Comparison of environmental responding strategies between Ulva prolifera and Sargassum horneri: an in-situ study during the co-occurrence of green tides and golden tides in the Yellow Sea, China in 2017*
2021-12-09XinyuZHAOYiZHONGHuanxinZHANGTongfeiQUChengzongHOUChenGUANFengLIUXuexiTANGYingWANG
Xinyu ZHAO , Yi ZHONG , Huanxin ZHANG , Tongfei QU , Chengzong HOU ,Chen GUAN , Feng LIU , Xuexi TANG ,**, Ying WANG ,**
1 College of Marine Life Sciences, Ocean University of China, Qingdao 266003, China
2 Qingdao National Laboratory for Marine Science and Technology, Qingdao 266237, China
3 College of Geography and Environment, Shandong Normal University, Jinan 250000, China
4 CAS Key Laboratory of Marine Ecology and Environmental Sciences, Institute of Oceanology, Chinese Academy of Sciences,Qingdao 266071, China
Abstract Large-scale green tides in the Yellow Sea occurred for 13 consecutive years since 2007.The unusual co-occurrence of green tides and golden tides occurred in the Yellow Sea in 2017. The causative species are Ulva prolifera and/or Sargassum horneri. Previous studies on physiological response characteristics of U. prolifera and S. horneri are done in the laboratory mainly, and there is no in-situ comparative study in this regard. In this study, the in-situ physiological response characteristics of both species were measured. The results indicated that cyclic electron f low and antioxidant system play more important roles in protecting U. prolifera, while non-photochemical quenching is more important for adapting to the environment in S. horneri. U. prolifera has a stronger ability to utilize nutrients to rapidly increase its biomass under a suitable condition compared to S. horneri.
Keyword: Ulva prolifera; Sargassum horneri; environmental response strategy; in-situ study
1 INTRODUCTION
In the 1970s, the biomass of macroalgae along the coast of industrialized countries began to increase. By the 1990s, macroalgae had become a public hazard in many coastal areas. Harmful algal blooms caused by large seaweeds around the world increased during the 2000 (Ye et al., 2011). Although macroalgae does not produce toxic substances by themselves, they can cause many serious eff ects. The macroalgae can block the channel. Large-scale expansion of macroalgae can cause damage to the ecological balance of estuarine communities, and may cause economic losses to f isheries and tourism (Keesing et al., 2016).
Harmful algal blooms caused by macroalgae show a signif icant impact on the Yellow Sea. Large-scale green tides occurred for thirteen consecutive years since 2007. The occurrence of green tides in the Yellow Sea has typical characteristics. The origin of green tides is in the southern Yellow Sea, while the location of the bloom is in the northern Yellow Sea(Liu et al., 2010). Long-duration of f loating drift is the typical characteristic of green tides. The causative species of green tides in the Yellow Sea isUlvaprolifera(Ye et al., 2008). Large-scale f loatingSargassumhorneriwere observed at the site during the Yellow Sea green tides outbreak in 2017 (Liu et al., 2018). The emergence of a large-scale f loatingS.hornericaused a new kind of harmful algal bloom(golden tides). The green tides and golden tides always occur separately. The unusual co-occurrence of the green tides and golden tides could be observed in the Yellow Sea in 2017, which increased the diffi culty of monitoring and controlling of harmful algal blooms.

Table 1 Information of the sampling site in the Yellow Sea
Diff erent species of macroalgae show diff erent environmental responding strategies. Cyclic electron f low (CEF), non-photochemical quenching (NPQ),and antioxidant system are important environmental responding strategies inU.prolifera. CEF can enhance the desiccation tolerance ofUlvasp. (Gao et al., 2011). Photosystem I (PSI) shows a higher tolerance to osmotic stress than photosystem II (PSII)inU.prolifera(Gao et al., 2014).U.proliferacan utilize CO2in the atmosphere directly for rapid growth(Huan et al., 2016). NPQ plays an important role in protectingU.proliferaunder high light stress (Mou et al., 2013). Antioxidant system also plays an important role in protectingU.proliferaunder the environmental stress (Wang et al., 2012). These characteristics enhance the photosynthetic plasticity and environmental adaptability ofU.prolifera. Many studies have also studied the response characteristics ofS.horneri. Moderately elevated temperatures,especially under elevated CO2levels at the same time,could signif icantly enhance the growth ofS.horneri(Li et al., 2020). The demand ofS.hornerifor nitrogen increased in spring, and the high temperature and nitrogen concentration in spring leaded to the high biomass accumulation of drifting thalli (Yu et al.,2019). The existing researches were f inished in the laboratory, and there was no in-situ comparative study on the physiological response characteristics ofU.proliferaandS.horneri. The in-situ comparative study aboutU.proliferaandS.horneriis meaningful.
In this study, the in-situ environmental indicator parameters were monitored. We also measured the insitu chlorophyll f luorescence and pigment changes inU.proliferaandS.horneri. Furthermore, we compared the antioxidant capacity of the two kinds of macroalgae, and the relationship among the development of green tides & golden tides and the environmental factors were analyzed.
2 MATERIAL AND METHOD
2.1 Study area and f ield sampling
Three sampling locations were distributed along each of the two transects (34°25′N-36°00′N) in the Southern Yellow Sea between 120°30′E-121°30′E.Three replicates of bothU.prolifera(N-U and S-U)andS.horneri(N-S and S-S) were sampled at each site, which resulted in eighteen samples (N1-1, N1-2,N1-3, N2-1, N2-2, N2-3, N3-1, N3-2, N3-3, S1-1, S1-2, S1-3, S2-1, S2-2, S2-3, S3-1, S3-2, and S3-3) in total for each species (Fig.1; Table 1).
