Upregulation of ASPP2 expression alleviates the development of proliferative vitreoretinopathy in a rat model
2021-12-17YanKunYueXiaoLiChenShanLiuWuLiu
Yan-Kun Yue, Xiao-Li Chen, Shan Liu, Wu Liu
1Department of Ophthalmology, Fuxing Hospital, Capital Medical University, Beijing 100038, China
2Department of Ophthalmology, Beijing TongRen Hospital,Capital Medical University, Beijing 100730, China
Abstract
● KEYWORDS: proliferative vitreoretinopathy; apoptosisstimulating p53 protein 2; epithelial-mesenchymal transition;autophagy; ARPE-19
INTRODUCTION
Proliferative vitreoretinopathy (PVR) is a severe eye disease, which usually causes failure and vision losses after surgeries for retinal detachment[1]. PVR can be characterized by the formation of fibrotic epiretinal membranes,and sometimes subretinal and intraretinal membranes. Despite remarkable advances in surgical technique, vision outcome for PVR is still unsatisfactory. Moreover, there is no effective pharmacologic agents for PVR treatment partly due to its unclear and complicated pathogeneses[2-3].PVR can be caused by many factors and retinal pigment epithelial (RPE) cells are considered as a crucial one. Although no consensus is achieved, a major component of PVR pathophysiology could be roughly depicted[4-5]. First, a retinal tear happens and RPE cells migrate into the vitreous cavity.Subsequently, the RPE cells transit to myofibroblasts/fibrotic cellsviaepithelial-mesenchymal transition (EMT). Lastly,those fibrotic cells in epiretinal membranes could provide contractile properties, leading to retinal detachment and vision loss. Nevertheless, one big obstacle for PVR research is the lack of widely-accepted animal models which can fully mimic human PVR.
Till now, there is no ideal animal model for PVR studies.Experimental animal models including rabbits, rats and mice have applied either the injection of different cell types or retinal trauma to promote PVR membranes formation or tractional retinal detachment[4,6]. Commonly, PVR membranes can successfully exist within 1-4wk after intravitreal injection.We also established a rat model of PVR by vitreous injection of ARPE-19 cells pretreated with apoptosis-stimulating p53 protein 2(ASPP2)-siRNA, which promoted experimental PVR progression[7]. ASPP2 is a famous member of the evolutionarily conserved ASPP family, which has characteristic sequences of ankyrin repeats, an SH3 domain, and a proline-rich region[8]. ASPP2 has been regarded as a pluripotent molecule including activator for p53[8], binding partner for PAR3[9],tumor suppressor and EMT regulator[10]. Numerous studies have confirmed that ASPP2 is critical to cell life probablyviaapoptosis, autophagy and EMT[11-12]. Besides, low expression level of ASPP2 is associated with various cancers, such as pancreatic cancer and hepatocellular carcinoma, indicating a poor clinical outcome[13-14]. Our previous study also suggested that PVR membranes of human specimens had decreased expression of ASPP2 and ASPP2 knockdown promoted PVR formationin vivopartlyviathe EMT regulation[7].However,the effects of ASPP2 upregulation during PVR development have not been investigated.
In this study, we tried to explore the function of ASPP2 upregulation on the progression of experimental PVR in a rat model.Specifically, ARPE-19 cells pretreated with ASPP2-lentivirus were injected into the vitreous cavities of Brown Norway(BN) rats to investigate its role in the PVR pathogenesis.Understanding the pathophysiologic role of ASPP2 in PVR disease may potentially provide new therapy targets.
MATERIALS AND METHODS
Ethical Approval All experiments were adhered to the Association for Research in Vision and Ophthalmology(ARVO) statements for the Use of Animals in Ophthalmology and Vision Research. The animal studies were approved by the Animal Care Use Committee of TongRen and FuXing Hospital.
