration and necrosis of dopaminergic neurons in the dense part of substantia nigra pars compacta, and there is no effective radical treatment. The occurrence rate of PD among people over 65 is about 1%~2% and almost 25 million people nowadays are suffering from it worldwide, and 5 million PD patients in China.6-Hydroxydopamine (6-OHDA) can cause neurodegeneration progressive changes, aging and death, resulting in nerve endings damage and transmitter reduction. PC12 cells are rat adrenal medulla pheochromocytoma cells and the main target cells of oxidative stress substances. The effect of 6-OHDA on PC12 cells is a common cell model of Parkinson's disease.
Immature bitter orange is made out of the drying unripe fruits of citrus of rutaceae family and its variants or sweet orange. Studies have shown that the traditional effect of immature bitter orange is to dispel qi and eliminate the accumulation, reduce phlegm and disperse the ruffian, it is clinically used to strengthen the spleen and appetizer, regulate qi and reduce inversion and block phlegm turbidit. Flavonoids are the most characteristic components of immature bitter orange, which have antioxidant, anti-inflammatory,anticancer, antibacterial and other pharmacological activities. The separation of neohesperidin from immature bitter orange will produce a large number of flavonoids at the same time. Studying the composition,content and function of these substances can improve economic benefits and add new additional products for the development and utilization of immature bitter orange.
Immature bitter orange were used as raw material to extract and separate flavonoids, the components and contents of total flavonoids were detected by HPLC. The protective effects of flavonoids extracts from immature bitter orange on PC12 cells injured by 6-OHDA were studied by CCK8 method, flow cytometry and western blotting. It provided a new idea for the utilization of the remaining flavonoids of immature bitter orange after the production of hesperidin and for the further prevention and treatment of neurodegenerative diseases related to aging.
2. Materials and Methods
2.1. Test materials
Immature bitter orange were provided by Lianyuankanglu High-tech Biopharmaceutical Company (identified asCitrus aurantium
L.);narirutin, naringin, neohesperidin, naringenin,hesperetin, sinensetin, nobiletin and tangeretin standards were purchased from Sinopharm Chemical Reagent Co., Ltd; heperidin, didymin standards both were purchased from Beijing Solarbio Technology Co., Ltd; chromatographic grade methanol, dimethyl sulfoxide and acetic acid were purchased from Merck; PC12 cells were provided by the cell bank of Shanghai Institutes for Biological Sciences; fetal bovine serum was purchased from Hangzhou Sijiqing bioengineering Material Co., Ltd; 1640 medium was purchased from Sigma; cell counting Kit-8 was purchased from Japanese Tongren.The main instruments were IC-20AT high performance liquid chromatograph (Shimadzu Company, Japan), EL204-IC electronic balance(METTLER TOLEDO), CKX41SF fluorescent inverted microscope (Japan Olympus), MK3 enzymelabeled instrument (America Thermo Company),Cytoflex flow cytometer (America Beckman Company), water jacket type II cell incubator (America Thermo Company), B-290 spray drier (Switz Buchi Company), SHB-III circulating water multi-purpose vacuum pump (Zhengzhou Great Wall Technology Industry and Trade Co., Ltd), UV-1700 ultraviolet visible spectrophotometer (Japan Shimadzu).
2.2. Methods
2.2.1. Extraction of flavonoids from immature bitter orange
500 g immature bitter orange was crushed then added 6 times of 90% ethanol, heated and refluxed for extraction twice, each time for 2 h, vacuum concentration, the extract was separated with BA-8 macroporous resin, eluent was evaporated and added 5.0% NaCl to separate out and generate coarse crystalline which can be used to generate neohesperidin. DA201-C macroporous resin was used to desalinize the solution and eluent was dried to obtain flavonoids powder and then the sample was weighed.
