Coping withextremes: lowered myocardial phosphofructokinase activities andglucose content butincreased fatty acids content inhighland Eurasian Tree Sparrows
2022-01-07BoyangDingYuliangZhaoYanfengSunQianZhangMoLiGhulamNabiYuefengWuChuanJiangandDongmingLi
Boyang Ding, Yuliang Zhao, Yanfeng Sun,2, Qian Zhang, Mo Li,3, Ghulam Nabi, Yuefeng Wu,Chuan Jiang* and Dongming Li*
Abstract
Keywords: Energy utilization, Eurasian Tree Sparrow, Metabolic enzyme, Myocardium, The Qinghai-Tibet Plateau
Background
High-altitude environments are characterized by low oxygen partial pressure and low ambient temperature(Storz et al. 2010). Theoretically, high-altitude endotherms have to cope with the hypobaric-hypoxia conditions by enhancing oxygen delivery and oxidative phosphorylation for metabolic thermogenesis relative to their low-altitude counterparts (Cheviron et al. 2014). For lowland dwellers, a suppressed overall metabolic intensity and enhanced glycolysis capacity are believed to be biochemical responses to hypoxic conditions due to the lowered blood O-delivery capacity (Cartee et al. 1991;Zinker et al. 1994; Azevedo Jr et al. 1995; Holden et al.1995; Roberts et al. 1996a, 1996b). In contrast to their lowland counterparts, high-altitude natives have possessed a suite of traits (e.g. bigger body size, larger lung,higher blood-Oaffinity, and more capillaries in organs)to overcome the consequences of hypoxic environments(Storz and Moriyama 2008; Qu et al. 2020). Furthermore,previous “Omics” studies have shown that strong positive selection in lipids metabolism is identified in those native endotherms of highland areas, such as birds (Ground TitParus
humilis
) (Qu et al. 2013), and mammals (YakBos mutus
and Plateau ZokorMyospalax
baileyi
) (Qiu et al.2012; Shao et al. 2015). Such an enhanced lipid metabolism is consistent with that of endotherms coping with cold, which suggests that the cold, rather than hypoxia, is the main stressful factor for free-living highland natives(Weber 2011). In humans, highland dwellers (i.e. Tibetans and Sherpas) also expressed a suite of convergent adaptations (e.g. larger motor organs and enhanced hemoglobin-Oaffinity) that are similar to those highland natives. However, they exhibited a strong positive selection in carbohydrate metabolism rather than lipid metabolism (Ge et al. 2012; Qu et al. 2015; Horscroft et al. 2017; Jing et al. 2021). Therefore, these findings suggest that evolutionary history, phylogeny, and habitat characteristics jointly play important roles in shaping the diversified strategies of high-altitude adaptations.The heart is the core engine for metabolism, which can drive the delivery of Oand metabolic substrates from the blood to target tissues and metabolic wastes from target tissues to excretory organs. For high-altitude animals,an efficient heart, therefore, is a proxy for the cardiopulmonary system under strong selective pressure of chronic low-oxygen environments (Ivy and Scott 2015; Sun et al.2016; Tate et al. 2017; Qu et al. 2020). Of importance, the heart has great flexibility on substrate usage that allows it to switch back and forth between fatty acid and carbohydrate oxidation, depending on the energy requirements and nutritional state of the organism, and the availability of glycolipid metabolic substrates to myocardium (Lopaschuk et al. 2010). Whether highland animals improve their metabolic capacities in the myocardium relative to their lowland counterparts is of interest and remains largely unclear.
In the myocardium, the mitochondrial oxidative phosphorylation contributes to approximately,95% of adenosine triphosphate (ATP) generation, and glycolysis contributes to the remainder (Ventura-Clapier et al. 2004). The carbohydrates (glucose and lactate) and fatty acids are the two primary energy substrates used by mitochondrial oxidative phosphorylation (Kodde et al.2007). In human, carbohydrates are more efficient than fatty acids, which can generate more contractile power for any given rate of myocardial oxygen consumption(Neglia et al. 2007). An increase in the uptake of glucose (Glu) and lactate is believed to enhance myocardial metabolic efficiency under acute cardiac stress, especially the contribution of glycolysis to the myocardial ATP production (Gertz et al. 1988; Camici et al. 1991;Bagger et al. 2000). In laboratory model mammals, the myocardial ischemia induced by hypoxia can increase the glycolysis to fulfill the workload of the heart, with a relative decrease in lipids uptake (Gertz et al. 1988; Fuxe et al. 1989; Camici et al. 1991; Bagger et al. 2000). On the other hand, myocardial fatty acids account for 60–90% of the total ATP generated in the resting fasting state (van der Vusse et al. 1992), which derives from the circulating triglyceride (TG) (Wang et al. 1998). However, there is limited knowledge on the substrate preference of either Glu or lipids utilization in the myocardium of highland animals.