Cell Culture and Treatment Dulbecco’s modified Eagle’s medium (DMEM)/F12 medium (HyClone; Grand Island, NY,USA) supplemented with 10% fetal bovine serum (Gibco,Grand Island, NY, USA) were used for ARPE-19 cells (CRL-2302; American Type Culture Collection) culture. The ASPP2-lentivirus-glial fibrillary acidic protein (GAFP) and scrambledlentivirus-GAFP were synthesized and provided by Shanghai GeneChem Company (Shanghai, China). ASPP2-lentivirus or scrambled-lentivirus were transfected into ARPE-19 cells according to the manufacturer’s instructions. In brief,cells were seeded at the recommended density for 12h and lentivirus suspension were added accordingly. At 72h after the transfection, protein was extracted from the cells and Western blot was employed. Stable cell lines were obtained after selection with 6 μg/mL of puromycin for 10d as previously described[15].
Cell Counting Kit-8 Assay ARPE-19 cells with lentivirus treatment for 48h and corresponding stable cell lines were plated on a 96-well plate with 100 μL liquid of DMEM/F12.Cell counting kit-8 assay (CCK-8; Beyotime, Jiangsu, China)was used to determine the cell viability at 12, 24, 48, 72, and 96h after transfection. Cells were starved for 24h and each well was given 10 μL CCK-8 reagents subsequently. After that, cells were incubated for 1.5h at 37°C under a humidified atmosphere with 5% CO2. Finally, a test wavelength of 450 nm was chosen to read the absorbance of cells.
Western Blotting After 72h of lentivirus transfection,overexpression of ASPP2 in the cultured cells was confirmed by Western blotting. Protein of cultured cells were extracted with lysis buffer and frozen at -80℃ until use. Protein concentrations of the samples were measured by bicinchoninic acid (BCA) protein assay kit (Pierce, USA) following the manufacturer’s instructions. We loaded 30 μg total proteins in each lane on the 12% sodium dodecyl sulfate polyacrylamide gel. Then we incubated membranes with primary antibody and with secondary antibody and visualized with enhanced chemiluminescence detection reagents (Pierce). The primary antibody used was polyclonal antibody against ASPP2 (sc-53861; Santa Cruz). On the day 28 after the intravitreal injection for PVR induction, rats were sacrificed and retinas were gained from the eyeball carefully. Then retinas were given lysis buffer and protein concentration was measured by BCA assay. A total of 50 μg proteins were loaded and incubated with primary antibodies of alpha-SMA (ab32575; Abcam) and LC3-II/I (CST#4108; Cell Signaling Technology). Immunoblot analyses were performed by Quantity One software.
Immunocytochemistry ARPE-19 cells were grown on glass coverslips. After culture for 48h, we washed coverslips in phosphate buffer saline (PBS), fixed cells in 4% PFA for 15min and in 10% goat serum for 30min. Then ASPP2 antibody was incubated overnight at 4℃, followed with incubation of secondary fluorescent antibody for 1h at 37℃ in avoidance of light. After that, DAPI (4, 6-diamino-2-phenylindole) was used for cell nuclei counterstain. Images were visualized using the fluorescence microscope (Leica). Experiments were repeated three times.
Intravitreal Injection and PVR Induction All rat surgeries were under sodium pentobarbital anesthesia. We made best efforts to minimize the rats’ sufferings. PVR experimental models of rats were established as previously described[7]. In brief, a total of 40 specific pathogen-free BN rats (200±10 g,6-7wk, male) were used. They were assigned to two groups according to intravitreal injection of ARPE-19 cells pretreated with ASPP2-lentivirus or scrambled-lentivirus. Rats were given anesthesia and pupil dilation. Injection of 1×106stable cell lines of ARPE-19 cells with lentivirus transfection (5 μL) into the vitreous cavity of BN rats was through a Hamilton syringe fitted with a 32-gauge microneedle. On 1, 3, 7, 14, 21, and 28d after the injection, two masked ophthalmologists examined all rat eyes. Phoenix Micron IV Retinal Imaging Microscope were used for fundus documentary of each rat. PVR was classified according to the criteria suggested by Behar-Cohenet al[16].