2.2.2. Determination of flavonoids from immature bitter orange
10 different types of flavone products were mixed up and resolved in 10 mL methanol and DMSO(1 ∶1 in volume ratio). Ultrasonic extraction was done for 30 min, centrifugation was done at 10 000 r/min for 10 min, collected the supernatant and the above operation was repeated twice, then the supernatants extracted three times were combined, centrifugation was done at 10 000 r/min for 10 min and constant volume was achieved to 50 mL. After filtration by a 0.22 μm microporous organic membrane, C18 chromatographic column (4.6 mm × 150 mm, 5 μm)was used in HPLC system and chromatographic separation was performed. Elution was performed using a two mobile phase gradient of methanol (phase A) and 0.2% acetic acid solution (phase B), the elution procedure was as follows: 0~8 min, 40%~60% B;8~25 min, 70%~30% B; 25~32 min, 40%~60% B. The speed of flow was 1 mL/min, the temperature of the column was 25℃ and the wavelength was 283 nm. By measuring the retention time and peak area of mixed standard, the composition and content of flavonoids in the sample were determined, and the content of total flavonoids was determined by spectrophotometry.
2.2.3. Detection of the cell viability by CCK8
PC12 was cultured in 1640 medium containing 10% fetal bovine serum, inoculated in 96-well plates and the inoculation density of each well was 5×l0cells, 100 μL per hole, 6-OHDA and the flavonoids extracts of immature bitter orange with different mass concentrations were added respectively after the cells attached, three repeat holes were set in each group.After 24 h incubation, the medium was removed and 100 μL CCK8 culture medium was added in each hole, then after incubation for 4 h, the absorbance was measured at 450 nm by an enzyme-labeled instrument.
2.2.4. Detection of cellular reactive oxygen species(ROS)
PC12 cells were inoculated in 6-well plates at a density of 1.0 ×10cells/mL, and cultured for 12 h at 37℃and 5% CO. 50 μmol/L 6-OHDA and different mass concentrations of fructus aurantii immaturus extracts (3.75, 7.5, 15 μg/mL) were added, respectively.The concentration of DCFH-DA was diluted to 10 μmol/L with serum-free medium. The cell culture liquid was removed carefully and then added 1 mL DCFH-DA (10 μmol/L) and incubated for 20 min at 37℃ and 5% CO. The cells were washed for 3 times by serum-free medium, collected by trypsin digestion and ROS levels were detected by flow cytometry.
2.2.5. Determination of antioxidant enzyme activity
PC12 cells were inoculated in 6-well plates at a density of 1.0 ×10cells/mL and 2 mL each hole, and cultured for 12 h. After adherence, 50 μmol/L 6-OHDA and different mass concentrations of fructus aurantii immaturus extracts (3.75, 7.5, 15 μg/mL) were added,respectively. After the cell culture liquid was removed and washed 3 times by PBS with pH 7.4, then 100 μL cell lysate was added and sample was centrifuged for 10 min with 2 000 r/min at 4℃. Then, the supernatant was retained to be tested. The levels of MDA and the activities of SOD, CAT and GSH-Px were measured according to the instructions of the kit.
2.3. Statistical analysis
All data were presented as mean values. SPSS software was used for one-way ANOVA,P
<0.05 indicated significant differences.3. Results and Analysis
3.1. Content and composition of total flavonoids in flavonoids extracts from immature bitter orange
The extraction rate of flavonoids extracts from immature bitter orange was 12.46% and the content of total flavonoids was 35.82%. As can be seen from Fig. 1, there were 9 kinds of flavonoids extracted from immature bitter orange, including narirutin, naringin,hesperidin, neohesperidin, naringenin, hesperitin,sinensetin, nobiletin, tangeretin. Among them, the content of naringin was the highest, 473.9 g/kg,followed by neohesperidin (153.7 g/kg) and narirutin(59.6 g/kg).
