Egg rejection and egg recognition mechanisms in Oriental Reed Warblers
2022-01-07LaikunMaandWeiLiang
Laikun Ma and Wei Liang
Abstract
Keywords: Cuckoo parasitism, Discordancy, Egg recognition mechanism, Egg rejection, True recognition
Background
Avian brood parasitism is a specific reproductive behavior in which parasitic birds lay eggs in the nests of other birds (hosts), transferring the costs of incubation and raising offspring to the host (Payne 1977). Successful nest parasitism results in significant reproductive losses to the host, prompting the host to evolve a range of antiparasitic strategies (Davies 2000; Soler 2014). Egg recognition and egg rejection are among the most common antiparasitic strategies and are important indicators of host adaptation to nest parasitism (Davies and Brooke 1989a; Moksnes et al. 1991b; Soler et al. 2017;Yang et al. 2020, 2021). For example, in cuckoo parasitic systems, bird species lacking a history of cuckoo parasitism has low or no egg recognition ability, whereas cuckoo hosts often have varied egg recognition abilities(Davies and Brooke 1989a, b; Moksnes et al. 1991a, b).Egg recognition ability acquired by hosts can be maintained for a considerable period of time (Peer et al.2007, 2011; Yang et al. 2014b; Yi et al. 2020), even in the absence of cuckoo brood parasitism (Honza et al. 2004;Lahti 2005, 2006). However, the level of host egg recognition ability is subject to variation under different parasitic pressure and coevolutionary time, with inter- and intra-specific variations in recognition and rejection of foreign eggs among hosts in the same area or among geographic populations of the same host (Brooke et al.1998; Lindholm and Thomas 2000; Moskát et al. 2002,2012; Li et al. 2016; Liang et al. 2016).
In general, the process of egg rejection by host consists of at least three steps: first, the recognition of foreign eggs; second, the decision to discard eggs or not;and last, the rejection of foreign eggs (Soler et al. 2012,2017). There are two plausible views on the mechanism of host recognition of foreign eggs; one is templatebased recognition, also known as true recognition(Rothstein 1974, 1975; Hauber and Sherman 2001), in which the host knows the characteristics of its own eggs through inheritance or learning and recognizes foreign eggs, independent of whether its own eggs are present or predominant in the nest (i.e., Moskát and Hauber 2007; Moskát et al. 2010; Yi et al. 2020). The second is recognition by discordancy, the simplest form of egg recognition, which determines the least similar eggs as foreign based on the discordancy of egg types within the nest (Rensch 1925; Rothstein 1974, 1975;Davies and Brooke 1989a; Yang et al. 2014c).
