ln depth understanding of retinitis pigmentosa pathogenesis through optical coherence tomography angiography analysis: a narrative review
2021-12-17BingWenLuGuoJunChaoGaiPingWuLiKeXie
Bing-Wen Lu, Guo-Jun Chao, Gai-Ping Wu, Li-Ke Xie
Department of Ophthalmology, Ophthalmology Hospital of China Academy of Traditional Chinese Medicine, Beijing 100040, China
Abstract
● KEYWORDS: retinitis pigmentosa; optical coherence tomography angiography; vascular dysfunction; microglia activation
INTRODUCTION
Progress degeneration of photoreceptors in retinitis pigmentosa (RP) eventually leads to blindness. The worldwide incidence of RP is about 1 in 4000, making it one of the most common causes of visual impairment[1]. Patients with RP typically experience impaired dark adaption, night blindness, followed by progressive visual field constriction,and eventually central vision deterioration[2].
A major goal for RP research is to determine how various rod specific gene mutations lead to subsequent cone degeneration,accompanied by retinal ganglion cells (RGCs) and retinal pigment epithelium (RPE) changes. Fundus examination shows peripheral bone-spicule deposits and reduced retinal blood vessels[3]. Electroretinogram (ERG), which is the golden diagnostic standard, demonstrates reduced rod and cone response amplitudes coupled with a delay in their timing[4]. Visual field (VF) loss is an important indicator of disease progression and treatment efficacy, changing from patchy loss of peripheral VF to a ring scotoma, tunnel vision,and eventually blindness[5]. Vascular dysfunction has been recognized to play a role in RP development recently. Imaging evidence from the novel high-resolution optical coherence tomography angiography (OCTA) of retinal and choroidal vasculature may be essential for elucidating the progression of retinal degeneration in this disease and developing effective therapies[6].
Reductions in retinal blood flow have been established in RP using OCTA, however, questions have yet to be answered regarding the relationship between vascular dysfunction and RP and the underlying pathogenesis. In this review, we propose a hypothesis of RP pathogenesis linked with the summarily findings of OCTA studies in RP patients. We also address pertinent future perspectives, providing an overview of how animal studies may help, how OCTA technology should develop, and what potential treatment options can be anticipated with the enhanced understanding.
WHAT ARE THE POSSIBLE FACTORS RELATED TO RETINITIS PIGMENTOSA PATHOGENESIS?
Hereditary degenerations of the human retina are a diverse group of clinically and genetically heterogeneous blinding diseases with more than 260 causal genes identified to date[7]. The pathogenesis of RP is complicated, which is mainly related to photoreceptor genetic alterations, leading to primary degeneration of rods, and secondary degeneration of cones. Further changes in RGCs and RPE, the inner retina disorganization as well as the vascular supply attenuation,following the outer retina damage ultimately cause the vision loss.The role of microglial activation in RP, a common hallmark of many retinal disease[8-10], has been proved by both clinical and pre-clinical studies[11-12]. In the normal retina, microglia mainly accumulate in the plexiform layers (restricted to the inner layers of the retina) with long protrusions continuously monitoring the micro-environmental homeostasis; however,different triggers originating from photoreceptors degeneration rapidly alert microglia, leading to their migration into the damaged layers and transformation into phagocytes, interacting with infiltrating blood cells[8-12]. In addition to phagocytosis of degenerated cells, these reactive microglia in the outer retina exacerbate photoreceptor cell death as well as secrete large amounts of pro-inflammatory neurotoxic factors[8-12].
Also, changes in the retina vasculature and hemodynamics have long been associated with RP. Fundamentally, retinal blood vessels have a pronounced autoregulation ability,whereas the choroidal tissue is regulated by the sympathetic and parasympathetic nervous systems. Either hyperoxic or hypoxic state could trigger the autoregulation of blood vessels to maintain retinal homeostasis. Histopathological studies in RP eyes showed an important vascular remodeling in both retina and choroid[13]. Also, significant evidences have been established recently to support the theory that vascular dysfunction is associated with but not the cause of photoreceptors death in RP.