3.2. Effects of flavonoids extracts from immature bitter orange and 6-OHDA on the viability of PC12 cells
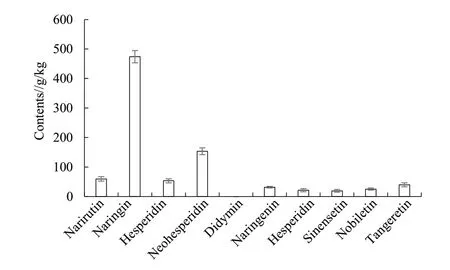
Fig. 1 Composition and content of flavonoids from fructus aurantii immaturus flavonoids extracts
Compared with the CK, 6-OHDA inhibited the growth of PC12 cells in a dose-dependent manner(Fig. 2); 3.75, 7.5 and 15 μg/mL flavonoids extracts from immature bitter orange had no apparent impact on the growth of PC12 cells, but high concentration of flavonoids extracts from immature bitter orange significantly inhibited the growth of PC12 cells (Fig.3). Therefore, 3.75, 7.5 and 15 μg/mL immature bitter orange flavonoids extracts should be further studied.As can be seen from Fig. 4, 3.75, 7.5 and 15 μg/mL immature bitter orange flavonoids extracts could significantly alleviate the damage caused by 6-OHDA to the growth of PC12 cells.
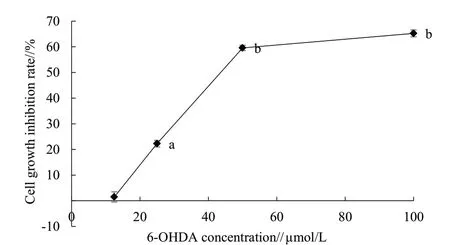
Fig. 2 Effects of 6-OHDA on the viability of PC12 cells
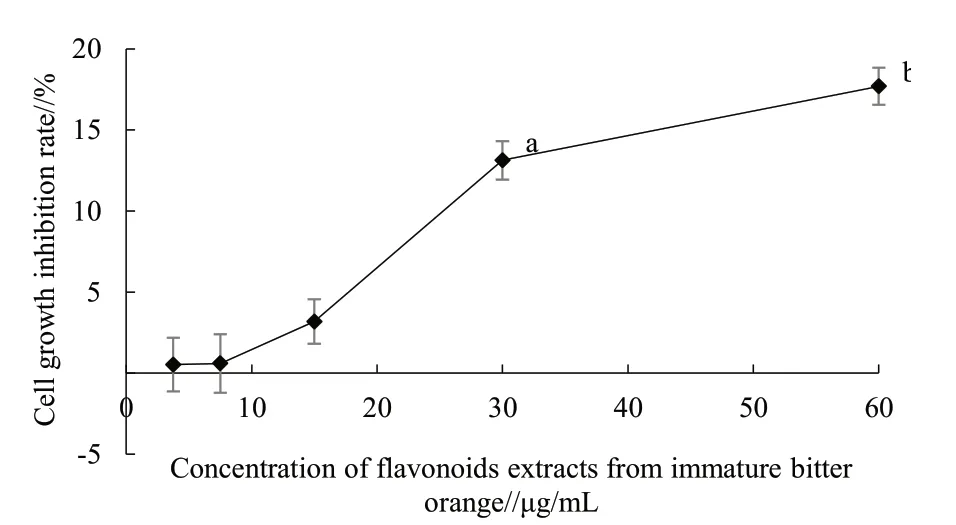
Fig. 3 Effects of flavonoids extracts from immature bitterorange on the viability of PC12 cells
3.3. Effects of flavonoids extracts from immature bitter orange and 6-OHDA on ROS level of PC12 cells
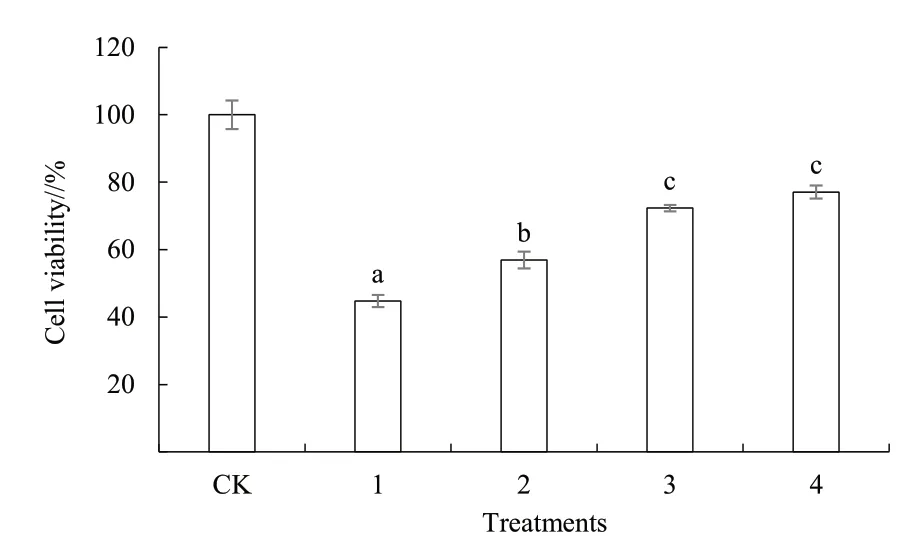
Fig. 4 Effects of flavonoids extracts from immature bitter orange on PC12 cell viability induced by 6-OHDA
The impact of flavonoids extracts from immature bitter orange and 6-OHDA on ROS level of PC12 cell were studied by flow cytometry. As can be seen from Fig. 5, compared with CK, 6-OHDA could induce ROS in PC12 cell release increase significantly;but 3.75, 7.5 and 15 μg/mL immature bitter orange flavonoids extracts could significantly alleviate the excessive production of ROS in PC12 cells by 6-OHDA.
