Protective effects of umbilical cord mesenchymal stem cell exosomes in a diabetic rat model through live retinal imaging
2021-12-17YanFuXiangGaoGuangHuiHeSongChenZhaoHuiGuYueLingZhangLiYingLi
Yan Fu, Xiang Gao, Guang-Hui He, Song Chen,3,4, Zhao-Hui Gu, Yue-Ling Zhang,Li-Ying Li
1College of Medicine, Nankai University, Tianjin 300071,China
2Department of Ophthalmology, Baoding No.1 Central Hospital, Baoding 071000, Hebei Province, China
3Tianjin Eye Hospital, Tianjin 300020, China
4Clinical College of Ophthalmology, Tianjin Medical University, Tianjin 300020, China
5Ophthalmic Center of Xinjiang Production and Construction Corps Hospital, Urumqi 830002, Xinjiang Uygur Autonomous Region, China
Abstract
● KEYWORDS: human umbilical cord mesenchymal stem cells; exosomes; diabetic retinopathy; fundus fluorescein angiography; optical coherence tomography; rat
INTRODUCTION
Diabetic retinopathy (DR), as a neurovascular complication that involves both microangiopathy and neuronopathy starting at an early stage[1-2]. The current therapies for DR are surgery and laser therapy combined with intravitreal injection of anti-vascular endothelial growth factor (VEGF) drugs[3].However, no effective treatments specifically we have known for mild DR except for glycemic control. Therefore, it is necessary to determine other effective early treatment methods against DR.
Due to their immunomodulatory and proangiogenic characteristics, mesenchymal stem cells (MSCs) show specific therapeutic potential in the treatment of eye diseases. Previous studies have determined the function of MSCs on the restore of retinal vasculature and neurons in DR[4]. Intravenous administration of MSCs to patients with DR is safe and feasible[5]. According to the previous studies, MSCs works mainly dependent on exosoms because they have the ability to rapidly diffuse through the retina due to their nanosize. These characteristics of exosomes make injection of them a better way to treat DR injury[6-7]. Several studies have demonstrated that administration of MSC-derived exosomes may be a suitable therapeutic strategy for several models of retinal disease.
Based on our understanding of DR and the protective effect of MSC-derived exosomes, we focus on the protective effect of human umbilical cord mesenchymal stem cell exosomes(hucMSC-Exs) against early DR.
MATERIALS AND METHODS
Ethical Approval All experiments were conducted in accordance with the Association for Research in Vision and Ophthalmology (ARVO) statement for the use of Animals in Ophthalmic and Vision Research. All procedures were conducted in accordance with the Tianjin Medical Experimental Animal Care, and animal protocols were approved by the Institutional Animal Care and Use Committee of Yi Shengyuan Gene Technology (Tianjin) Co., Ltd.
Culture, Isolation, and Identification of hucMSC-Exs P3-P5 hucMSCs (Saier Biological Company, Tianjin, China) were cultivated in an incubator with 5% CO2at 37℃ in serum-free culture medium (CM; Gibco, Grand Island, NY, USA) for 48h.To obtain the CM of hucMSCs centrifugation at 1000 g and 4℃ for half an hour was performed. Thereafter, a series of centrifugation (300 g for 10min, 2000 g for 20min, 10 000 g for 30min) was performed. Then, the 0.22 μm filters were used to filter the supernatant. Then, exosomes were precipitated through ultracentrifuging the supernatant for 70min at 100 000 g. The exosomes were resuspended with phosphate buffered saline(PBS) for the further experiments.
The FEI Tecnai G2 TEM (Philips, Amsterdam, Netherlands)was used to characterize the morphology of hucMSC-Exs.NTA was chose for the detection of the size distribution of exosomes (Particle Metrix, Meerbusch, Germany). We assessed the protein concentration of the exosomes using BCA method. PBS was diluted according to the instructions.Western blot was conducted to detect the expression of exosome markers, such as CD63, CD9, and Calnexin. After cell lysis, the total protein was extracted from exosomes.
Experimental Animals Twenty-four 6-8 weeks old male Sprague-Dawley (SD) rats weighing 180-220 g (Beijing Vital River Laboratory Animal Technology Co., Ltd.) were maintained at 23℃±3℃ with a humidity of approximately 55%. A 12h light/dark cycle was applied. Animals were access to food and water freely throughout the feeding process.