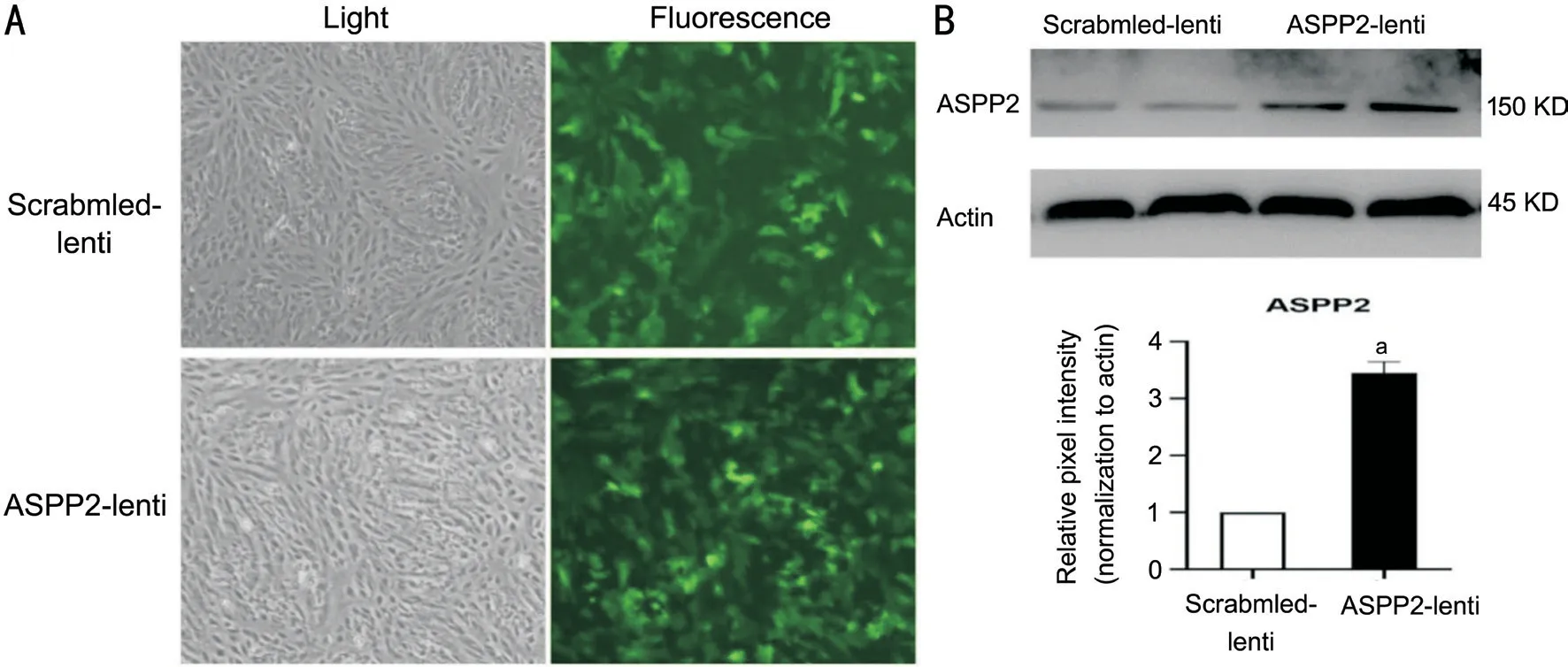
Figure 1 ASPP2-lentivirus efficiently upregulated ASPP2 expression in ARPE-19 cells A: The fluorescence intensity shows the efficacy of lentivirus transfection to ARPE-19 cells. Green fluorescence represents the GAFP in the scrambled-lentivirus and ASPP2-lentivirus. B:Representative band of Western blot shows the enhanced protein expression of ASPP2 in cells with ASPP2-lentivirus transfection for 72h.Repetitive bands of both scrambled-lentivirus and ASPP2-lentivirus transfection groups were shown for robust validation. β-actin is selected for internal reference. The relative ASPP2 protein expression in scrambled-lentivirus and ASPP2-lentivirus group normalized to β-actin. Data are depicted as the mean±SD, n=3. aP<0.01. Scrambled-lentivirus group is set to 100%.