3.4. Effects of of flavonoids extracts from immature bitter orange and 6-OHDA on MDA and antioxidant enzymes activity
As can be seen from Fig. 6, compared with CK,the content of MDA in 6-OHDA group increased significantly (Fig. 6A), while the activities of SOD,CAT and GSH-Px decreased significantly (P
<0.05);compared with the model group ( added only 6-OHDA), the content of MDA decreased significantly with the increase of the concentration of flavonoids extracts from immature bitter orange and the enzyme activities of SOD, CAT and GSH-Px all dramatically increased (P
<0.05), whereas 7.5 and 15 μg/mL flavonoids extracts from immature bitter orange had no significant dose effect on enhancing the antioxidant enzyme activity of PC12 cells induced by 6-OHDA.4. Conclusions and Discussions
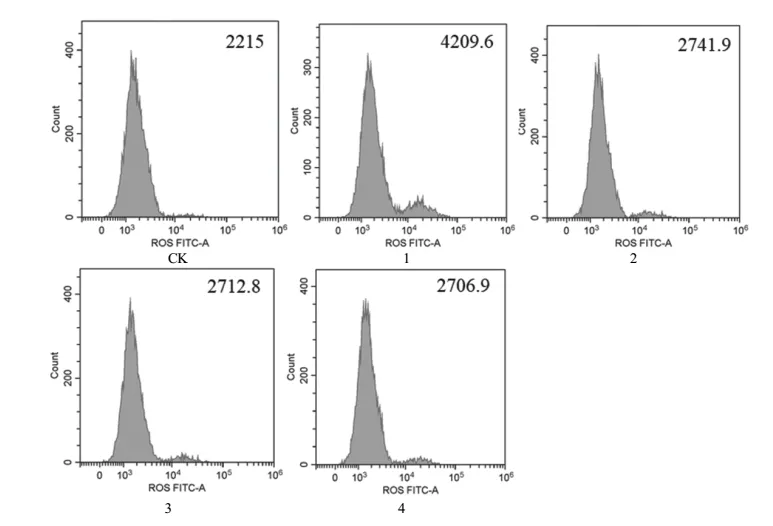
Fig. 5 Effects of flavonoids extract from immature bitter orange on ROS level of PC12 cells induced by 6-OHDA
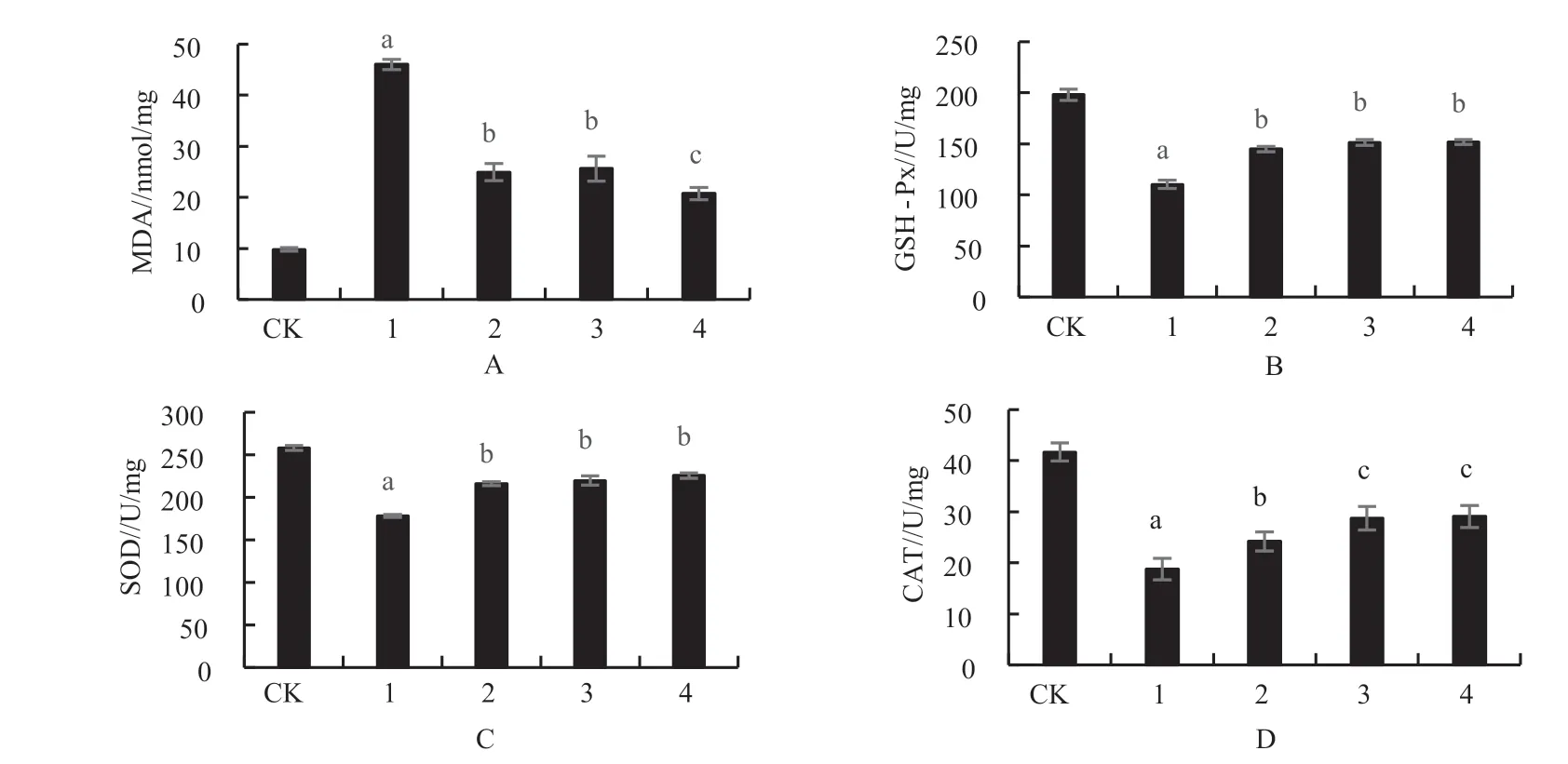
Fig. 6 Effects of flavonoids extracts from immature bitter orange on MDA content and antioxidant enzyme activity of PC12 cell induced by 6-OHDA
As the hydroxydated derivates of the neurotransmitter, dopamine, 6-OHDA has neurotoxicity to many species. It has been found that 6-OHDA shows strong oxidative damage abilityin vivo
andin vitro
,it can induce cells to produce a large number of substances such as hydrogen peroxide, reduce the activity of related antioxidant enzymes and promote the rapid accumulation of ROS. When the production of reactive oxygen species exceeds the clearance capacity