Rat Model of Diabetic A single intraperitoneal injection of streptozotocin (STZ) with the concentration of 60 mg/kg was carried out to establish diabetes model. Seven days after STZ administration, animals with the glucose levels in 3 consecutive days higher than 16.7 mmol/L were selected for the further experiments. Sixteen eligible DR model rats were randomly divided to two groups, the diabetic group and the hucMSC-Ex group. In addition, 8 normal age-matched rats used as control were treated with an ordinary diet.
Intravitreal Injections Thirty days later, hucMSC-Ex therapy was performed through intravitreal injection. After anesthesia with isoflurane (2%-3% in oxygen), 1 µL hucMSC-Ex was injected into the right pars plana of rats using a sterile 33-G needle attached to a Hamilton syringe. PBS injection was used as control.
FFA and OCT were performed to observe pathological changes in the diabetic retina. And the rats’ eyes were removed after imaging for hematoxylin-eosin (HE) staining.
Optical Coherence Tomography Changes in retinal structure and thickness were determined using spectral-domain optical coherence tomography (SD-OCT; Optovue, USA) 4wk after intravitreal injection. Data were collected using Retina Map Scan protocol. Retinal thickness was restricted into 5×5 mm2grid. The scan data were collected for the retinal segmentations from the center of the optic nerve head.
Fluorescein Fundus Angiography After intravenous injection with fluorescein regents (Sigma-Aldrich, USA),fundus images within 5min was performed.
Ocular Histology The eyes were fixed with 4% paraformaldehyde.The 5 µm thickness of tissues was cut. HE staining was performed for histological analysis.
Statistical Analyses Data were evaluated using Graphpad8.0 and represented as mean±SD. The comparison difference was analyzed using ANOVA and LSD.Pvalues less than 0.05 was deemed as statistical significance.
RESULTS
Identification of hucMSC-Exs Figure 1A showed that vesicles was found with round membrane, which was characteristics of exosomes. Moreover, exosome surface markers, including CD9 and CD63 was overexpressed.Moreover, transmission electron microscopy (TEM) revealed nanoparticles with a typical spheroid morphology ranging in size from 50 to 150 nm in diameter (Figure 1A). WB revealed the expression of characteristic exosome surface markers,including CD9, CD63 and Calnexin (Figure 1B). NTA showed that the exosomes were similar in size, with a mean diameter of approximately 129.1 nm (Figure 1C). These results dictated that hucMSC-Exs were well-established.
hucMSC-Exs Injection Abated the Decrease of Blood Glucose Levels Induced by DR As shown in Table 1, Body weight and blood glucose levels were significantly decreased in diabetic group; however, hucMSC-Exs injection significantly increased body weight and blood glucose levels.
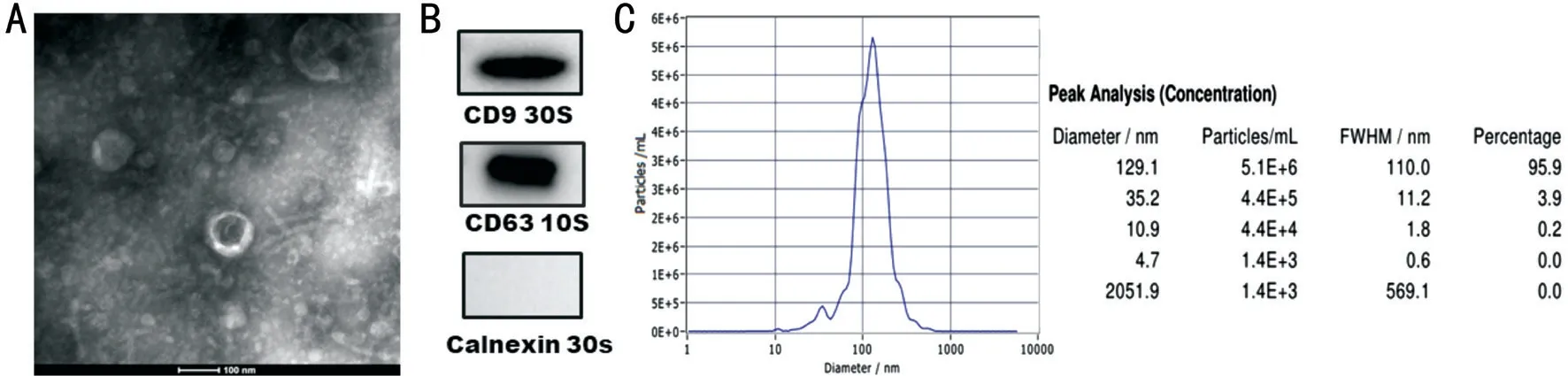
Figure 1 Identification of hucMSC-Exs A: Electron microscopy showed that the hucMSC-Exs were spheroid. Scale bar: 100 nm; B: WB demonstrated that hucMSC-Exs expressed the characteristic exosome markers CD63, CD9 and Calnexin; C: Analysis of the size distribution of hucMSC-Ex with a NanoSight analysis system showed a peak size of 129.1 nm.