The cardiovascular systems of birds and mammals are highly similar in form and modulation (Burggren et al.2011). However, birds have larger hearts, cardiac outputs,and stroke volumes but lower heart rates than mammals when body mass is controlled (Calder 1968; Grubb 1983).Furthermore, birds also have higher blood-Oaffinities(Storz and Moriyama 2008), do not have a hypoxic pulmonary vasoconstriction response (Scott et al. 2011), and are insensitive to hypercapnia (Faraci et al. 1984; Durmowicz et al. 1993; Groves et al. 1993; Ge et al. 1998; Sakai et al. 2003). These characteristics are critical for birds to fulfill Outilization for organs (tissue) under hypobaric hypoxia conditions.
图库一体化能在一个软件平台上,以一套数据完成建库和出图2种成果的更新生产,直接对库体数据进行基于范围的更新,通过一些显示技术实现,做到建库和出图的高度统一,实现数据的联动更新和快速成图。图库一体化模式用同一个软件操作,大大提高了更新效率,缩短了更新周期,能更好地满足对地理信息现势性的需求。
The Qinghai-Tibet Plateau (QTP) is the largest and highest plateau worldwide, featuring typical hypobaric hypoxia conditions (Thompson et al. 2000). Previous studies have found that some birds on the QTP genetically increased hemoglobin-Oaffinity and exhibited stronger hypoxia tolerance than their lowland counterparts (Scott and Milsom 2007; Zhu et al. 2018; Hao et al. 2019). As a small-sized passerine, the Eurasian Tree Sparrow (Passer
montanus
) is widely distributed from the lowland areas to the QTP (Summers-Smith 2018).As a typical human commensal species, this small-sized passerine bird is ideal for studying adaptative strategies to high-altitudes, owing to having almost similar highaltitude adaptation history with the Tibetans. It is known that Eurasian Tree Sparrows on the QTP have larger body sizes and wings, greater size-corrected pectoralis and lungs (Sun et al. 2016), larger myofibers in pectoralis but not in the myocardium (Qu et al. 2020), have similar patterns of stress responses relative to their lowland counterparts (Li et al. 2011).It was estimated that Eurasian Tree Sparrows had occupied the QTP after agricultural civilization (about 2600 years; Qu et al. 2020). Based on those findings in native Tibetans or those lowland animals acclimatizing to high-altitude environments showing a relatively lower ratio of lipid utilization but a higher ratio of carbohydrate utilization (Young et al. 1982, 1992; Sutton et al.1983; Green et al. 1989; Brooks et al. 1991, 1992; Roberts et al. 1996a), we hypothesized that the QTP Eurasian Tree Sparrows would enhance carbohydrate metabolism in the myocardium rather than lipid metabolism than their lowland counterparts. To validate our hypothesis,we compared the maximal activities of three rate-limiting enzymes in glycolysis (hexokinase, HK; phosphofructokinase, PFK; pyruvate kinase, PK), one key enzyme of the TCA cycle (citrate synthase, CS), a rate-limiting enzyme for transporting long chain free fatty acid (FFA)into the mitochondria (carnitine palmitoyl transferase 1, CPT-1); maximal activities for lactic dehydrogenase(LDH, a measure of tissue damage) and creatine kinase(CK; a measure of cardiac functions) and metabolic substrate contents for Glu, TG, and FFA in the myocardium between the QTP and lowland Eurasian Tree Sparrows.
Methods
Study area and sample collection
A total of 29 adult Eurasian Tree Sparrows were sampled using nylon mist nets from the QTP (8 males and 9 females; Gonghe County, Qinghai Province, 36.28°N,100.62°E, altitude of 3230 m; average temperature range:– 4 to 3 °C) and lowland area (7 males and 5 females;Pingshan County, Hebei Province, 38.25°N, 114.20°E,altitude of 80 m; average temperature range: of 3–6 °C)during the wintering stage between December 2014 and February 2015. Within 3–4 h post-capture, the birds were anesthetized by injecting with phenobarbitone (7.5 μL/g body mass) and humanely sacrificed. The left ventricle of hearts was rapidly dissected, washed with ice-cold phosphate buffer. All the samples were rapidly frozen in liquid nitrogen and stored at – 80 °C until assay.