Hence, we propose a pattern of etiologic and pathogenic factors leading to retinal degeneration of RP based on the current understandings, highlighting the two key factors(Figure 1).
WHAT IS OUR HYPOTHESIS ON RETINITIS PIGMENTOSA PATHOGENESIS WITH OCTA FINDINGS?
We propose our hypothesis of the possible pathogenesis of RP linked with current OCTA findings in RP patients as well as histopathological findings (Figure 2). The whole process is likely to include four stages: the initiate stage, the early stage,the middle stage, and the advanced stage. In correspondence to the time course of the progression of the VF defects in RP patients detected by Goldmann kinetic perimetry, the VF progression of the four stages changes beginning with no VF loss, scotomas in the peripheral regions, scotomas in the midperipheral regions, and the end stage when only the central VF remains[14].
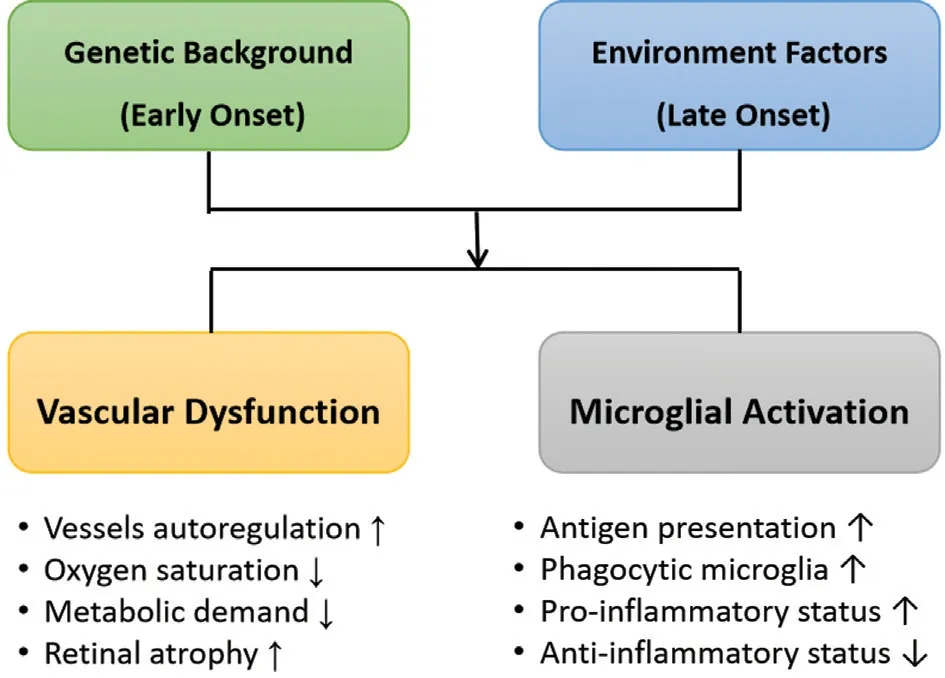
Figure 1 Pattern of etiologic and pathogenic factors leading to retinal degeneration of retinitis pigmentosa.
Initiate Stage-Retinal Hyperoxia Primary defect of RP lies in rod degeneration caused by various rod specific gene mutations. The reduction of the oxygen consumption due to photoreceptors loss has been suggested to cause oxygen diffusion from the choroidal vessels into the inner retina,which decreases the need for oxygen delivery from the retinal circulation in the pathology of eyes with RP, leading to a hyperoxic state[15]. We consider it as the initiative trigger of subsequent consecutive changes leading to irreversible retinal degeneration.
Early Stage-Oxidative Stress and Microglial Activation Excess reactive oxygen species (ROS) are produced consequently under hyperoxic environment which eventually leads to microglial activation[16]. Apoptosis of rods is followed by the migration of microglia from the inner retina to the outer retina. Activated microglia participate in phagocytosis of dead rods, which also exacerbate cone injury through synaptic changes conversely.
