Aqueous angiopoietin-like levels correlate with optical coherence tomography angiography metrics in diabetic macular edema
2021-12-17JieYanWuJunLiYaZhouQinXuanYuQiuLiQinJingMingLi
Jie Yan, Wu-Jun Li, Ya-Zhou Qin, Xuan-Yu Qiu, Li Qin, Jing-Ming Li
1Department of Ophthalmology, the First Affiliated Hospital of Xi’an Jiaotong University, Xi’an 710061, Shaanxi Province,China
2Department of Ophthalmology, Yulin Hospital of Traditional Chinese Medicine, Yulin 719000, Shaanxi Province, China
Abstract
● KEYWORDS: diabetic retinopathy; diabetic macular edema; optical coherence tomography angiography;angiopoietin-like; vascular leakage
INTRODUCTION
The incidence of diabetic retinopathy (DR), an ocular microvascular complication of hyperglycemia, increases with the prevalence of diabetes[1-3]. Specifically, diabetic macular edema (DME) is the leading cause of vision loss in patients with diabetes and can occur at any DR stage[4]. It has been reported that 2 million people experience vision problems caused by DME worldwide[5]. The main pathophysiological event in DME is the disruption of the blood-retinal barrier,caused by vascular endothelial growth factor (VEGF) and other pro-inflammatory cytokines, which in turn leads to retinal blood vessel leakage[5]. Major therapeutic measures for DME involve the intravitreal injection of VEGF inhibitors and corticosteroids[4]. Given that corticosteroids are associated with ocular adverse effects such as cataracts and glaucoma, anti-VEGF therapy is generally regarded as the first-line option for patients with DME[6]. Although anti-VEGF drugs have been efficacious in improving not only the prognosis, but also best corrected visual acuity[7], some patients do not respond to this therapy[8]. Therefore, investigating the role of other cytokines in the pathogenesis of DME is necessary.
Angiopoietin-like (ANGPTL) proteins are a class of secreted glycoproteins with a structure similar to that of angiopoietin.The eight members of this class are associated with angiogenesis and vascular leakage[9-10]. Of these, ANGPTL3 has been shown to induce corneal angiogenesis in rats[11].Additionally, patients with type-2 diabetes mellitus show elevated serum ANGPTL3 levels, which correlate positively with DR severity[12]. ANGPTL4 has been a research hot spot in recent years. Although the role of this protein in promoting angiogenesis and increasing vascular permeability is still debated[13], anti-ANGPTL4 therapy has shown positive effects on ischemic retinopathy, including DR[14-16]. A recent study has demonstrated that targeted inhibition of ANGPTL4 can decrease vascular permeability significantly in mice[10]. Oikeet al[17]have found that ANGPLT6, another proangiogenic factor,inducesin vitroandin vivoskin and corneal angiogenesis and promotes skin vascular leakage in mice. Despite these data,the effects of ANGPTL3, 4, and 6 on DR have not been fully determined yet.
Optical coherence tomography angiography (OCTA) is a noninvasive fundus imaging technique that has attracted much attention due to its potential to substitute fundus fluorescein angiography (FFA) in the diagnosis of retinal vascular diseases.OCTA obtains blood vessel images at different segmentation slabs and quantitatively estimates the macular capillary loss in patients with diabetes[18-20]. Therefore, quantifying microvascular changes using OCTA greatly contributes to the screening, diagnosis, and disease surveillance of DR.
In this study, we aimed to measure aqueous ANGPTL3,ANGPTL4, and ANGPTL6 levels in patients with DME and assess their correlation with OCTA metrics.
SUBJECTS AND METHODS
Ethical Approval All subjects provided written informed consent before specimens were collected. The study was approved by the Institutional Ethical Review Board of the First Affiliated Hospital of Xi’an Jiaotong University and abided by the formulation of the Helsinki Declaration.