Assays of metabolic enzyme activities and substrate concentrations
Samples of cardiac muscle were pulverized with a ceramic mortar and pestle under liquid nitrogen to powder. The powder was suspended by exacting solution (1:10, w/v) in an ice water bath. The mixed liquor was centrifuged at 11,000×g
for 10 min at 4 °C, and the supernatant was transferred to the new EP tube. Total protein content was measured by the BCA method with commercially available kits (CW0014, CoWin Biotech Co. Ltd., Jiangsu, China), and using BSA as standard.The maximal enzyme activities for all supernatant samples were measured in duplicate according to the manufacturer’s protocols. The kits for CPT-1 measurement were purchased from Enzyme-linked Biotechnology Co.Ltd. (Shanghai, China). The kits for the measurement of CS, HK, PFK, and PK were purchased from Ke-Ming Corp (Suzhou, China). Maximal enzyme activities of LDH and CK measured by an automated biochemistry analyzer (Mindray, mode BS-180, Shenzhen, China)using commercially available kits (Mindray, Shenzhen,China). The substrates were measured in 25 μL supernatant samples diluted with dHO (1:39) using an automatic biochemical analyzer (Mindray BS-180) with commercially available kits (Mindray Corp., Shenzhen, China).All samples were measured in duplicate. Intra- and interassay variation were 3.1% and 8.2% (Glu), 9.6% and 10.1%(TG), 4.2% and 6.3% (FFA), respectively. Assay sensitivity was 0.3 mmol/L (Glu), 0.03 mmol/L (TG), 0. 03 mmol/L(FFA).
Statistical analysis
The effects of altitude, sex, and the interaction of altitude and sex on the maximal enzyme activities of CS, CPT-1,HK, PFK, and PK, and substrate concentrations of Glu,TG, and FFA in the myocardium were determined using a generalized linear model (GLM), respectively. If a statistically significant effect was detected, we conducted multiple comparisons in each of the two groups using Bonferroni post hoc tests. AP
value < 0.05 represents a statistically significant difference. All the analyses were performed using IBM SPSS Statistics 25 (IBM Inc., NY,USA), and figures were generated using GraphPad Prism 8.4 (GraphPad Software Inc., CA, USA). The data are expressed as the mean ± SEM.Results
Effects of altitude and sex on metabolic enzyme activities in the myocardium
The myocardial PK, CPT-1, CK, and LDH activities of Eurasian Tree Sparrows did not vary with altitude, sex,and the interaction of altitude and sex (Table 1). The myocardial PFK did not vary with sex and the interaction of altitude and sex but varied with altitude.Post hoc
results showed that high-altitude sparrows had significantly higher PFK activities relative to low-altitude ones(Table 1; Fig. 1a). The myocardial HK and CS did not vary with altitude and the interaction of altitude and sex but varied with sex.Post hoc