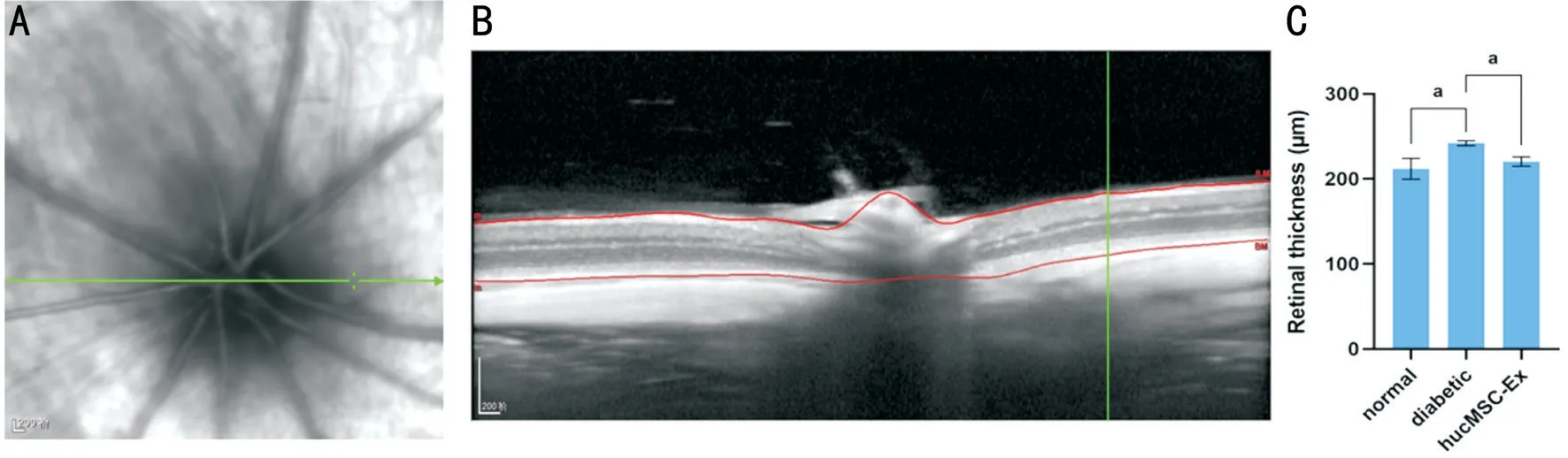
Figure 2 Representative SD-OCT image of the retina A: Dynamic OCT scanning process of the rat fundus; B: The total thickness of the rat retina from the inner limiting membrane to the RPE was measured by OCT; C: The retina of the diabetic group was significantly thicker than that of the control group (aP<0.05). Compared with the diabetic group, the hucMSC-Ex diabetic group demonstrated significant thinning of the retina(aP<0.05).