Subjects This cross-sectional study included 39 patients (47 eyes) who underwent cataract surgery or intravitreal injection for DME in the Department of Ophthalmology of the First Affiliated Hospital of Xi’an Jiaotong University, China. All patients underwent OCTA imaging and ultra-wide field fundus photography. According to Diabetic Retinopathy Disease Severity Scale and center-involved DME (CI-DME) definition by Diabetic Retinopathy Clinical Research Network (DRCR.net), patients were categorized into one of the following three groups: a control group, which included patients with no diabetes; a non-diabetic retinopathy (NDR) group, which included diabetic patients without apparent retinopathy; and DME group, which included diabetic patients with CI-DME.Patients with a history of intraocular surgery (including intravitreal injection of anti-VEGF drugs), other oculopathies(such as uveitis, retinitis pigmentosa, glaucoma, agerelated macular degeneration, and progressive hypertensive retinopathy), or serious systemic conditions (including heart,liver, kidney, and autoimmune diseases) were excluded from the study.
Aqueous Humor Collection, Preservation, and Assessment Before cataract surgery or intravitreal injection, an anterior chamber puncture was performed through the cornea limbus to obtain 0.05 mL of aqueous humor using a 1 mL insulin disposable syringe. The samples were injected into a labeled eppendorf tube, placed on ice, and transferred to a -80°C freezer. The levels of ANGPTL3, ANGPTL4, and ANGPTL6 in the aqueous humor were detected using suspension array technology (LXSAHM-13, R&D Systems, Minneapolis, MN,USA).
Image Acquisition Protocol High-quality (signal strength ≥7)OCTA images were obtained using a Zeiss Cirrus 5000 HDOCT Angioplex device (Carl Zeiss Meditec, Inc., Dublin, CA,USA) at half an hour after mydriasis. Each eye was scanned using a 3×3-mm2protocol in the superficial capillary plexus(inner limiting membrane to inner plexiform layer) and a macular cube 512×128 protocol (inner limiting membrane to retinal pigment epithelium) in the macular area. Retinal vessels were automatically measured using the built-in software(CIRRUS 11.0, Carl Zeiss Meditec). CIRRUS macular scan regions were derived from the Early Treatment Diabetic Retinopathy Study (ETDRS) grid. Therefore, OCTA metrics consisted of central, inner, and full vessel density (CVD, IVD,and FVD, respectively); central, inner, and full perfusion density (CPD, IPD, and FPD, respectively), foveal avascular zone (FAZ) area, FAZ perimeter, and FAZ circularity index(FAZ-CI). Additionally, central