results showed that male sparrows had significantly higher myocardial HK and CS activities than females (Table 1; Fig. 2a, b).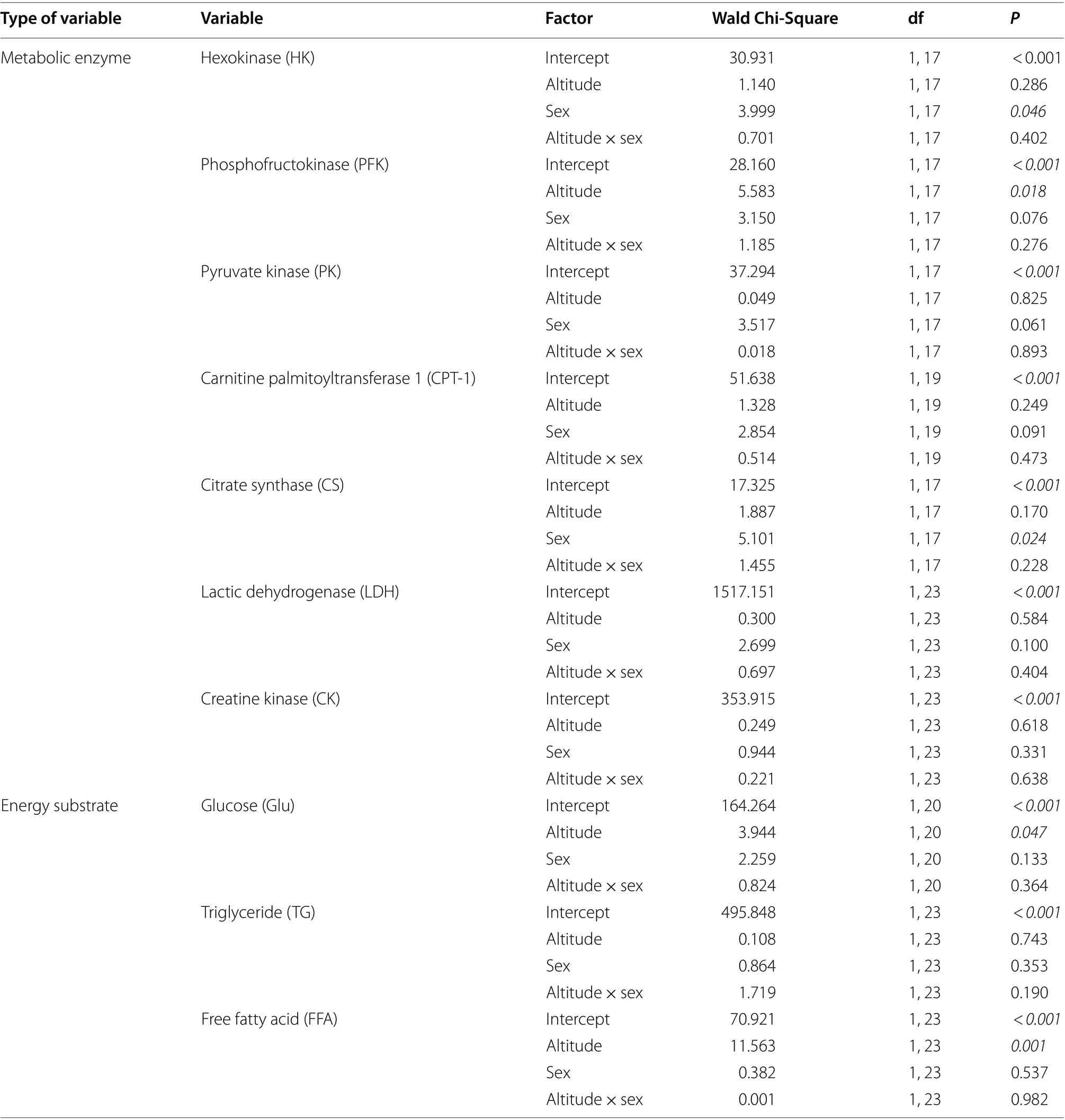
Table 1 Effects of altitude, sex, and interaction of altitude and sex on myocardial metabolic enzymes and energy substrates in Eurasian Tree Sparrows (Passer montanus)
Effects of altitude and sex on glucolipid substrates in the myocardium
The myocardial TG did not vary with altitude, sex,and the interaction of altitude and sex (Table 1). The myocardial Glu and FFA contents did not vary with sex and the interaction of altitude and sex but varied with altitude.Post hoc
results showed that high-altitude sparrows had significantly lower myocardial Glu but higher FFA concentrations (Fig. 1b, c).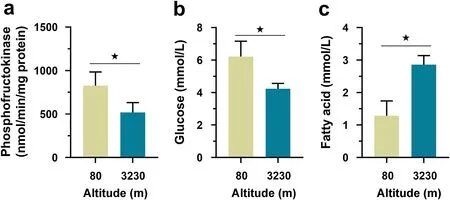
Fig. 1 Comparisons of maximal phosphofructokinase (PFK) activity (a), glucose (Glu) content (b), and free fatty acid (FFA) content (c) in themyocardium of Eurasian Tree Sparrows (Passer montanus) between high altitude (3230 m) and low altitude (80 m) areas. The statistical results are shown in Table 1. The asterisk represents a significant difference (P < 0.05) between groups
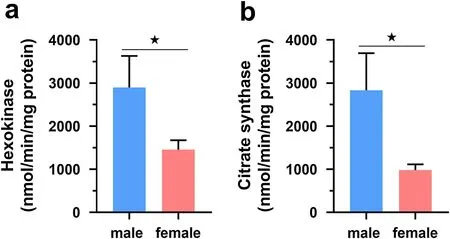
Fig. 2 Comparisons of maximal hexokinase (HK) activity (a) and citrate synthase (CS) activity (b) in the myocardium of Eurasian Tree Sparrows(Passer montanus) between sexes. The statistical results are shown in Table 1. The asterisk represents a significant difference (P < 0.05) between groups
Discussion
In the present study, we found that maximal enzyme activities of myocardial HK and PK in Eurasian Tree Sparrows did not vary with altitude, but the QTP population had significantly lower maximal enzyme activities of PFK and glucose content in the myocardium. As a critical enzyme for phosphorylating fructose 6-phosphate to fructose 1, 6-bisphosphate of glycolysis, the PFK is negatively inhibited by ATP and citrate (fuel substrate of the TCA cycle). Theoretically, enhanced PFK activity will consume more Glu and indirectly result in a reduction of Glu levels, which will eventually lead to mobilizing other fuel substrates. Our observed lower Glu contents and decreased maximal PFK activities in the myocardium,along with lower plasma Glu levels of the QTP Eurasian Tree Sparrows relative to their lowland counterparts (Li et al. unpublished data), suggesting highland sparrows may weaken the glycolytic pathway through lowering maximal PFK activity and tend to depend on other fuel substrates.