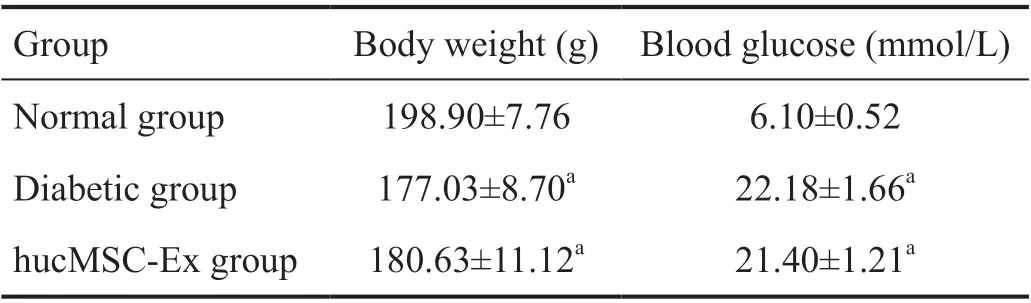
Table 1 A comparison of blood glucose levels and body weight among groups
Effects of hucMSC-Exs on Retinal Thickness OCT was performed to evaluate retinal structural alterations and the details of rat retina scans (Figure 2A). Representative images of the retinal layers from all experimental groups (Figure 2B),which showed the details of each layer of rat retina, were obtained by OCT. The average thickness of all retinal layers[from the internal limiting membrane to the retinal pigment epithelium (RPE)] was 211.80±12.28 μm in the normal group,241.17±2.94 μm in the diabetic group, and 220.34±5.50 μm in the hucMSC-Ex group. The normal group did not show any notable changes in retinal layer thickness in our study. The total retinal thickness of the diabetic group was significantly increased compared with that of the normal group. There was a significant difference in retinal thicknesses between the diabetic group and the hucMSC-Ex group, with the hucMSCEx group showing a decreased total retinal thickness compared with the diabetic group (P<0.05). Intravitreal injection of hucMSC-Exs significantly attenuated the diabetes-induced increase in total retinal thickness (Figure 2C).
hucMSC-Exs Attenuated Diabetic-Induced Retinal Microvascular Changes FFA was used to analyze the effects of hucMSC-Exs on the retinal vasculature. The normal group showed a clear full fundus and fluorescence. Diabetic rats displayed microvascular changes consistent with DR. There were widespread dilated and tortuous vessels on the retina in the diabetic group (Figure 3A). Hemorrhage and venous beading were observed in the diabetic group. Dilation and tortuosity of the vessels was greatly improved in the hucMSCExs group compared with the diabetic group at 4wk following intravitreal injection. Moreover, vessel expansion and tortuosity were reversed in the hucMSC-Ex group (Figure 3B). There was no neovascularization in the diabetic group or hucMSC-Ex group at any time point.
Effects of hucMSC-Exs on Diabetes-Induced Histological Damage to the Retinal Structure To determine the effects of hucMSC-Exs on diabetes-induced pathological changes in the retinal structure, HE was performed. The retinal surface was smooth in the normal group. The cells in each layer were neatly arranged and densely distributed. In contrast, significant structural disturbances in the retina and cell loss were observed in the diabetic rat group, cells were disordered, and the nerve fiber layer was edematous. The total number of nuclei in the inner nuclear layer (INL) and outer nuclear layer (ONL) was significantly lower in the diabetic rat group then the normal group. However, hucMSC-Ex administration rescued diabetesinduced abnormalities. The retinal thickness was decreased in the hucMSC-Ex group compared with the diabetic group (Figure 4).
DISCUSSION
DR is characterized by microaneurysms, exudation,hemorrhage, and complications such as retinal neovascularization and inter-retinal edema. The anti-VEGF intravitreal injection is an available therapy used to protect central vision. However,anti-VEGF agents do not prevent vision loss as the result of retinal ischemia and degeneration. Anti-VEGF agents are efficacious in the advanced stage of DR but are not suitable for the treatment of early DR. Since DR is characterized by impairment of the neurovascular unit starting at a very early stage[8], a safe cell-based therapy that can protect against retinal vascular damage as well as retinal neurodegeneration would be beneficial.