subfield thickness (CST),cube volume (CV), and cube average thickness (CAT) were measured using a macular cube 512×128 protocol. Vessel density (VD) was defined as the total length of perfused blood vessels per unit area in the region of measurement, perfusion density (PD) was defined as the total area of perfused blood vessels per unit area in the region of measurement, and FAZCI was defined as the ratio of the FAZ area to a perfect circle that had the same perimeter as the FAZ.
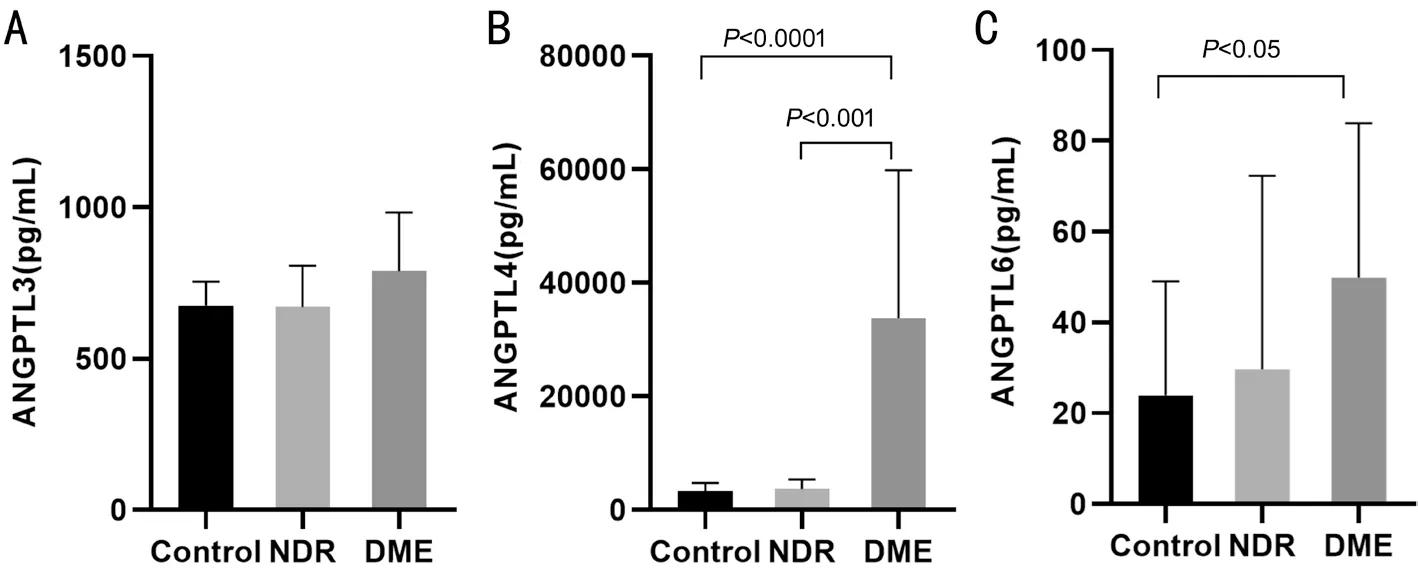
Figure 1 Bar graphs showing the mean aqueous levels of ANGPTL3 (A), ANGPTL4 (B), and ANGPTL6 (C), in the control, NDR, and DME groups NDR: Non-diabetic retinopathy; DME: Diabetic macular edema.
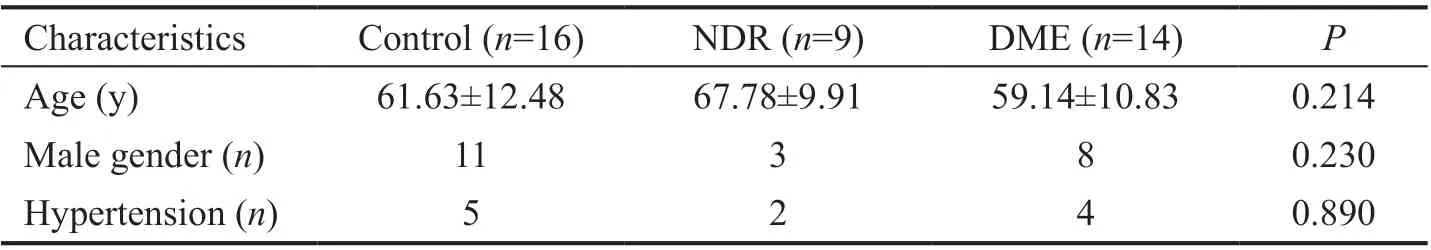
Table 1 Baseline characteristics of the study subjects in each group mean±SD
Statistical Analysis All statistical analyses were performed using GraphPad Prism 8.0.2 (GraphPad, San Diego, CA,USA). Classified data were compared using the Chi-square test. Continuous data are expressed as mean±standard deviation (SD) and were evaluated with a one-way ANOVA test followed by Bonferroni post hoc tests. Multivariate or Kruskal-Wallis tests were conducted for normally and nonnormally distributed variables, respectively. The Spearman’s test was used to perform correlation analysis. The test standard was α=0.05, and statistical significance was considered atP<0.05.
RESULTS
Demographic Features The study included 16 non-diabetic patients (20 eyes, control group) and 23 diabetic patients(27 eyes). Of the latter, 9 (11 eyes) were categorized into the NDR group and 14 (16 eyes) into the DME group. Baseline characteristics did not significantly differ between groups(Table 1).
Aqueous ANGPTLs Levels The control, NDR, and DME groups had comparable mean aqueous ANGPTL3 levels(675.8±78.93, 670.6±135.9, and 789.2±194.5 pg/mL,respectively;P>0.05 for all comparisons; Figure 1A). Mean aqueous ANGPTL4 levels were 3380±1373, 3717±1623,and 33744±26078 pg/mL in the control, NDR, and DME groups, respectively (Figure 1B). The DME group exhibited significantly higher ANGPTL4 levels than the control and NDR groups (P<0.0001 andP<0.001). The difference between the control and NDR groups in this regard was not statistically significant (P>0.05). Finally, the mean aqueous ANGPTL6 levels in the control, NDR, and DME groups were 23.93±25.09, 29.69±42.57, and 49.87±34.04 pg/mL,respectively (Figure 1C). The DME group had significantly higher ANGPTL6 levels than the control group (P<0.05). The other two comparisons were not statistically significant.