Electroretinography Electroretinography (ERG) was carried out before intravitreal injection, and 7/14d after the injection of ARPE-19 cells for the evaluation of retinal function. The rats were given 8-hour dark adaption, and then anaesthetized with sodium pentobarbital. After that, rat eyes were given 1% tropicamide for pupillary dilatation and 1%proparacaine for topical anaesthetization, followed by one drop of methylcellulose applied for electrodes settings. Briefly,we placed the corneal jet electrodes on the cornea and the reference electrode subcutaneously between the base of the ear and lateral canthus. Then we put the ground electrode on the occiput subcutaneously with dim red light. According to the ISCEV guidelines, we conducted and analyzed ERG recordings.Statistical Analysis Statistical analysis was conducted with Graph Pad Prism 8 (GraphPad Software, Inc., US). Data were described as mean±SD. CCK-8 results were analyzed by oneway analysis of variance (ANOVA) method and PVR grades were compared by non-parametric Mann-WhitneyUtest. ERG and Western blot data were analyzed usingt-test. AP<0.05 was regarded as statistically significant.
RESULTS
Confirmation of ASPP2 Upregulation in ARPE-19 Cells ASPP2-lentivirus were transfected to ARPE-19 cells successfully, and protein expression level of ASPP2 showed a significant increase compared to the scrambled-lentivirus controls (Figure 1). Upregulation of ASPP2 expression was further confirmed by immunocytochemistry assay (Figure 2).Effect of ASPP2 Upregulation on the Viability of ARPE-19 Cells Since ASPP2-lentivirus transfection significantly increased theprotein expression level of ASPP2, we further investigate its effect on cell viability of ARPE-19. In the CCK-8 assay, cell viability decreased in ASPP2-lentivirus group at 12h but increased at 24h after transfection (P<0.01 andP<0.05,respectively), compared with scrambled-lentivirus group.However, there was no significant differences between ASPP2-lentivirus and scrambled-lentivirus groups at 48, 72, and 96h.Therefore, we chose 48h after the transfection as a timepoint for the studies afterwards. As we used stable cell lines with ASPP2-lentivirus and scrambled-lentivirus transfection for thein vivoexperiment, we also measured the viability of those stable cell lines by CCK-8. Our results showed a similar trend of cell viability between stable cell lines and ARPE-19 cells with 48h lentivirus transfection (Figure 3).
Upregulation of ASPP2 Impeded Induction of Experimental PVR in Rats As shown in Table 1, the severity and rate of PVR had a significant decrease in rats with vitreous injection of ARPE-19 cells pretreated with ASPP2-lentivirus compared to those pretreated with scramble-lentivirus. The grading scheme of PVR severity was similar to the previous studies[7,16]. We used fundus imaging to detect the changes of retinal morphology and PVR formation (Figure 4).Three rats were excluded because of cataract and vitreous hemorrhage after the injection. Therefore, in total 18 rats in ASPP2-lentivirus group and 19 rats in scrambled-lentivirus group.On day 7 after intravitreal injection, we observed vitreous haze and strands (stage 1) in 2 rats (10.53%) in the scrambledlentivirus group while none in the ASPP2-lentivirus group. On day 14, six rats (31.58%) in the scrambled-lentivirus group progressed to stage 1 and three rats (15.79%) to stage 2, but only 3 rats (16.67%) in the ASPP2-lentivirus group entered stage 1. The difference between the two groups was statistically significant (P<0.05). Similar changes in severity of PVR were observed on day 21 and day 28 respectively (P<0.05).

Figure 2 Upregulation and localization of ASPP2 was confirmed in ARPE-19 cells by immunocytochemistry Fluorescence micrograph showing the ASPP2 expression (red) in ARPE-19 cells and cell nuclei are counterstained with DAPI (blue). The white arrows show the cell junctions. DAPI: 4,6-diamino-2-phenylindole. Scale bars: 20 µm.