Meanwhile, we found the QTP sparrows had comparable TG contents and maximal CPT-1 activities but increased FFA levels in the myocardium relative to lowland ones. In the hearts, the increased FFA supply through hydrolysis of circulating lipoproteins (via lipoprotein lipase) is considered as a way to compensate for the diminished contribution of glucose as an energy substrate (Pulinilkunnil and Rodrigues 2006). Therefore, our results suggest that the QTP sparrows should enhance the FFA transportation from blood to compensate for the lowered Glu contents in the heart. This preference for metabolic substrate utilization is similar to that of Deer Mice (Peromyscus
leucopus
), a highland native small mammalian species (Sears et al. 2006; Vaillancourt et al.2009; Cheviron et al. 2012). Previous studies have shown that the QTP sparrows have greater body weight and heart size (Sun et al. 2016) relative to the lowland ones,which is believed to be fundamental for preventing heat dissipation and enhancing energy efficiency. Considering that lipids have a much higher energy density than carbohydrates (Berg et al. 2002; Hochachka and Somero 2002; Belitz et al. 2009), the increase in FFA and decrease in Glu influx in the myocardium might be a metabolic strategy to enhance energy efficiency for coping with the extreme environments. Whether this strategy occurs in other organs (tissues) remains to be further determined.We detected both highland and lowland sparrows had similar maximal LDH and CK activities, which means similar maximal velocity on lactate metabolism for both populations. There are two possible explanations: (1)highland sparrows are able to counter the adverse effect of anaerobic metabolism, which is more easily induced by hypoxic environment (Samaja et al. 1997; Pilarski et al.2009), on acid–base equilibrium in cardiomyocytes via lower glycolysis level (Burggren and Cameron 1980; Casiday and Frey 2012; Hebisz et al. 2016); (2) both population possessed similar maximal capacity on production and regeneration of ATP by lactate metabolism (Ingwall et al. 1985; Kodde et al. 2007), which indicate that the highland sparrows, compared to their lowland counterparts, could better cope with the extra demand of ATP when the cardiac workloads increased but the aerobic metabolism is suppressed. Since the leakage of LDH and CK into the cardiovascular system are documented as indexes of myocardial injury (Kaur et al. 1997), our results showed that the cardiac functions of both the highland and lowland sparrows were unlikely to be injured. Considering that limited information on cardiac functions is available between high and low altitude animals, other parameters such as heart beat rate and blood pressure are also fundamental for better understanding the physiological and ecological strategy of high-altitude adaptation,warrant to be further investigated.