The thalli were cleaned gently with a brush and sterile seawater to remove any attached sediment,small grazers, and epiphytes. The samples were used directly for measuring chlorophyll f luorescence. The thalli were then placed in a freezer at -80 °C for further study. In-situ salinity and temperature were measured by using an YSI Professional Pro Meter (YSI Inc.,United States). Seawater samples were collected by using Niskin bottles, which were then f iltered through precombusted (460 °C, 6 h) Whatman GF/F f ilters.The f iltrates were collected using polyethylene bottles at -20 °C for nutrient analysis. The concentrations of NO3ˉ and NO2ˉ were determined by using indophenol blue method, and concentration of NH4+was measured using indophenol blue method. The concentration of PO43ˉ-P was measured by using molybdate blue method. Dissolved inorganic nitrogen (DIN) was obtained by calculating of the sum of NO3ˉ , NO2ˉ, and NH4+. Total dissolved nitrogen (TDN) and phosphorus(TDP) were measured through persulfate oxidation.The concentration of dissolved organic nitrogen(DON) was calculated based on the concentrations of TDN and DIN, and the concentration of dissolved organic phosphorus (DOP) was calculated based on those of TDP and PO43ˉ-P (Grasshoff et al., 1999).
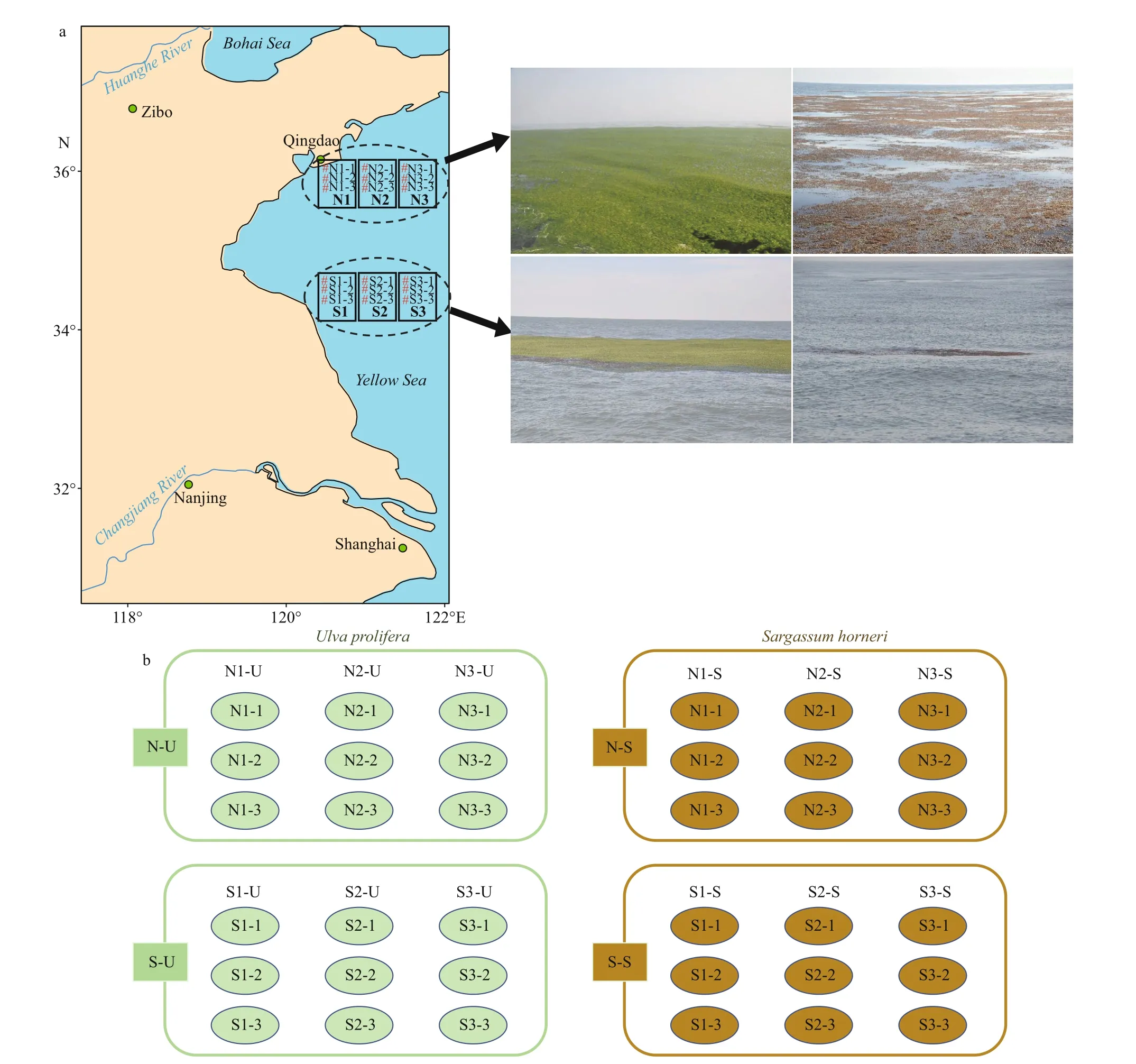
Fig.1 The sampling deployments
2.2 Pigment extraction
Samples of macroalgae were ground in liquid nitrogen, and then 90% (v/v) acetone buff er was used to extract the pigments. The mixture was centrifuged at 10 000×gat 4 °C, and the supernatant was extracted and conserved for further analyses. To determine the chlorophyll and carotenoid contents, a TU-1800 ultraviolet-visible spectrophotometer (PERSEE,China) was used. The optical densities (OD), i.e.,OD470, OD645, and OD663, were measured separately.The following equations were used to obtain the concentrations of chlorophylla(Chla), chlorophyllb(Chlb), total chlorophyll (total Chl), and carotenoid(Car) contents:
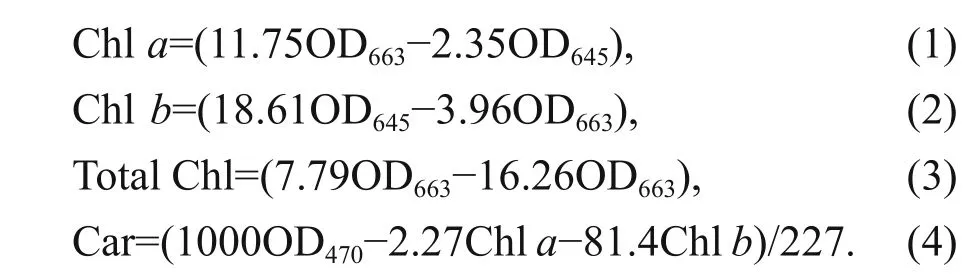
All experiments were performed as three replicates;the units of the results are given as milligrams per gram (mg/g) of fresh weight (FW) (Arnon, 1949).