of neurons, the lipid membrane structure in cells will be attacked and hence it lead to the generation of neurotoxicity. In this experiment, the in vitro PD model of PC12 cell injury was successfully constructed with 6-OHDA. Compared with the CK,the viability of PC12 cells decreased significantly after adding 6-OHDA. The levels of ROS and MDA increased significantly, while the activities of related antioxidant enzymes decreased significantly.Oxidative damage was one of the main mechanisms of PD occurrence and development.Oxidative stress initiated and aggravated PD response,which was mainly due to the excessive production of ROS. As a strong chemical media, ROS can act on cell membrane receptor kinase, regulate a variety of cell signal transduction pathways, damage the macromolecular structure and attack the lipid membrane of cells, hence produce neurotic poison,which finally contributes to the occurance of PD.Studies had shown that plant extracts can inhibit the excessive release of ROS induced by 6-OHDA, such as salidroside can reduce the excessive production of ROS in SH-SY5Y cells induced by 6-OHDA and played its antioxidant role. The study also found that 6-OHDA could induce a large increase in ROS and decrease the viability of PC12 cells, while the addition of different concentrations of flavonoids extracts from immature bitter orange could reduce the level of ROS in PC12 cells induced by 6-OHDA.
The excessive increase of ROS would aggravate the degree of cell oxidative damage. Normally,antioxidant enzymes such as SOD, CAT and GSH-Px could regulate the level of free radicals and the degree of cell damage could be reflected by MDA content.The