Additionally, sex conflict can occur in antiparasitic behavior due to differences in the role division between males and females during breeding (Požgayová et al.2009; Trnka and Prokop 2010; Trnka et al. 2013), with males showing more aggressive behavior towards nest invaders (Montgomerie and Weatherhead 1988). A study by Li et al. (2015) on Oriental Reed Warblers(Acrocephalus orientalis
) found that males were more aggressive in nest defense, while females were more likely to detect intruders. Studies of Great Reed Warblers (Acrocephalus arundinaceus
) found that males were primarily responsible for territory defense, while females were responsible for incubation and egg recognition (Požgayová et al. 2009; Trnka and Prokop 2010).Another study of Ashy-throated Parrotbills (Paradoxornis alphonsianus
) with egg color polymorphism found that both males and females have egg recognition ability, but they may use different recognition mechanisms (Liang et al. 2012).The Oriental Reed Warbler, a favorite host of Common Cuckoos (Cuculus canorus
) (hereafter cuckoos) in China,is at a high stage of co-evolution with cuckoos (Yang et al.2014a; Li et al. 2016; Ma et al. 2018a). Egg recognition ability of Oriental Reed Warblers is known to be high,and the Common Cuckoo has formed a highly mimetic reed warbler clade (gens) in the local population (Li et al.2016; Yang et al. 2016, 2017). Previous work showed that only females incubate eggs, while males are responsible for guarding nearby during the incubation period (Lotem et al. 1992). In this study, we tested egg rejection and egg recognition mechanism of Oriental Reed Warblers by controlling the ratio of experimental eggs and its own eggs in its nests. In addition, we explored whether there were sexual differences in egg recognition by Oriental Reed Warblers.Methods
Study area
The study area is located in the Yongnian Depressional Wetland (36° 40′–36° 41′ N, 114° 41′–114° 45′ E)in Yongnian County, Hebei Province, China, which is a natural depression in the alluvial plain of the Fuyang River. The Yongnian Depressional Wetland has a welldeveloped water system, numerous tributaries, and yearround waterlogging, at an altitude of only 40.3 m. The average annual rainfall is 527.8 mm, mostly in summer,and the average annual temperature is 12.9 °C. The main vegetation type of the wetland is reeds (Phragmites australis
), which are interspersed mainly with cattails (Typha latifolia
) (Ma et al. 2018a, b).Sexing of Oriental Reed Warblers in the field