2.3 Measurement of chlorophyll f luorescence
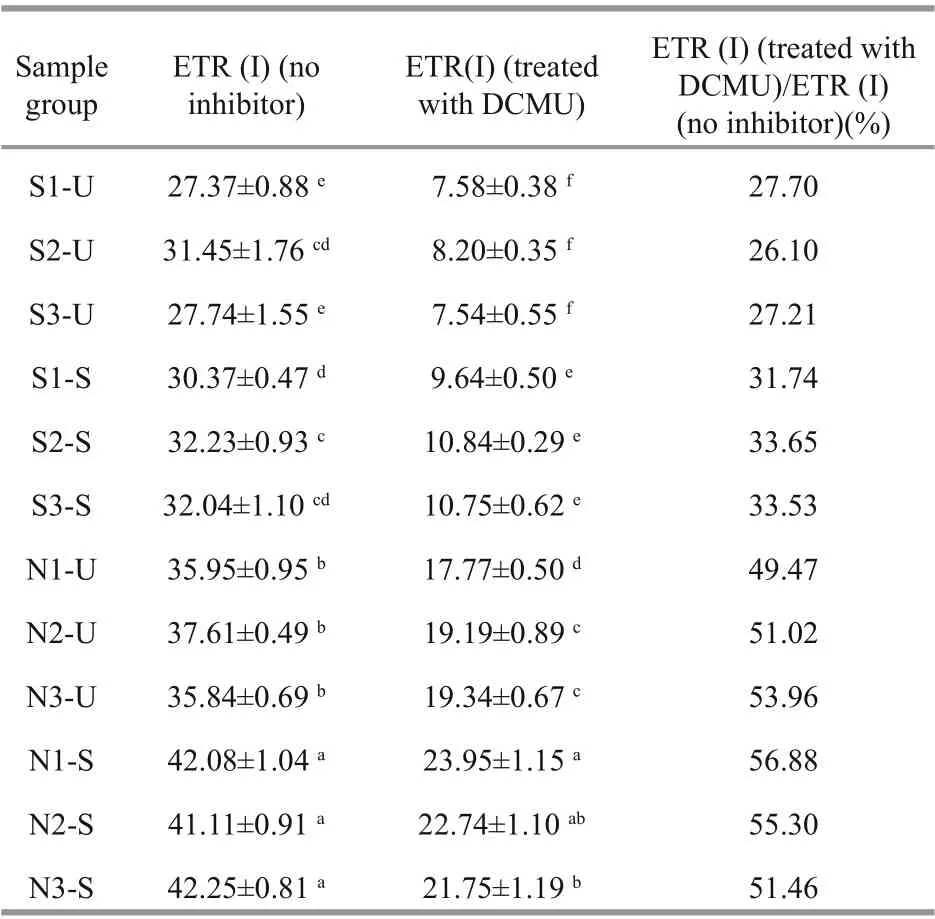
A Dual-PAM-100 f luorometer (Walz, Germany)was used to measure the chlorophyll f luorescence and P700 parameters. Before the experiments, the thalli were placed in the dark for 15 min. The settings of the Dual-PAM-100 f luorometer were as described in Zhao et al. (2016). The automated induction, recovery curve routine, and repetitive application of saturation pulses were used to obtain the results of chlorophyll f luorescence. After the saturating pulse,F0andFmwere determined.F0is the minimal f luorescence after dark acclimation.Fmis the maximal f luorescence after saturation f lashes in the dark-acclimatized sample. The parameterFv/Fm(PSII maximum quantum yield) was calculated by the equation:Fv/Fm=(Fm−F0)/Fm, which represent the PSII maximum quantum yield in the thalli.Y(II) is the eff ective quantum yield of PSII, which can also be used for evaluating the photosynthetic activity inU.prolifera.Y(NPQ) (quantum yield of regulated non-photochemical energy loss) andY(NO) (quantum yield of non-regulated energy loss) represent the negative eff ects onU.proliferafrom environmental stress.Y(I) can represent the eff ective quantum yield of PSI. The ratio of the eff ective quantum yield of PSI can represent the energy distribution between PSI and PSII (Huang et al., 2013). Furthermore,3-(30, 40-dichlorophenyl)-1,1-dimethylurea (DCMU)was used for determining the PSI-driven CEF in the thalli. DCMU is a kind of inhibitor, which can abolish the linear electron f low (LEF) (Joët et al., 2002). ETR(I) (photosynthetic electron transport rate of PSI)(treated with DCMU) and ETR (I) (no inhibitor) were determined by us, and the ETR (I) (treated with DCMU)/ETR (I) (no inhibitor) was calculated for studying the ratios of cyclic electron f low to total electron f low.