Previous studies have shown the therapeutic efficacy of MSC administration as a cell-based therapy for DR[9]. The experiments of our group also proved the protective effect of hucMSC in DR[10]. Due to ethical concerns and issues regarding the safety of MSC transplantation and the possible risks, such as proliferative vitreoretinopathy, vitreous opacity,and vision loss[11], the use of MSC transplantation is still controversial[12-13]. The therapeutic efficacy of MSCs can be attributed to exosomes, and MSC-derived exosomes have been shown to exert beneficial effects in models of ocular diseases[14], such as corneal diseases[15], autoimmune uveitis[16],glaucoma models[17], and retinal damage caused by ischemia[18].And compared with other cells, MSC produces more exosomes usually[19]. After summarizing the experimental effects of exosomes in a variety of eye diseases, we investigated the protective effect of hucMSC-Exs on the function and structure of the retina in a rat model of DR.

Figure 3 Vascular abnormalities identified through FFA A:Diabetic group; B: hucMSC-Ex group. The arrows indicate dilated and tortuous vessels.
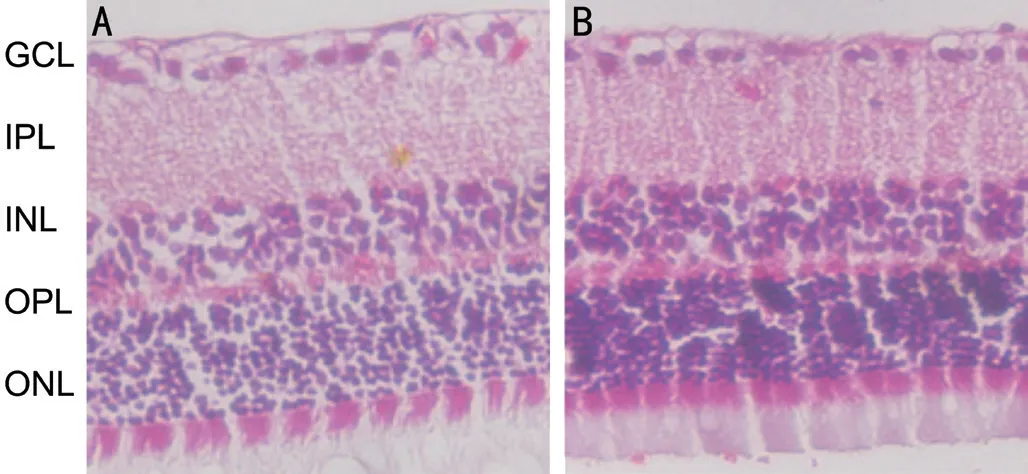
Figure 4 Ocular histological examination of the effects of hucMSC-Ex on STZ-induced diabetic retinopathy Representative images of HE staining showing the morphology of the retina from the diabetic group and hucMSC-Ex group. A: Diabetic group; B:hucMSC-Ex group.
In this study, we used the classic STZ-induced diabetic model to characterize the effects of hucMSC-Exs on the retina at the earliest stage of DR. The experimental results confirmed that hucMSC-Exs did attenuated neuropathy and vasculopathy caused by diabetes at the earliest stage. To assess the effects of hucMSC-Exs, we first analyzed the retinal microvasculature using FFA and found dilated and tortuous vessels, hemorrhage,and venous beading in the diabetic group. Administration of hucMSC-Exs had protective effects on the retinal vessels.Vessel expansion and tortuosity were markedly improved in the hucMSC-Ex group compared with the diabetic group. The retinal hemorrhage was absorbed. OCT and HE staining were applied to evaluate retinal structural alterations. Morphometric examination by OCT demonstrated a significantly increased total retinal thickness in the diabetic group compared with the normal control group. Compared with the diabetic group, the hucMSC-Ex group showed a decrease in total retinal thickness,demonstrating that intravitreal injection of hucMSC-Exs was associated with a significant decrease in retinal thickness.Morphological analysis by HE staining further confirmed these results. Finally, the results of HE staining indicated that the total number of nuclei in the INL and ONL was significantly lower in the diabetic rat group. Intravitreal injection of hucMSC-Exs significantly attenuated retinal structure damage.In addition, hucMSC-Ex administration significantly attenuated the loss of INL and ONL cells. Our analysis indicated that hucMSC-Exs had ameliorative effects on retinal vessels and structure. Additionally, intravitreal injection of hucMSC-Exs had no effect on blood glucose levels, and the body weight of the hucMSC-Ex group was comparable to that of the untreated diabetic group, suggesting that hucMSC-Exs exert unique protective effects against DR that are independent of blood glucose regulation.