Changes in OCTA Metrics The complete data on OCTA metrics are shown in Table 2. The CVD of the DME group was remarkably lower than that of the control group (P=0.002),and there was no significant difference among the other groups(bothP>0.05). The IVD and FVD were significantly lower in the DME group than in the NDR and control groups. All PD metrics were significantly lower in the DME group compared to the control group, while being similar between the DME and NDR groups. The FAZ area of the DME group was significantly higher than that of the control group, but similar to that of the NDR group. The FAZ perimeter and CI of the DME group were significantly worse than those of the control and NDR groups.
Correlation between Aqueous ANGPTL3, ANGPTL4,and ANGPTL6 Levels and OCTA Metrics Finally, we investigated the correlation between aqueous ANGPTL3,ANGPTL4, ANGPTL6 levels, and OCTA metrics (Table 3).Pearson’s correlation analysis showed that aqueous ANGPTL3 levels correlated negatively with IVD, FVD, IPD, and FPD, and positively with CV and CAT in all patients. In turn, aqueous ANGPTL4 levels correlated negatively with CVD, IVD, FVD, CPD, IPD, FPD, and positively with FAZ perimeter, CST, CV and CAT in all patients. Finally, aqueous ANGPTL6 levels correlated negatively with IVD, FVD, IPD,FPD, and FAZ-CI, and positively with CST, CV, and CAT.