Both sexes of Oriental Reed Warblers are similar. Our observations show that males have towering head feathers that form a small crest, whereas the females have gentle head feathers and no crest under normal status.This was confirmed with video monitoring showing that incubating parents had the plumage characteristics of females, and it is known that only females incubate eggs(Lotem et al. 1992). In addition, most cases of egg rejection were carried out obviously by incubating individuals without crest. Therefore, the sex of Oriental Reed Warblers can be determined by the pattern of head feathers from videos (Fig. 1).
Egg recognition experiments
Fieldwork was conducted during the 2016 and 2017 breeding seasons. Because parasitism by common cuckoo usually occurs in the laying or early incubation cycle of host nests for advantage of prior hatching(Geltsch et al. 2016; Wang et al. 2020), egg recognition experiments (one model egg or one real foreign egg was added to an experimental nest) were carried out during the early incubation period, along with video recording using a minicamera. Blue and white model eggs were produced based on Li et al. (2016) using a highly plastic synthetic soft clay to the size of local cuckoo eggs (Table 1).The real eggs used were white unfertilized Budgerigar(Melopsittacus undulatus
) eggs coming from artificially bred parrots.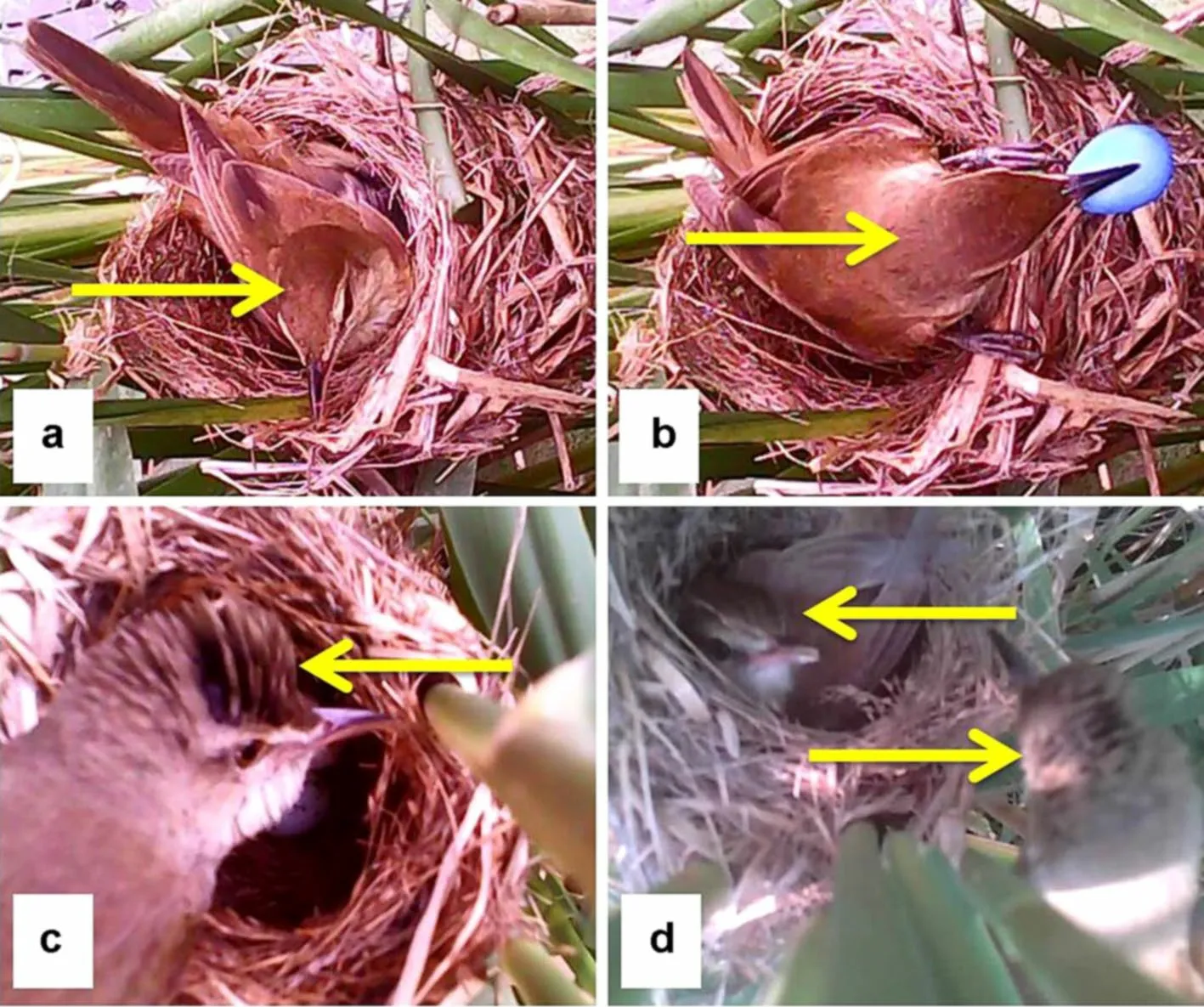
Fig. 1 Comparison of male and female individuals of Oriental Reed Warblers. a refers to a female incubating in the nest; b refers to a femalerejecting a blue model egg; c refers to a male checking the nest and d refers to a female incubating and a male checking the nest. Yellow arrows show the top of the head of both sexes, with the female being flat and the male having a crest
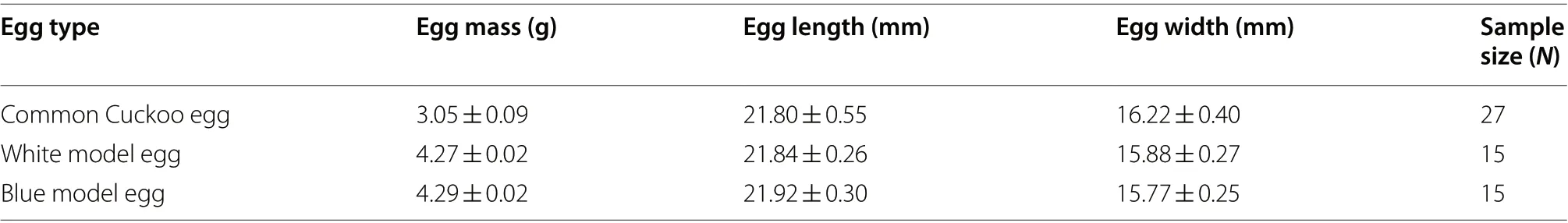
Table 1 Parameters of cuckoo and experimental eggs used in this study
Previous studies have shown that replacement with or direct addition of one experimental egg does not affect the egg rejection of the host (Davies and Brooke 1988), so the egg recognition ability of Oriental Reed Warblers was tested by direct addition of a blue model egg (highly nonmimetic) to the host nest (Fig. 2a). The host’s response to experimental eggs within six days was observed (cf. Moksnes et al. 1991b): if the host did not reject the experimental egg within six days, it was recorded as accepted;if the egg was pecked, disappeared or deserted within six days, it was considered rejected. Experimental nests that were predated or destroyed because of bad weather within six days were not counted in the results of the experiment (see also Yang et al. 2019).