2.4 Analysis of lipid peroxidation and H 2 O 2 content
Thalli samples of 0.1 g FW were ground in liquid nitrogen, to which 1-mL 5% (w/v) trichloroacetic acid (TCA) was added. The mixture was then centrifuged at 12 000×gfor 10 min at 4 °C. The content of H2O2was determined using H2O2kits according to the manufacturer’s instructions (Nanjing Jiancheng, China), and the level of lipid peroxidation(MDA) was determined using thiobarbituric acid(TBA)-reacting substance contents (Buege and Aust,1978).
2.5 Determination of antioxidant enzyme activity
Thallus samples of 0.1 g FW were ground in liquid nitrogen and then extracted with 1 mL of 0.05-mol/L potassium phosphate buff er (pH 7.0). The mixture was centrifuged at 12 000×gfor 10 min at 4 °C. The supernatant was used for measuring the total soluble protein (TSP) and the activities of antioxidant enzymes. The TSP content was determined using the Coomassie blue dye binding assay (Bradford, 1976).The activities of the antioxidant enzymes superoxide dismutase (SOD), catalase (CAT), and guaiacol peroxidase (GPX) were measured using commercial kits, following the manufacturer’s instructions(Nanjing Jiancheng, China).
2.6 Statistical analysis
All values in this research were obtained from random collected samples. The mean values and standard deviations (mean±SD) were calculated for the replicates of each treatment condition (n=3). The results are tested in a one-way ANOVA using SPSS 22.0 statistical software (IBM Corp., United States),and Bivariate Pearson’s correlation analysis was performed to assess the relationships of the parameters for photosynthetic and antioxidant systems inU.proliferaandS.horneri. Data were examined with Levene’s test for homogeneity and the Shapiro-Wilk test for normality. Student-Newman-Keuls post-hoc multiple comparison test and Duncan post-hoc test were used if ANOVA indicated a signif icant eff ect.Diff erences were judged to be signif icant atP<0.05 andP<0.01, respectively. The f igures included in this study were generated using SigmaPlot 12.5 software(Systat Software Inc., USA).
3 RESULT
3.1 Pigment analysis
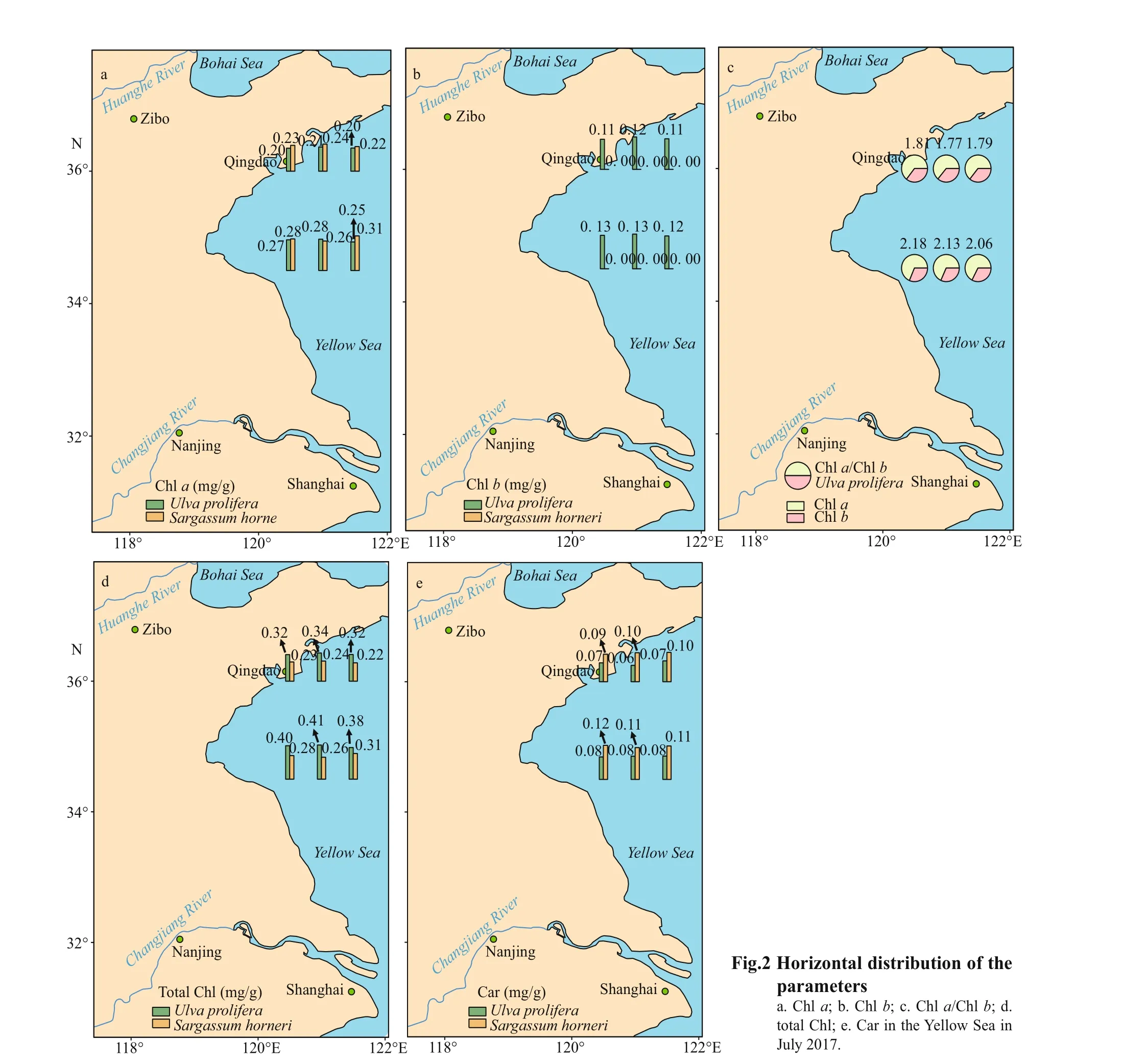