DISCUSSION
This study showed that patients with DME presented higher aqueous ANGPTL4 and ANGPTL6 levels than control subjects. Regarding OCTA metrics, patients with DME showed a lower VD, PD, and FAZ-CI and a larger FAZ areaand perimeter. Although not significantly different between groups, ANGPTL3 levels had a positive correlation with CV and CAT, and a negative correlation with inner and full vessel and perfusion densities. Notably, ANGPTL4 and ANGPTL6 levels exhibited a significant correlation with most alterations in OCTA metrics.
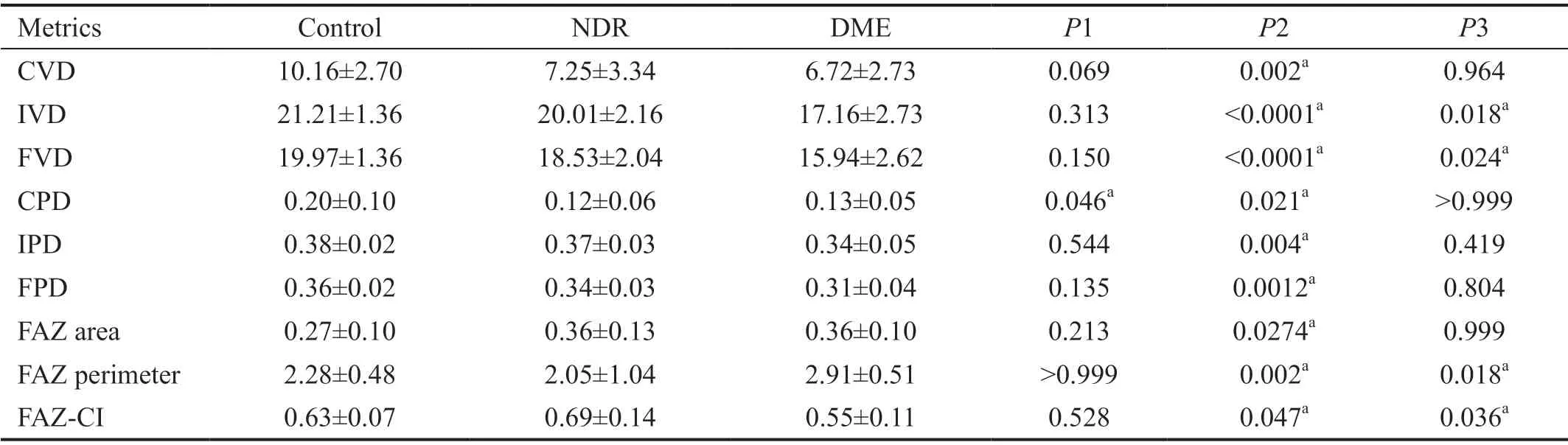
Table 2 Descriptive statistics for OCTA metrics in the control, NDR, and DME groups mean±SD
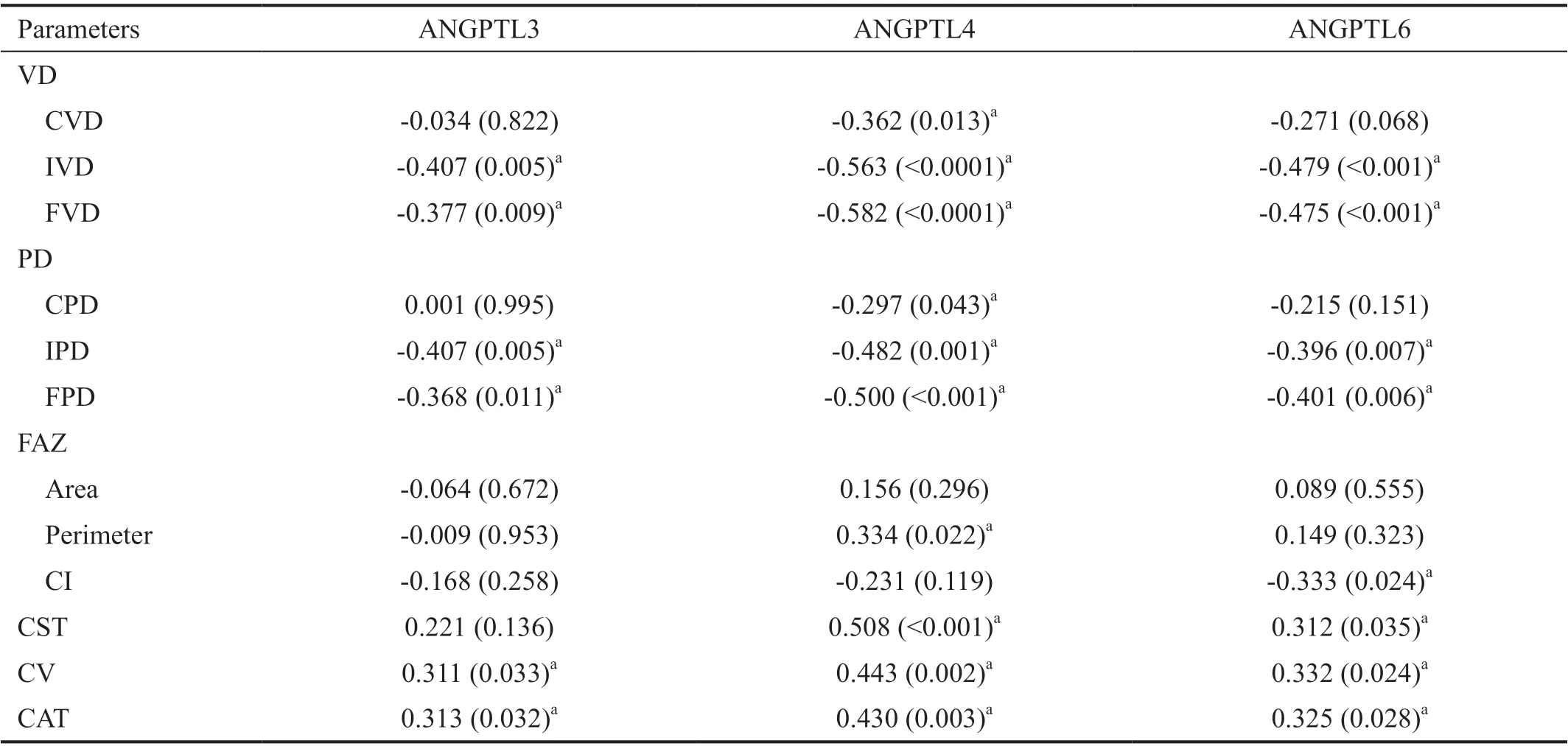
Table 3 Correlation coefficients between OCTA parameters and aqueous levels of ANGPTLs in the complete cohort r (P)
Although patients with DR were shown to have elevated serum ANGPTL3 levels in a previous study[12], we could not demonstrate that aqueous ANGPTL3 levels were significantly higher in patients with DME than in the control group. This might have been due to the limited quantity of aqueous humor specimens and the insufficient expression of ANGPTL3 in the aqueous humor.