Egg recognition mechanism experiments
In 2017, two sets of experiments were conducted. In the first experiment, the number of Oriental Reed Warbler eggs and experimental eggs were controlled to both be two to maintain the same proportion. Host eggs were removed provisionally so that there were only two, and two experimental eggs were added to the nest. Experimental eggs used either two white model eggs (Fig. 2b)or two white budgerigar eggs (Fig. 2c). This experiment is thus referred to as the 2 + 2 experiment. Eggs were observed for 6 days, and the results were recorded. In the second experiment, the number of Oriental Reed Warbler eggs and experimental eggs was controlled to be one and three, respectively. That is, the number of host eggs was controlled to be lower than that of experimental eggs, because Oriental Reed Warblers are likely to abandon the nest when only one egg is left in the nest.This experiment is thus referred to as the 1 + 3 experiment. In a pre-experiment, we found that Oriental Reed Warblers were able to quickly recognize and reject experimental eggs in the 1 + 3 experiment, and that the timely return of Oriental Reed Warbler eggs to the nest after the experiment could avoid nest desertion, so we checked the experimental results within half an hour after starting the experiment. The experimental eggs used in the 1 + 3 experiment were white budgerigar eggs (Fig. 2d). The recognition mechanism experiment was performed only once for each nest.

Fig. 2 Egg recognition experiments in nests of the Oriental Reed Warbler. a refers to experimental nest with adding one blue model egg; b and c refer to experimental nests with two experimental eggs and two host eggs (i.e., 2 + 2), while experimental eggs in b are white model eggs and c are white budgerigar eggs; d refers to experimental nests with one host egg and three budgerigar eggs (i.e., 1 + 3)
Data analyses
Statistical analyses were performed using IBM SPSS 20.0 for Windows. Fisher’s exact test was used for comparison between different probabilities and Wilcoxon rank sum test was used to compare the number of nest-returns and egg checks between males and females before egg rejection. All tests were two-tailed, with a significance level ofP
< 0.05, and data are presented in the form of mean ± standard deviation (mean ± SD).Results
Egg rejection
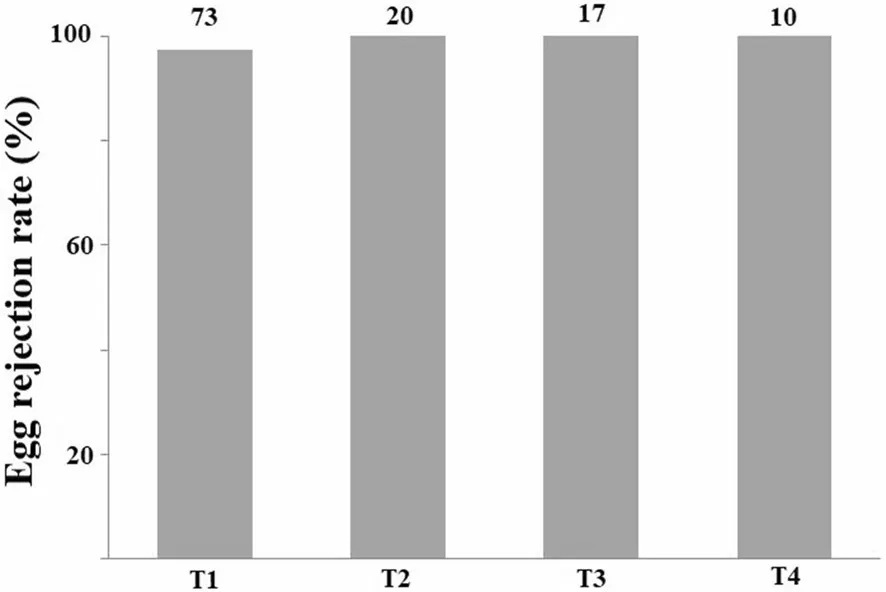
Fig. 3 Egg rejection rates by Oriental reed warblers in egg recognition mechanism experiments. T1 refers to experimental group with one blue model egg added to the nest; T2 refers to experimental group with two host eggs and two white model eggs; T3 refers to experimental group with two host eggs and two white budgerigar eggs; T4 refers to experimental group with one host egg and three white budgerigar eggs. Numbers above bars indicate the sample size
Egg recognition experiments with non-mimetic blue model eggs were performed in 75 nests of Oriental Reed Warblers. Blue mode eggs were rejected 73 times and accepted two times, at a rejection rate of 97.3% (Fig. 3;Additional file 1: Video S1). Furthermore, Oriental Reed Warblers rejected all foreign eggs with a rejection rate of 100% in 11 nests which one foreign egg was added (4 nests with a budgerigar egg, 6 nests with a black-painted Budgerigar egg, and one nest with a Reed Parrotbill (Paradoxornis heudei
) egg, Additional file 2: Video S2).Egg recognition mechanisms
The 2 + 2 experiment was performed in a total of 20 nests with white model eggs and 17 nests with white Budgerigar eggs. The 1 + 3 experiment was conducted in 10 nests with white budgerigar eggs. Across all experimental nests, Oriental Reed Warblers were able to accurately recognize its own eggs while rejecting the foreign eggs,with a recognition rate of 100% and no recognition errors were found, showing the true recognition mechanism(Fig. 3).