Chlorophyllashowed signif icant sampling locality and latitude eff ects; Chlb, total Chl, and Car showed signif icant species eff ect (Table 2). Figures 2 & 3 show the contents of pigment in two kinds of macroalgae at each site. There was no signif icant diff erence in the content of Chlain the same kind of macroalgae at the same latitude (Fig.2a; one-way ANOVA,P> 0.05). Similar trend could also be observed in Chlb, total Chl, and Car (Fig.2b, d, & e).For Chla, no signif icant diff erence was seen between S-U and S-S or between N-U and N-S (Fig.3a; oneway ANOVA,P>0.05). No signif icant diff erence in Chlawas seen between S-S and N-S (Fig.3a; oneway ANOVA,P>0.05), while the content of Chlain S-U was signif icantly higher than that of N-U (Fig.3a;one-way ANOVA,P< 0.05). There was no signif icant diff erence in Chlbbetween S-U and N-U (one-way ANOVA,P>0.05), and Chlbcould not be measured in S-S and N-S (Fig.3b). No signif icant diff erence in total Chl was observed between S-U and N-U or S-S and N-S (Fig.3c; one-way ANOVA,P>0.05). Values of total Chl in S-U and N-U were signif icantly higher than those of S-S and N-S, respectively (Fig.3c; oneway ANOVA,P< 0.05). There was no signif icant diff erence in Car between S-U and N-U or S-S and N-S (Fig.3d; one-way ANOVA,P>0.05). No signif icant diff erence could be observed between S-U and S-S or N-U and N-S (Fig.3d; one-way ANOVA,P>0.05).

Table 2 Results of one-way ANOVA for all the chemical indicators

Fig.3 Box plot of all the parameters
3.2 Analysis of chlorophyll f luorescence
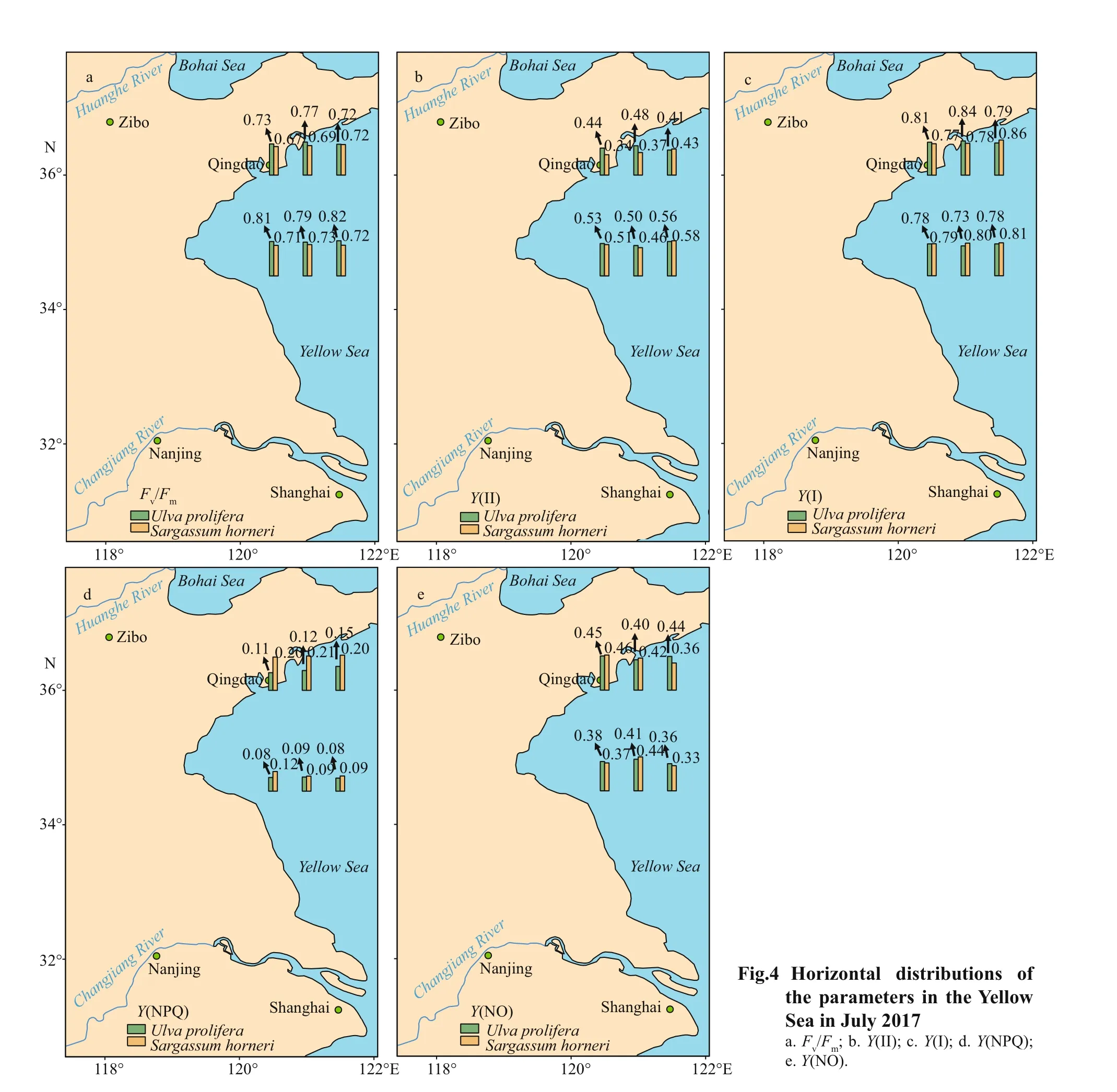
Fv/FmandY(NPQ) showed signif icant species,sampling locality, and latitude eff ects, and there was not signif icant eff ect ofY(II),Y(NO), and ETR (I)(treated with DCMU)/ETR (I) (no inhibitor) in species(Table 2). The values of chlorophyll f luorescence could be seen in Figs.4 & 5.Fv/Fmof S3-U and N3-S were signif icantly higher than those of S2-U and N1-S, respectively (Fig.4a; one-way ANOVA,P< 0.05).The values ofY(II) inS.horneriin the eastern part was higher than those of western part and central part,while no similar results could be observed inU.prolifera(Fig.4b). There was no remarkable diff erence among the S-U, S-S, N-U, and N-S inY(I)(Fig.5c; one-way ANOVA,P>0.05).Y(NPQ) of N3-U was signif icantly higher than that of N1-U (Fig.4d;one-way ANOVA,P< 0.05).Y(NO) of S2-U, S1-U,S3-U signif icantly decreased in turn. The ascending sequence ofY(NO) was S3-S
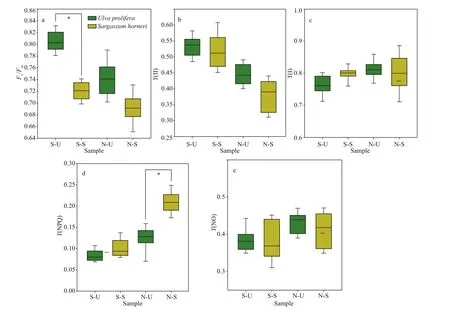
Fig.5 Box plot of photosynthetic parameters and the mean (±SD) of three independent experiments