ANGPTL4 participates in many body processes including glycolipid metabolism[21], energy homeostasis[22], tumorigenesis[23],angiogenesis, and increased vascular permeability[24]. A study found that ANGPTL4 caused endothelial cell junction breakage bothin vitroand in the lungs, increased vascular permeability,and promoted lung metastasis of breast cancer cells[25]. Using gene expression analysis, Xinet al[10]determined that HIF-1α, which is a transcription factor involved in angiogenesis[26],increases the expression of ANGPTL4 in hypoxic Müller cells and ischemic retinal tissues, and ANGPTL4 promotes vascular permeability, bothin vivoandin vitro. Additionally, ANGPTL4 knock-out mice exhibit immature endothelial tight junctions in the retinal vascular plexus[27]. Furthermore, ANGPTL4 results in activation of the Rho/ROCK signaling pathway, cell connection disruption, and retinal vascular leakage in mice by binding to neuropilins[16], while the inhibition of the HIF-1a/ANGPTL4 signal transduction pathway reduces hypoxiainduced cell permeability in ARPE-19 cells[28]. Our results showed that aqueous ANGPTL4 levels were significantly higher in patients with DME than in healthy patients and diabetic patients without DR. These findings are consistent with those of a previous report by Kwonet al[29]that showed that aqueous ANGPTL4 levels were significantly higher in patients with DME (including those with proliferative and non-proliferative DR) than in healthy controls. Other studies analyzing patients with DR have revealed similar results[30-31].Based on this evidence, we speculate that ANGPTL4 induces retinal vascular permeability in DME patients.
In a previous study[17], ANGPTL6 promoted angiogenesis and induced vascular leakage in mice. Researchers showed that when ANGPTL6 was injected into the dermis, a large amount of Evans blue dye leakage was found at the injection site.To the best of our knowledge, this is the first study to assess ANGPTL6 levels in the aqueous humor of diabetic patients.Our results showed that these were significantly higher in the DME group than in the control group. Considering the previous evidence, we speculate that ANGPTL6 is associated with retinal microvascular leakage in patients with DME.
Retinal ischemia and DME are the main features of DR. Before the advent of OCTA, FFA was the main method for assessing retinal ischemia, which was mainly diagnosed by the increment of the FAZ area and loss of capillaries in the macular area[32].Considering that FFA is invasive and time-consuming, and can not recognize DME and distinguish retinal microvascular changes, the emergence of OCTA represents a significant advancement that compensates for these shortcomings. OCTA can not only obtain images of retinal blood vessels quickly and non-invasively, but can also distinguish DME from capillary nonperfusion[33]. Various OCTA metrics are currently used to evaluate macular ischemia, including VD, PD, FAZ area, FAZ circumference, and FAZ-CI[34]. The Zeiss OCTA 5000 offers three scan sizes: 3×3, 6×6, and 8×8 mm2. In this study, we chose the 3×3-mm2scan based on a report[35]that suggests that this scan size may be the best predictive sensitivity for DR, just like the finding in patients with DME.