plant extracts can make the amount of MDA decrease by improving the activity of the antioxidant enzyme to achieve the effect of treating PD. For example, antrodia cinnamomea polysaccharide showed good therapeutic effect on Parkinson's mice,its effect was related to regulating the activities of antioxidant enzymes SOD, CAT and GSH-Px and reducing the content of MDA. It was found that the flavonoids extract of immature bitter orange could significantly inhibit the increase of MDA content in PC12 cell induced by 6-OHDA. It was also found that it could significantly increase the activities of antioxidant enzymes such as GSH-Px, SOD and CAT,and these changes were positively correlated with the dose.
In conclusion, it was confirmed by cell biology and molecular biology analysis that flavonoids extracts from immature bitter orange colud effectively inhibit the damage of PC12 cells induced by 6-OHDA, which provided a theoretical basis for the application of immature bitter orange flavone, and its specific antinerve injury mechanism needed to be further studied.
杂志排行
Agricultural Science & Technology的其它文章
- Screening of an Antagonistic Strain of Phytophthora nicotianae and Its Application Potential
- Characterization of Specific Spoilage Bacteria and Volatile Flavor Compounds of Flavored Crayfish
- Effects of Boron Application on Growth and Quality of Flue-cured Tobacco NC297 under Different Potassium Supply Levels
- Efficient Extraction of Tea Saponin from Tea Seed Meals by 60Co γ-Irradiation
- Community Characteristics of Cardamine in Brassicaceae
- Effects of 4 Types of Remediation Agents on Reducing Cd Contents in Soil and Rice on Cd-contaminated Farmland