Primary photoreceptor death and secondary death of the rods causes thinning of the outer retina[17]. The inner retina including the ganglion cell-inner plexiform layer (GCIPL) and retinal nerve fiber layer (RNFL) maintains gross integrity with retinal remodeling longer than the photoreceptor layer in RP[18].
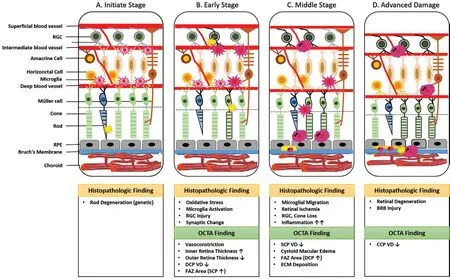
Figure 2 Hypothesis of retinitis pigmentosa pathogenesis A: The initiate stage; B: The early stage; C: The middle stage; D: The advanced stage. RGC: Retinal ganglion cell; RPE: Retinal pigment epithelium; FAZ: Foveal avascular zone; SCP: Superficial capillary plexus; DCP: Deep capillary plexus; ECM: Extracellular matrix; CCP: Choroicapillaries plexus; VD: Vessel density; INL: Inner nuclear layer.
Meanwhile, such change of oxygen diffusion (the hyperoxic state) in turn results in a reflex vasoconstriction of retinal arteries at the level of superficial capillary plexus (SCP), which possess high autoregulatory properties[19-22]. Histopathological studies showed that the features of RP included vessel narrowing and sclerosis, followed by thickening of the blood vessel wall and finally, lumen occlusion[23]. Reduced retinal blood flow velocity and vascular diameter were demonstrated with the usage of magnetic resonance imaging (MRI)[24]as well as colour doppler flow imaging (CDFI)[25]in RP patients.A reduced flow starts at the level of deep capillary plexus(DCP), which can be explained by a vascular constriction at the level of DCP due to the reduced oxygen demand by photoreceptors, as well as a redistribution of blood flow in DCP located near the inner nuclear layer (INL) in order to meet the needs of a high metabolic demands. In agreement with this, Battaglia Parodiet al[26]has shown a more profound involvement of the deep layer with reduced parafoveal vessel density (VD; DCP,P=0.001; SCP,P=0.009). This finding was later proved by Sugaharaet al[27], whose study included 110 eyes of RP patients and 32 control eyes, showing that the more severe vascular impairment happened in the DCP (parafoveal VD: DCP,P<0.001; SCP,P=0.66). Also, Takagiet al[28]demonstrated that flow area in the deep retinal layer was more easily to be affected when compared to that of the SCP and choriocapillaris plexus (CCP; DCP,P=0.004; SCP,P=0.007;CCP,P=0.353). Most recently, Falfoulet al[29]also verified that vascular alteration in RP might begin at the level of DCP,while the change of the SCP would occur later in the evolution of the disease.
Many studies have suggested that macular microvasculature changes caused by decreased blood flow might be indicated in the development of RP including foveal avascular zone(FAZ) enlargement. Although not remarkably significant,FAZ area was found to be firstly enlarged in the superficial retinal layer at the early stage when compared to normal subjects (DCP,P=0.309; SCP,P=0.890), but not the deep layer until mid-to-late stages[30]. Most recently, quantitative OCTA biomarkers that describe the abnormalities of geometric vascular features, including vessel density, vessel tortuosity(VT), vessel dispersion (VDisp), vessel rarefaction (VR),vessel diameter index and increased vessel length density(VLD) were developed to analyze vascular alterations in RP patients for early detection[31-33]. Comparing RP patients and controls, RP patients showed higher VDisp, VR, and lower VT in both retinal layers (P<0.01)[32]. Also, larger vessel diameter indexes and decreased VLDs in both SCP and DCP were shown in RP patients (P<0.001)[31]. These parameters considered to be associated with different RP clinical forms,RP pathophysiology, as well as with different progression.