Sex roles in egg recognition
Egg rejections were recorded on videos in 30 nests lasting about two hours until the power run out, including 19 nests for recognition mechanism experiments, and 11 nests in which one foreign egg was added for egg recognition experiments. All eggs were ejected and completed by female Oriental Reed Warblers, 16 of which had males returning and checking eggs prior to egg rejection.Females had significantly more numbers of returning the nest (Female: 8.10 ± 8.38, Male: 0.87 ± 1.33,N
= 30) and checking eggs (Female: 56.33 ± 67.01, Male: 1.70 ± 3.23,N
= 30) than males for all nests before egg rejection(Z
= − 4.643,P
< 0.01;Z
= − 4.585,P
< 0.01).Discussion
Egg recognition is one of the most effective means for hosts to combat parasitism, but even different geographic populations of the same host can exhibit significant geographic variation in the recognition and rejection of foreign eggs due to differences in the history of co-evolution and reciprocal pressure (Brooke et al. 1998; Lindholm and Thomas 2000; Moskát et al. 2002, 2012; Li et al. 2016;Liang et al. 2016). In this study, Oriental Reed Warblers,as one of the most common hosts of cuckoos, have a nearly 100% rejection rate for non-mimetic eggs, similar to the population in northeastern China, which rejected both blue (n
= 15) and white (n
= 24) model eggs at a rate of 100% (Wang et al. 2021) and the Japanese population (94%,n
= 33; Lotem et al. 1995), suggesting that the Oriental Reed Warbler possesses an extremely strong egg recognition ability. However, egg rejection of another population of Oriental Reed Warblers (Li et al. 2016,2020) was slightly lower than that of this population, possibly because the material and size of experimental eggs used may influence on the host’s completion of egg rejection (Roncalli et al. 2017; Li et al. 2020).In examination of recognition mechanisms of the Oriental Reed Warbler, we found that regardless of the ratio of warbler eggs and experimental eggs in the nest, the Oriental Reed Warbler was able to recognize 100% of experimental eggs and reject them quickly, suggesting that the Oriental Reed Warbler uses template memory to recognize foreign eggs and shows a true recognition mechanism. Template recognition is a ubiquitous recognition mechanism in hosts, and discordancy recognition has not been found in isolated cases to date, but often coexists with template recognition (Lyon 2007; Moskát et al. 2014; Yang et al. 2014c; Wang et al. 2015). Moskát et al. (2010) found that the European Great Reed Warbler uses both template and discordancy recognition, which was unlike the present results. This may be due to differences in geographic factors that cause the two hosts to be at different stages of coevolution with cuckoos, as was found in a study of European Great Reed Warblers and the Japanese Oriental Reed Warblers, where the two were clearly at different stages of coevolution (Moskát et al. 2012). Another possible reason is that the two studies used different experimental eggs, with the degree of egg mimicry being an important factor in host recognition and rejection (Davies and Brooke 1988; Stokke et al.2004; Antonov et al. 2006). Moskát et al.’s (2010) experiments used the host’s own real egg painted with spots as experimental eggs, and the similarity between the two egg types may have led to difficulties in host recognition,e.g., the rejection rate of a single experimental egg by the host in the experiment was only 32% (6/19), which was much lower than the previously reported rejection rate of non-mimetic eggs by the same host species (Moskát et al.2008). Clearly, these need to be confirmed further in the future to use similar and mimetic experimental eggs to examine the recognition mechanism in warbler hosts.