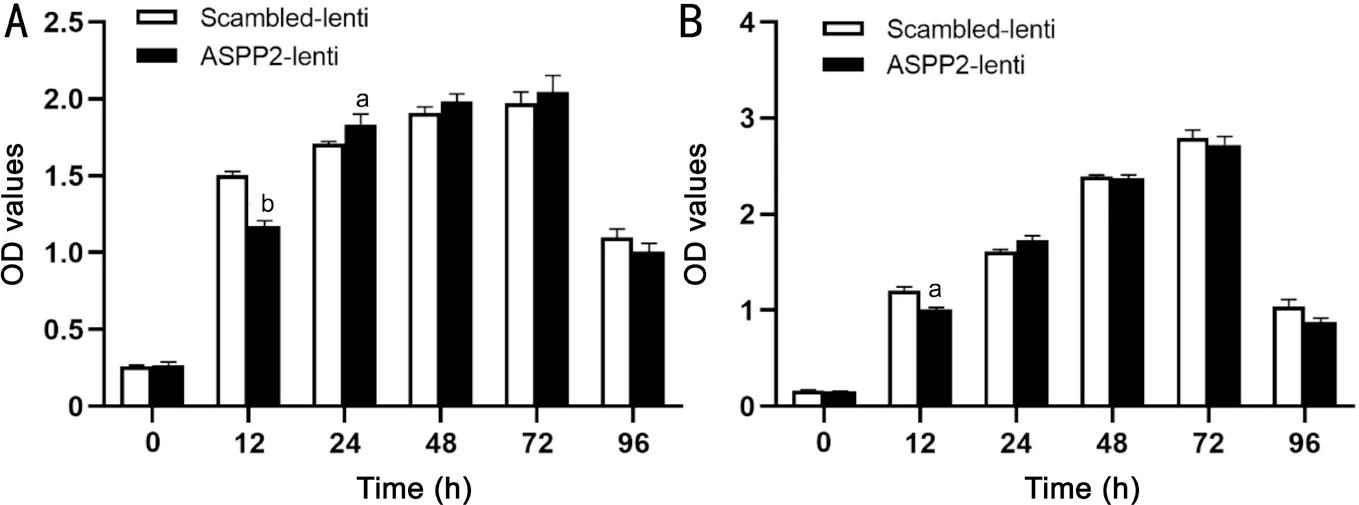
Figure 3 Cell viability changes induced by lentivirus transfection to ARPE-19 cells A: ARPE-19 cell viability is measured at 0, 12, 24, 48,72, and 96h after transfection by CCK-8 assay; B: The viability of stable cell lines with ASPP2-lentivirus and scrambled-lentivirus transfection were detected respectively by CCK-8 at 0, 12, 24, 48, 72, and 96h. Data are the mean±SD, n=3 experiments. aP<0.05, bP<0.01.
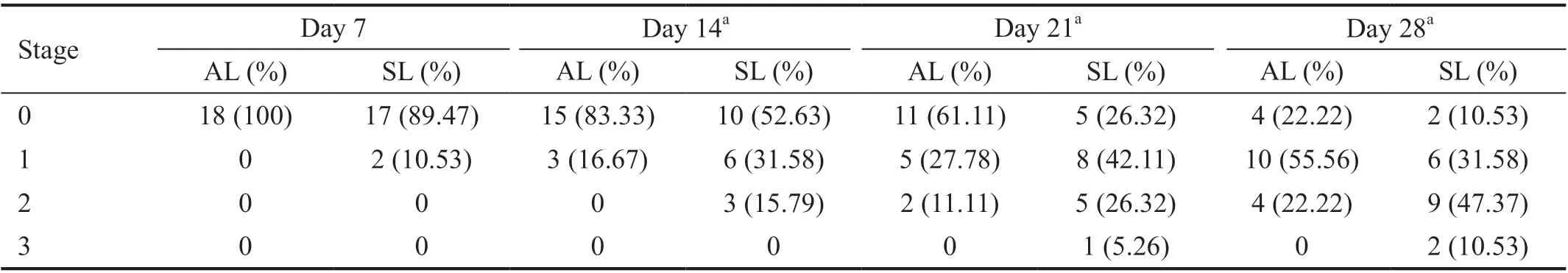
Table 1 PVR development in rats treated with ASPP2-lentivirus or scrambled-lentivirus
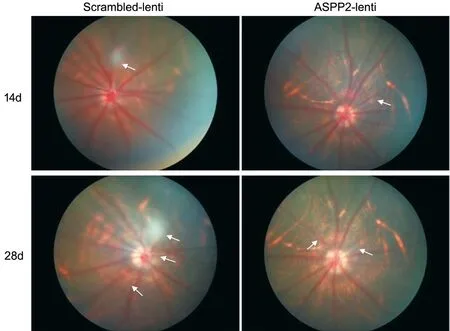
Figure 4 ASPP2 upregulation affected the retinal morphology in experimental rat PVR models Represent fundus images of BN rats at the 14-day and 28-day follow up. The white arrows indicate fibrous proliferation, which is observed in both the ASPP2-lentivirus and scrambledlentivirus group, but more pronounced in the scrambled-lentivirus group.