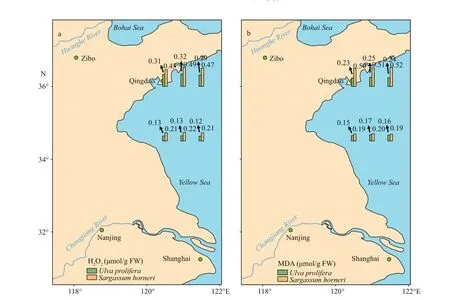
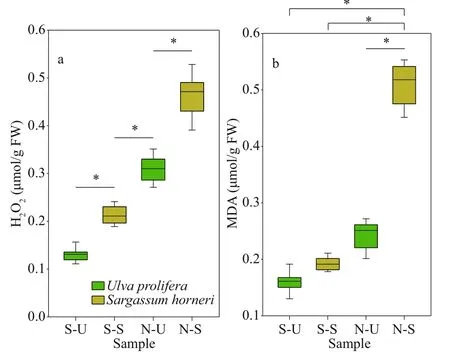
3.3 Results of H 2 O 2 and MDA
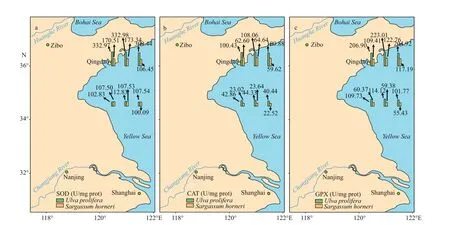
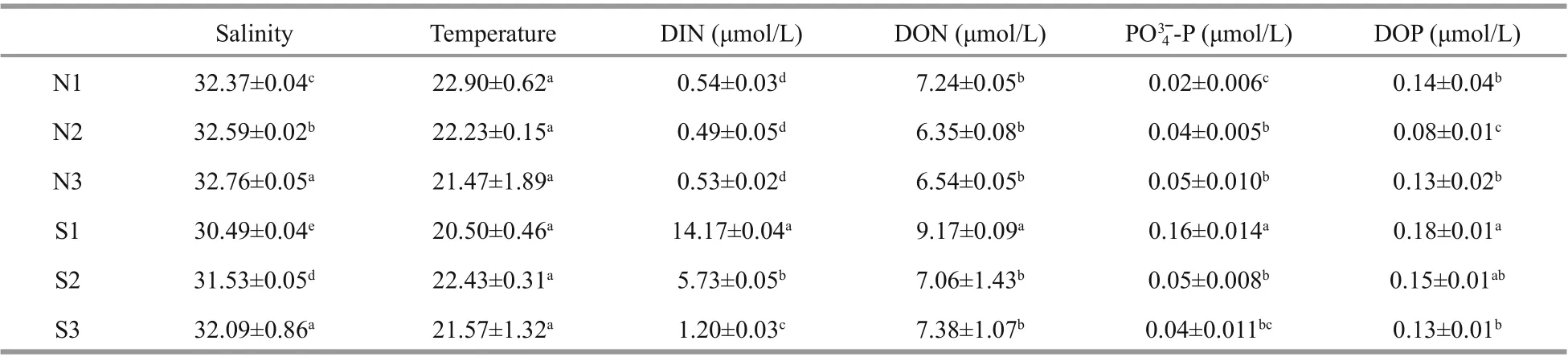
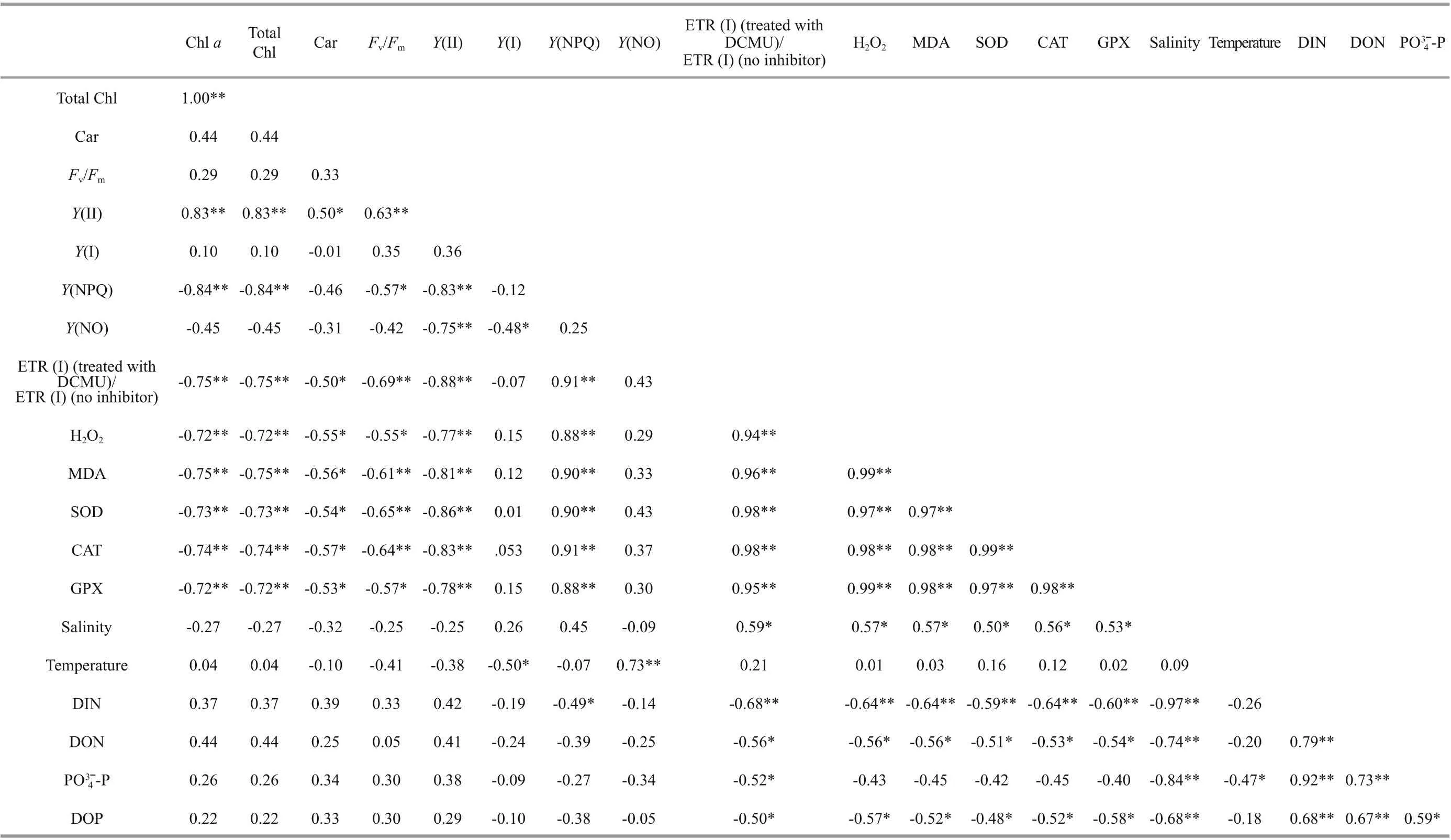
Both H2O2and MDA showed signif icant species,sampling locality, and latitude eff ects (Table 2). There was no signif icant diff erence in the content of H2O2and MDA in the same kind of macroalgae at the same latitude (Fig.6; one-way ANOVA,P> 0.05). The ascending sequence of content of H2O2was S-U There were signif icant eff ects of SOD, CAT, and GPX in species, sampling locality, and latitude (Table 2). No signif icant diff erence in activities of SOD,CAT, and GPX in the same kind of macroalgae at the same latitude could be seen in Fig.8 (one-way ANOVA,P> 0.05). Activity of SOD in the thalli of N-U was signif icantly higher than that of N-S, and that of N-S was signif icantly higher than those of S-U and S-S (Fig.9a; one-way ANOVA,P< 0.05). Activity of CAT in the thalli of S-U was signif icantly higher than that of S-S, and activity of CAT of N-U was signif icantly higher than that of N-S (Fig.9b; one-way ANOVA,P< 0.05). The trend similar to Fig.9b could also be seen in Fig.9c. Table 3 ETR (I) of the thalli treated with the inhibitor DCMU Fig.6 Horizontal distribution of the H 2 O 2 (a) and MDA (b) in Ulva prolifera and Sargassum horner in the Yellow Sea in July 2017 There were signif icant eff ects of salinity, DIN,DON, and DOP in species, sampling locality, and latitude but no signif icant diff erence of temperature in sampling locality and latitude (Table 2). The results of salinity, temperature, and nutrients are summarized in Table 4. Horizontal distribution of salinity during the harmful algal bloom was signif icantly variable among sites at the same latitude. There was no signif icant diff erence in temperature among diff erent sites. The results of DIN at southern 3 sites were signif icantly higher than those of northern sites, and ascending sequence of DIN at southern sites was S1 Fig.7 Boxplot ofthe H2 O2 (a)andMDA(b)with the mean(±SD) of three independentexperiments Fig.8 Horizontal distribution of SOD (a), CAT (b), and GPX (c) in Ulva prolifera and Sargassum horner in the Yellow Sea in July 2017 Fig.9 Box plot of SOD (a), CAT (b), and GPX (c) with