The egg rejection behaviors recorded on videos in this study all occurred in females, which may be due to the differential division of roles between male and female individuals during reproduction (Požgayová et al.2009; Trnka and Prokop 2010). Oriental Reed Warbler males are not involved in egg incubation, but in territory defense and nest defense (Li et al. 2015); whereas females are responsible for egg incubation. By contrast,in ashy-throated parrotbills, males and females take turns incubating eggs, and both males and females have egg recognition ability but use different recognition mechanisms to reject eggs to ensure maximum benefit(Liang et al. 2012; Yang et al. 2014c). Therefore, the sex for incubation seems to play an important role in the evolution of host egg recognition. Such sexual difference is also present in the anti-parasitic behavior of the host’s nest defense (Požgayová et al. 2009; Trnka and Prokop 2010; Li et al. 2015).
Conclusions
In summary, the present study showed that Oriental Reed Warblers possess a high degree of egg recognition ability and employs a true recognition mechanism. In addition, all egg rejection is conducted by female individuals with ejection. The sexual difference for host nest defense and egg incubation seems to play an important role in the evolution of egg recognition mechanisms,which needs more investigations in future work.
Supplementary Information
The online version contains supplementary material available at https:// doi.org/ 10. 1186/ s40657- 021- 00283-4.
Additional file 1: Video S1. A blue model egg rejected by the Oriental Reed Warbler host in the egg recognition experiment.
Additional file 2: Video S2. A Reed Parrotbill egg rejected by the Oriental Reed Warbler host in the egg recognition experiment.
在过去的几年里,由于缺乏统一的协作机制,即使签订了一些“协议”或“宣言”,开发工作仍然各自为政。旅游景区和旅游路线就好比珍珠和金线,想要做一串漂亮的手链,珍珠和金线同等重要。但大别山红色旅游业的珍珠和金线均打造不足。珍珠方面,大别山红色旅游项目多以静态观瞻性景点为主,旅游形式单一,缺乏看点和兴奋点。金线方面,虽然30条“红色旅游精品线”中大别山有两条,但还需要凝练出更多的精品旅游路线。由此构建基于SCS理念模型的大别山红军文化主题旅游产品显得尤为必要了。
Acknowledgements
We thank the Forestry Bureau of Yongnian County, Hebei Province, China, for permission to undertake this study. We are grateful to Canchao Yang and Jianping Liu for help with statistical analyses, Xiaodong Rao and Jianwei Zhang for assistance with fieldwork.
Authors’ contributions
WL designed the study; LM carried out field experiments, performed statistical analyses and wrote the draft manuscript. WL revised and improved the manuscript. Both authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China(No. 31970427 to WL and 32101242 to LM) and by the Open Foundation of Hebei Key Laboratory of Wetland Ecology and Conservation (hklk201903 to LM) and the Natural Science Foundation of Hebei Province of China(C2020101002 to LM).
Data availability statement
The data that supports the findings of this study are available in the supplementary material of this article.
Declarations
Ethics approval and consent to participate
The experiments comply with the current laws of China. Experimental procedures were in agreement with the Animal Research Ethics Committee of Hainan Provincial Education Centre for Ecology and Environment, Hainan Normal University (permit no. NECEE-2012-003).
Competing interests
The authors declare that they have no competing interests.
Author details
Department of Biology and Food Science, Hebei Normal University for Nationalities, Chengde 067000, China.Ministry of Education Key Laboratory for Ecology of Tropical Islands, College of Life Sciences, Hainan Normal University, Haikou 571158, China.Hebei Key Laboratory of Wetland Ecology and Conservation, Hengshui University, Hengshui 053000, China.
Received: 19 June 2021 Accepted: 7 September 2021
杂志排行
Avian Research的其它文章
- Taxonomic revision of the Savanna Nightjar (Caprimulgus affinis) complex based on vocalizations reveals three species
- Taxonomic status of grey-headed Yellow Wagtails breeding in western China
- The composition of mixed-species flocks of birds in and around Chitwan National Park,Nepal
- True grit: ingestion of small stone particles by hummingbirds in West Mexico
- Stopover behavior of Red-eyed Vireos (Vireo olivaceus) during fall migration on the coast of the Yucatan Peninsula
- Phylogeography and morphometric variation in the Cinnamon Hummingbird complex: Amazilia rutila (Aves: Trochilidae)