Interestingly, we found male sparrows had significantly higher activities of HK and CS than females, independent of altitude. Our results are inconsistent with the finding showing no differences in CS and HK activities in the myocardium between sexes in rats (Milerová et al. 2016). Generally, the sex-based differences for animals in behavior, neuroendocrinology, and physiology are generally related to stress response, locomotor ability and body composition, etc. (Blanchard et al. 1995;Koolhaas et al. 1995; Martinez et al. 1998). Furthermore,several researchers have reported sex-based differences in the human cardiovascular system, such as in cardiac growth, Ouptake, resting heart rate, myocardial contractile performance and modulation of arterial blood pressure (Vinet et al. 2003; Kolar and Ostadal 2013).These sex-dependent differences in the cardiovascular system are largely associated with sex hormones (Hayward et al. 2001; Lagranha et al. 2010; Murphy 2011). In our previous study, we did not detect sex-based differences in baseline and stress-induced corticosterone content (Sun et al. unpublished data) between highland and lowland Eurasian Tree Sparrows, which can rule out the metabolic impacts induced by glucocorticoids. Moreover,male sparrows tend to have longer wings, greater wing areas, and total flight muscle mass relative to females(Mónus et al. 2011; Sun et al. 2016, 2017). Whether increased overall cardiac metabolic capacity and myocardium glycolysis are associated with meeting the energy requirements of their vigorous metabolic organs for flight remains further investigation.
Conclusions
In the present study, we identified that the QTP Eurasian Tree Sparrows have lower maximal myocardial PFK activities and Glu content but higher FFA content relative to their lowland counterparts. Although this species is an invasive species on the QTP introduced along with humans, it exhibited reduced Glu utilization and enhanced FFA utilization in the myocardium, which differs from the Tibetans showing increased carbohydrate utilization but agrees with those small-sized high-altitude natives. Our results did not detect any differences in other measured metabolic enzyme activities between highland and lowland populations, indicating the maximal activities of these metabolic enzymes are highly conserved and independent of environmental conditions.These identified differences and similarities of metabolic enzymes and metabolic substrates in the myocardium of Eurasian Tree Sparrows contribute to uncovering the coping mechanisms of physiological adjustments for adapting to the extreme conditions of the QTP. Still, how highland Eurasian Tree Sparrows can express hypoxic tolerance and orchestrate energy substrate utilization in other organs and what is the underlying regulatory mechanism remains to be further determined.
Acknowledgements
We thank Zhipeng Ren, Yinchao Hao, Simeng Yu, and Haiqing He for their assistance with sample collection in the field.
Authors’ contributions
BD and YZ: methodology, validation; YS and ML: fieldwork and investigation;GN and YW: help of writing original draft; QZ, laboratory assays; CJ: conceptualization, supervision; DL: conceptualization, supervision, funding acquisition.All authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (NSFC, No. 31971413) to DL and NSFC (No. 31770445) to YW, the Second Tibetan Plateau Scientific Expedition and Research Program (STEP,2019QZKK0501), and the Natural Science Foundation of Hebei Province(NSFHB, C2020205038) to DL, the Foundation of Hebei Normal University(L2019B26) to CJ, and the Post-doctoral Research Programm to PD.
Availability of data and materials
The datasets used in the present study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The present study complies with the current laws of China. Fieldwork was carried out under permission from the Department of Wildlife Conservation(Forestry Bureau) of Hebei Province and Qinghai Province. Experimental procedures were permitted by the approval of the Institutional Animal Care and Use Committee (HEBTU2013-7) and the Ethics and Animal Welfare Committee (No.2013-6) of Hebei Normal University, China.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Author details
Key Laboratory of Animal Physiology, Biochemistry and Molecular Biology of Hebei Province, College of Life Sciences, Hebei Normal University,Shijiazhuang 050010, China.Ocean College, Hebei Agricultural University,066003 Qinhuangdao, China.College of Life Science, Cangzhou Normal University, 061001 Cangzhou, China.
Received: 19 April 2021 Accepted: 1 September 2021
杂志排行
Avian Research的其它文章
- Taxonomic revision of the Savanna Nightjar (Caprimulgus affinis) complex based on vocalizations reveals three species
- Taxonomic status of grey-headed Yellow Wagtails breeding in western China
- The composition of mixed-species flocks of birds in and around Chitwan National Park,Nepal
- True grit: ingestion of small stone particles by hummingbirds in West Mexico
- Stopover behavior of Red-eyed Vireos (Vireo olivaceus) during fall migration on the coast of the Yucatan Peninsula
- Phylogeography and morphometric variation in the Cinnamon Hummingbird complex: Amazilia rutila (Aves: Trochilidae)