Middle Stage-Retinal Ischemia The morphological transformation and migration of activated microglia mark the third stage of this response. At this stage, microglia accumulate in the damaged layers and interact with infiltrating blood cells.SCP is affected lateron during the progression of the disease,affected by the reduced flow at the level of DCP, causing ischemia of the inner retina and progressive RGC loss. OCTA studies have shown that both SCP and DCP vessel densities are significantly decreased in middle- and late-stage RP after comparison with healthy objects[34]. Vascular density of the SCP was 42.2%±3.4% in the RP group and 51.4%±2.3% in the control group (P<0.001), whereas those of the DCP were 42.7%±6.2% versus 56.6%±2.2%[34].
At this stage, FAZ area was found to be significantly enlarged at the level of DCP than SCP in RP eyes compared to normal controls (DCP,P<0.001; SCP,P=0.350)[26]. Linet al[35]addressed significant cone losses in mid-to-late stage RP patients with objectively quantified cone density (CD) in all retinal layers, demonstrating the macular structural and functional alterations. AttaAllahet al[36]demonstrated macular microvascular density reduction in all studied layers on OCTA as well as macular structural changes such as ellipsoid zone(EZ) disruption and FAZ enlargement. The reduction of parafoveal VD was more significant in the DCP and CCP when compared to controls (DCP,P<0.001; CCP,P<0.001; SCP,P=0.191)[36].
Expression of a variety of inflammatory factors, adhesion molecules and chemokines under retinal ischemia accelerate cells apoptosis and retinal edema. The presence of cystoid macular edema (CME) in eyes with RP is considered to occur mainly at this stage.
These recruited inflammatory cells, together with accumulated microglia seem to play an important role in the extracellular matrix (ECM) deposition, increased vascular permeability and on RPE atrophy.
Advanced Stage-Retinal Degeneration Pronounced RPE atrophy and breakdown of Bruch’s membrane occur in the more advanced stages, leading to additional clinical hallmarks of the disease such as attenuation of retinal vessels and intraretinal pigment migration. Vascular density of choroicapillaris is slightly attenuated due to loss of photoreceptor metabolism, allowing choroidal oxygen to reach the inner retina. Remarkably decreased choriocapillaris blood flow occurs at late stage of RP, which could in turn accelerate the late phase of retinal degeneration[37]. Early histopathology studies have shown the missing of choriocapillaris[23]. However,when analyzing the blood flow at the CCP layer, there were controversies on the OCTA findings among different studies.Vessel densities of CCP in mid-to-late stage RP patients were reported to be remarkably lower[34], while other studies reported no differences in CCP vessel densities between RP patients and controls[26,28]. These discrepancies could be explained by the limitation of conventional OCTA devices suffering from projection artifacts and penetrating depth. With advanced OCTA technology, accurate choroidal changes could be detected. Flow voids (FVs) in RP patients were significantly reduced, indicating the compromised choriocapillaris in pathogenesis[38]. For deep choroid closer to the Bruch’s membrane,a more significant reduction of vascular density could be found[39].Recently, wide-angle OCTA has been applied to investigate choriocapillaris defects, since the peripheral retina is more likely to be affected at the earlier stages in RP patients[40-41].Choroidal vascularity index (CVI), which was used to reflect middle/large choroidal vascularity, decreased in the perifoveal(P=0.003), pararetinal (P=0.001) and periretinal regions(P=0.002) in the RP eyes, compared to controls[41].
HOW WILL ANIMAL STUDIES PROVIDE EVIDENCES FOR THE HYPOTHESIS?