the mean (±SD) of three independent experiments Table 4 Horizontal distribution of salinity, temperature, and nutrients UlvaproliferaandS.horneriare the causative species of green tides and golden tides in the Yellow Sea (Ye et al., 2008; Liu et al., 2018). Previous studies have done a lot on the environmental responding strategies of these two kinds of macroalgae, while these studies are mainly f inished in the laboratories.Furthermore, green tides occurred for consecutive years in the Yellow Sea, while the golden tides only co-occurred with the green tides in the Yellow Sea since 2016. It is necessary to compare the environmental strategies of the two kinds of macroalgae. During blooms, large-scale macroalgae gathered and rapidly expanded (Liu et al., 2015, 2018). Both the f loatingU.proliferaandS.hornerishow strong photosynthetic adaptability for the rapidly expanding of the thalli. NPQ is a crucial photosynthetic mechanism that exists in macroalgae for adapting to the complex and changeable environment (Li et al.,2009). In the present study, the photosynthetic activity of the thalli decreased during the northward drift process, which still maintain a relatively high level(Figs.4-5). The photosynthetic activity of the f loatingU.proliferais always higher than that of f loatingS.horneriduring its northward drift process, and the photosynthetic activity ofU.proliferaandS.horneriwasn’t damaged signif icantly during the northward drift process (Fig.5). NPQ plays a key role inU.proliferaandS.hornerifor adapting to the environment, and NPQ plays a more important role inS.hornericompared toU.prolifera, especially at the northern 3 sites (Fig.5). There is also a remarkable correlation betweenY(NO) and ETR (I) (treated with DCMU)/ETR (I) (no inhibitor), which means the important role of CEF under extreme environment inU.proliferarather than inS.horneri(Tables 5-6). Pigment composition also plays an important role in macroalgae environmental adaptation (Praba et al.,2011). The change trend of Chl-acontent inU.proliferawas the same as that ofS.horneriduring the northward drift process (Figs.2-3). Chl-bcontent inU.proliferadoes not change signif icantly during the northward drift process, while Chlbwas not detected inS.horneri(Figs.2-3). The total Chl content inU.proliferawas always signif icantly higher than that inS.horneri. The