Table 2 A wave and b wave amplitude of ERG in rats treated with ASPP2-lentivirus and scrambled-lentivirus mean±SD, n=3
Effect of ASPP2 Upregulation on Retinal Function in PVR Models To evaluate the effect of ASPP2 upregulation on the retinal function, ERG data were collected on day 7 and day 14 after the intravitreal injection. Results showed that vitreous injection of ARPE-19 cells caused retinal function changes at early time (day 7) even though the fundus photographs were normal at that time. However, the difference was not N1/P1: The amplitude from N1 to P1; NA: Not applicable.aP<0.05 between two groups.significant between ASPP2-lentivirus and scrambled-lentivirus group (data not shown). At day 14, the difference is statistically significant as shown in Figure 5 and Table 2. The amplitudes of a wave and b waves in scotopic and photopic tests with different stimulus were significantly decreased in rats with scrambled-lentivirus treatment in comparison to ASPP2-lentivirus treatment (P<0.05, respectively).
ASPP2 Upregulation Attenuated Changes of Epithelialmesenchymal Transition and Autophagy Since PVR grades were less pronounced in rats with vitreous injection of ARPE-19 cells pretreated with ASPP2-lentivirus compared to those pretreated with scrambled-lentivirus, we next studied the EMT changes in rats’ retinas. As shown in Figure 6, protein expression of alpha-smooth muscle actin (alpha-SMA, an EMT marker) was down-regulated in the ASPP2-lentivirus group.Meanwhile, LC3-II/I, a well-known indicator of autophagy,displayed a decreased expression in the ASPP2-lentivirus group versus the scrambled-lentivirus group.
DISCUSSION
In the present research, we studied the effect of vitreous injection of ARPE-19 cells pretreated with ASPP2-lentivirus on the development of experimental PVR in BN rats. Our study indicated that ASPP2 upregulation significantly decreased the rate of PVR induction and degraded PVR severitiesin vivo.Many other studies suggested that the retinal function was damaged in PVR development[4,17-18]. In accordance with that,we also found that retinal function decreased during PVR progression in the control group, but ASPP2 upregulation alleviated the damage significantly. Moreover, although ASPP2 is reported to be related to cell viability[19], our study showed that ASPP2 upregulation did not affect the cell viability of APRE-19 at 48, 72, and 96h after transfection. What worth notify is that cell viability decreased at 12h but increased at 24h after transfection with ASPP2-lentivirus, which might reflect the cell adaption to lentivirus transfection. Specifically,ASPP2 upregulation may promote cell apoptosis in ARPE-19 cells but this effect was weakened by cell self-regulation.However, the exact pathway is unclear and need further study.As far as we concerned, the present study reported the effect of ASPP2 upregulation on PVR progression in a rat model for the first time. But our study has some limitations. First, we used ARPE-19 cells for vitreous injection which may cause some degree of inflammation, but there are other studies which also chose ARPE-19 cells injection for experimental PVR[4,20].The reason we chose ARPE-19 is that it is stable in different passages and may result in minimal variations. Another limitation is lack of research on the molecular mechanism of ASPP2 upregulationin vitro. As we previously reported,ASPP2 knockdown promoted PVR development possibly through the EMT changes of ARPE-19 cells[7]. However,in this study we did not find any obvious changes of EMT phenotype in ARPE-19 cells with ASPP2-lentivirus treatment(data not shown). Unlike ASPP2 knockdown, which may be a trigger for EMT, we suppose that ASPP2 upregulation alone may not cause EMT changes in ARPE-19 cells.