Since the literature lacks histopathological studies in the early phases of RP, it is still unknown whether vascular changes occur first during the disease, or secondary to the degeneration of photoreceptors due to the close interdependence. Moreover,it is unknown whether the retinal capillary plexuses can be reestablished after photoreceptors transplantation and RPE relocation because of the importance of vascular bed restoration to cell survival and function. Therefore, using animal models that recapitulate aspects of human disease with advanced retinal imaging technologies that can show retinal and choriodal microvasculature is of great value to prove the hypothesis of RP pathogenesis. At present, only one study investigated vascular impairment in wild-type (WT) andrd10mouse retinas with OCTA longitudinally[42]. Further animal studies could be designed with other transgenic mouse models with specific labels to correlate OCTA findings with retinal function, retinal oxymetry[43]and histopathology, enhancing our understanding of RP pathophysiology.
TO WHAT EXTENT WILL ADVANCED OCTA TECHNOLOGY IMPROVE UNDERSTANDING FOR RP?
As the arterial and venous system are differently affected in RP pathogenesis, classification of retinal vessels as arteries and veins is of high importance[44]. Differential artery-vein analysis in OCTA is still challenging to date, despite its highquality capillary level resolution. Several methods have been lately introduced for artery-vein differentiating on OCTA images guided by color fundus image[45], OCT[46], nearinfrared oximetry[47], or realized through incorporating the use of vortices in the DCP to identify venous origin[48]. Besides,benchmark data and clinically relevant metrics for OCTA retinal image segmentation have been established recently[49-52].We believe that the developing differential artery-vein analysis and vessel segmentation could increase the performance of OCTA detection, classification of various stages of RP and treatment evaluation.
WHAT ARE THE PROSPECTS FOR TREATMENT OPTIONS WITH BETTER UNDERSTANDING OF RP?
At present, there are no established treatments for RP. New encouraging treatments have been proposed for RP, including gene therapy, stem cell transplantation, neurotrophic growth factors, and retinal prosthesis. We believe that novel treatment options like microglia modulating therapy which involves either immune modulation and neuroprotection in early phases of activation may reduce the production of several proinflammatory mediators and may therefore result in broader therapeutic effects, while OCTA analysis can help with the identification of early phases of RP. Based on the conceptthat microglia modulation or deactivation can improve retinal function and survival, potential candidate compounds can be envisioned (Table 1)[53-59].

Table 1 Candidate compounds for microglia modulation approaches
CONCLUSIONS
As a healthy vascular system is required to support the cells in the retina, any forms of deterioration to retinal and choroidal vasculatures may restrict the impact of the available therapies for RP. Understanding the role of vascular dysfunction in retinal degeneration and the RP pathogenesis with the most advanced OCTA technology may help with the diagnostic,prognostic and potential therapeutic directions. Our hypothesis of RP pathogenesis combined with the OCTA findings suggests the important role of microglial activation and vascular dysfunction in the whole process of retinal degeneration.Further animal studies and longitudinal trials with improved OCTA technique are needed to prove provide evidence for this hypothesis.
ACKNOWLEDGEMENTS
Authors’ contributions: Lu BW contributed to the conception and design of the study, data collection, analysis, and interpretation of data, drafting of the manuscript. Supervision by Xie LK. All authors contributed to critical revision of the manuscript for important intellectual content.
Foundations: Supported by National Natural Science Foundation of China (No.82174445); China Post-doctoral Science Foundation in 2019 (No.2019M650987); Natural Science Foundation of Beijing of China (No.7192235).
Conflicts of Interest:Lu BW, None; Chao GJ, None; Wu
GP, None; Xie LK, None.
杂志排行
International Journal of Ophthalmology的其它文章
- Upregulation of ASPP2 expression alleviates the development of proliferative vitreoretinopathy in a rat model
- Mesenchymal stem cell-derived exosomes inhibit the VEGF-A expression in human retinal vascular endothelial cells induced by high glucose
- Protective effects of umbilical cord mesenchymal stem cell exosomes in a diabetic rat model through live retinal imaging
- New technique for removal of perfluorocarbon liquid related sticky silicone oil and literature review
- Quantitative analysis of retinal vasculature in normal eyes using ultra-widefield fluorescein angiography
- Evaluation of the long-term effect of foldable capsular vitreous bodies in severe ocular rupture