MSC-derived exosomes appear to be promising cell-based candidates and for the treatment of DR. Similarly, Safwatet al[20]showed that subconjunctival and intraocular injection of adipose MSC-Exs can ameliorate DR progression. Another study by Zhanget al[21]showed that platelet-rich plasmaderived exosomes can reduce the expression of inflammatory molecules in STZ-induced DR.
Exosomes, which range in size from 50 to 150 nm, contain mRNAs, miRNAs, lipids and proteins[22]. Exosomes deliver these expansive cargos to target cells, affect protein expression and exert significant therapeutic effects[23]. Transplantation of bone marrow-derived MSC exosomes into the vitreous of glaucoma model animals was shown to promote retinal ganglion cell (RGC) survival and prevent RGC functional decline[24-26]. This neuroprotective effect appeared to be associated with the delivery of miRNA cargo of exosomes to RGCs[17,27]. Exosome administration may serve as a suitable neuroprotective strategy for retinal disease. Exosomes derived from MSCs prevent retinal cell loss and dysfunction in mice subjected to retinal ischemic induced by hyperoxic conditioning[18], prevent neovascularization, suppress retinal vascular leakage[28], and suppress the inflammatory response[29].The mechanism of DR involves inflammation, oxidative stress,and vascular dysfunction[30]. However, the exact therapeutic effect of exosomes in a rat model of DR is still unknown.
In our study, we observed that STZ-induced diabetic rats with early-stage DR display retinal morphological alterations and retinal vasculature changes. While focused on the advanced stage of DR and on the effect of exosomesin vitro[31], we used FFA, OCT and HE staining to examine the influence of hucMSC-Exs on the retinal vasculature and retinal structurein vivo. We found that retinal vasculature and structure were preserved in the eyes of rats with DR.
This study has some limitations. First, we administered hucMSC-Exs at the earliest detectable stage of DR to investigate the protective effect of hucMSC-Exs, but the longterm effects of hucMSC-Exs on the retinas of diabetic rats are unclear. Second, an STZ-induced diabetic model was used to explore whether hucMSC-Exs are ideal cells for protecting against DR, but it should be noted that there are safety concerns related to intravitreal administration. Third, our analysis focused on changes in retinal function and structure to evaluate the effect of hucMSC-Exs on DR in the eyes of live rats. However, we did not explore the mechanisms of hucMSC-Exs. Future studies are still needed to determine the mechanism of hucMSC-Exs.
In the present study, we evaluated the effect of hucMSC-Exs on DR. hucMSC-Exs not only attenuated diabetes-induced retinal neuron degeneration but also inhibited microangiopathy.hucMSC-Exs significantly attenuated the loss of neuronal cells, as well as retinal vascular damage observed in diabetic rats. Although the long-term effects of hucMSC-Exs on the damaged retina were not studied, we speculated that hucMSCExs may have protective effects in the earliest stage of DR development.
ACKNOWLEDGEMENTS
Foundations: Supported by the S&T Program of Hebei (No.H2021104002); Tianjin Science and Technology Commission(No.14JCYBJC27400); Program of Tianjin Eye Hospital(No.YKZD1901); Science and Technology Project of Tianjin Municipal Health Bureau (No.2015KZ073)
Conflicts of Interest:Fu Y, None; Gao X, None; He GH, None;Chen S, None; Gu ZH, None; Zhang YL, None; Li LY, None.
杂志排行
International Journal of Ophthalmology的其它文章
- Upregulation of ASPP2 expression alleviates the development of proliferative vitreoretinopathy in a rat model
- Mesenchymal stem cell-derived exosomes inhibit the VEGF-A expression in human retinal vascular endothelial cells induced by high glucose
- New technique for removal of perfluorocarbon liquid related sticky silicone oil and literature review
- Quantitative analysis of retinal vasculature in normal eyes using ultra-widefield fluorescein angiography
- Evaluation of the long-term effect of foldable capsular vitreous bodies in severe ocular rupture
- Efficacy and safety of non-penetrating glaucoma surgery with phacoemulsification versus non-penetrating glaucoma surgery: a Meta-analysis