Figure 5 ERG values of rats treated with ASPP2-lentivirus and scrambled-lentivirus Represent ERG results of experimental PVR at day 14 after the induction. Retinal functions are better reserved in rats of the ASPP2-lentivirus group compared to the scrambled-lentivirus group.
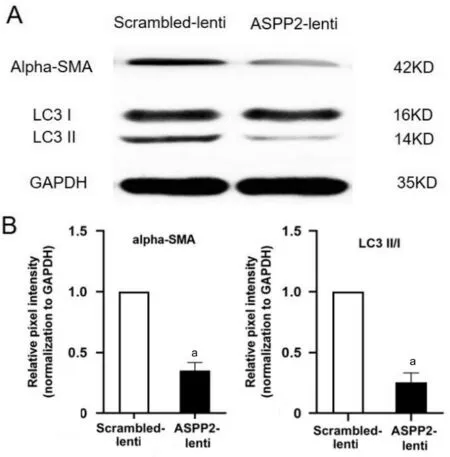
Figure 6 Western blot analysis of alpha-SMA and LC3-II/I in retinas of rats treated with ASPP2-lentivirus and scrambledlentivirus A: Representative Western band showing decreased expression of alpha-SMA and LC3-II/I in retinas of rats in ASPP2-lentivirus group compared to scrambled-lentivirus group. GAPDH was used as loading control. B: Bar graph shows the relative protein expression level of alpha-SMA and LC3-II/I, normalized to GAPDH,respectively. Data are depicted as the mean±SD, n=3. aP<0.01.Scrambled-lentivirus group is set to 100%.
Then, how can ASPP2 upregulation negatively affect PVR progression in rats? It might be rational to speculate that ASPP2 related autophagy may participate in this process.Autophagy is known as a lysosomal-dependent cellular degradation process, during which the cell digests its own proteins and organelles[13]. Autophagy plays an important role in the pathogenesis of diverse diseases, including neuronal degeneration, aging and cancer. Inhibition of autophagy resulted in developmental delayviaEMT reduction in the gastrulation of chick embryos[21]. Similarly, inhibition of autophagy significantly weakened the TGF-β2 mediated EMT in RPE cells, as reported by Miaoet al[22]. Our results are consistent to those findings, as decreased expressions of autophagy and EMT markers are found in the retinas of rats treated with ASPP2-lentivirus compared to those with scrambled-lentivirus.Meanwhile, there is increasing evidence demonstrating that ASPP2 is critical to autophagy. ASPP2 upregulation negatively regulates autophagic flux in pancreatic cancer[14], whereas downregulation of ASPP2 facilitates autophagic activity in hepatocellular carcinoma cells[13]. In addition, ourin vivoresults also suggest that ASPP2 upregulation might lead to reduced autophagy and attenuated EMT changes. However,there is a complex interaction between ASPP2, autophagy and EMT, which needs in-depth investigation.
In conclusion, our study indicated that ASPP2 upregulation impeded the development of experimental PVR and mitigated PVR severities in BN rats. ASPP2 might be a novel target for the prevention and treatment of PVR disease.
ACKNOWLEDGEMENTS
We want to thank Beijing Ophthalmology & Visual Sciences Key Laboratory for their support.
Foundation:Supported by National Natural Science Foundation of China (No.81800827).
Conflicts of Interest:Yue YK, None; Chen XL, None; Liu S,
None; Liu W, None.
杂志排行
International Journal of Ophthalmology的其它文章
- Mesenchymal stem cell-derived exosomes inhibit the VEGF-A expression in human retinal vascular endothelial cells induced by high glucose
- Protective effects of umbilical cord mesenchymal stem cell exosomes in a diabetic rat model through live retinal imaging
- New technique for removal of perfluorocarbon liquid related sticky silicone oil and literature review
- Quantitative analysis of retinal vasculature in normal eyes using ultra-widefield fluorescein angiography
- Evaluation of the long-term effect of foldable capsular vitreous bodies in severe ocular rupture
- Efficacy and safety of non-penetrating glaucoma surgery with phacoemulsification versus non-penetrating glaucoma surgery: a Meta-analysis