Although the DME group showed a significantly lower PD in the deep capillary plexuses compared with the non-DME group, this metric was not significantly different in the superficial capillary plexuses[36]. In this study, vessel and perfusion density metrics were considerably lower in DME eyes than in control eyes. These findings are consistent with those of several previous studies[37-39]. Specifically, Tinget al[38]showed that the PD was lower in DME patients than in non-DME patients, regardless of whether the superficial capillary plexuses or deep capillary plexuses were affected.Additionally, Kimet al[39]found that among patients with mild non-proliferative DR, patients with DME exhibited significantly lower vessel and perfusion density than non-DME patients. In accordance with our results, previous studies[36-37,40]found that the FAZ area was significantly higher in patients with DME than in healthy subjects or diabetic patients without DME. Additionally, this study showed that DME eyes had a larger FAZ perimeter and lower FAZ-CI than control eyes.These results suggest that OCTA metrics may be helpful for evaluating macular ischemia. It is worth noting that, compared with the NDR group, DME IVD, FVD, and FAZ-CI were significantly lower and FAZ parameters were higher in the DM subgroup, which may indicate that microvascular alterations in the macula may constitute risk factors for DME. Our research team will conduct a long-term follow-up to verify this hypothesis.
Based on the observation that aqueous ANGPTL4 levels correlated positively with the capillary non-perfusion zone,Kwonet al[29]suggested that expression of this protein is induced by retinal ischemia. In our study, aqueous ANGPTL4 levels correlated negatively with vessel and perfusion densities of all partitions, while ANGPTL3 and ANGPTL6 did so with all vessel and perfusion metrics except CVD and CPD.Given that, as previously mentioned, decreases in vessel and perfusion densities are indicative of macular ischemia,our results support the fact that ANGPTL3, ANGPTL4, and ANGPTL6 are induced by retinal ischemia.
Studies have confirmed that ANGPTL3 promotes podocyte permeability[41]. In this study, aqueous levels of the three ANGPTL proteins correlated positively with CV and CAT,while ANGPTL4 and ANGPTL6 levels did so with CST as well. It is widely known that CST, CV, and CAT are all closely related to macular edema, which in turn can be caused by vascular leakage. In previous studies, aqueous ANGPTL4 levels correlated positively with CV in DME patients[29]and with CST and CV in patients with macular edema caused by retinal vein branch occlusion[15]. These results further support the hypothesis that ANGPTL3, ANGPTL4, and ANGPTL6 promote vascular permeability and induce DME.
This study had several limitations. First, because of the small sample size, patients who had undergone panretinal photocoagulation could not be excluded; this might have affected the OCTA measurement results. Finally, whether the various OCTA metrics are the most efficient indicators for evaluating macular ischemia still needs to be confirmed by large-scale studies. Additionally, the OCTA measurement range was small, which made the application of ultra-widefield OCTA particularly important.
In conclusion, this study showed that compared with control subjects, patients with DME had higher aqueous ANGPTL4 and ANGPTL6 levels, a lower VD, PD, and FAZ-CI, and a larger FAZ area and FAZ perimeter. Most importantly, ANGPTL4 and ANGPTL6 levels showed a significant correlation with alterations in OCTA parameters (such as CST, CV, CAT, and vessel and perfusion densities) that are indicative of macular edema. These results strongly suggest that ANGPTL4 and ANGPTL6 are associated with vascular leakage in DME. We speculate that these proteins could be potential targets in DME therapy. In addition, OCTA metrics may help evaluate macular ischemia in DME.
ACKNOWLEDGEMENTS
Foundations: Supported by National Natural Science Foundation of China (No.81741058); Key Research and Development Program of Shaanxi Province (No.2021SF-155);First Affiliated Hospital of Xi’an Jiaotong University(No.2019QN-05).
Conflicts of Interest:Yan J, None; Li WJ, None; Qin YZ,None; Qiu XY, None; Qin L, None; Li JM, None.
杂志排行
International Journal of Ophthalmology的其它文章
- Upregulation of ASPP2 expression alleviates the development of proliferative vitreoretinopathy in a rat model
- Mesenchymal stem cell-derived exosomes inhibit the VEGF-A expression in human retinal vascular endothelial cells induced by high glucose
- Protective effects of umbilical cord mesenchymal stem cell exosomes in a diabetic rat model through live retinal imaging
- New technique for removal of perfluorocarbon liquid related sticky silicone oil and literature review
- Quantitative analysis of retinal vasculature in normal eyes using ultra-widefield fluorescein angiography
- Evaluation of the long-term effect of foldable capsular vitreous bodies in severe ocular rupture