total Chl content inU.proliferadoes not change signif icantly, while total Chl content inS.horneridecreases signif icantly during the northward drift process (Figs.2-3).Furthermore, a signif icant correlation amongFv/Fm,Chla, and total Chl could be found inU.proliferarather than inS.horneri(Tables 5-6). Chlbwas not detected inS.hornerias it did not exist in Phaeophytes(Larkum et al., 2012). The Car content in f loatingS.horneriwas always higher than that ofU.prolifera,and the Car content in the two kinds of macroalgae does not change signif icantly during the northward drift process (Fig.3). MDA content is the parameter of lipid peroxidation,and higher H2O2content can lead to higher lipid peroxidation (Luo and Liu, 2011). The H2O2content in the two kinds of macroalgae increase signif icantly during the northward drift process, and H2O2content inS.horneriwas always signif icantly higher than that inU.prolifera(Fig.7a). MDA content in the two kinds of macroalgae also increases signif icantly during the northward drift process (Fig.7b). There was no signif icant diff erence in MDA content between the two kinds of macroalgae at the southern 3 sites,while the MDA content inS.horneriwas signif icantly higher than that inU.proliferaat the northern 3 sites(Fig.7b). Furthermore, a signif icant positive correlation could be observed among H2O2, MDA andY(NO), which conf irmed the close relationship of higher levels of H2O2and MDA with irreversible damage inU.prolifera(Tables 5-6). SOD, CAT, and GPX are all important antioxidant enzymes in plants (Imlay, 2003). The activities of CAT and GPX inU.proliferawere signif icantly higher than those ofS.horneriat the south of 35°N,while there was no signif icant diff erence in SOD activity between the two macroalgae (Fig.9). The activities of SOD, CAT, and GPX in the two kinds of macroalgae increase signif icantly during the northward drift process, and the activities of the four kinds of enzymes inU.proliferaare signif icantly higher than those inS.horneri(Fig.9). The signif icant positive correlation among SOD, CAT, GPX, andY(NO) could be observed inU.prolifera, which further conf irmed more important roles of antioxidant system inU.proliferathanS.horneri(Tables 5-6). Table 5 Pearson’s correlation coeffi cients in the test index in the thalli of U. prolifera Table 6 Pearson’s correlation coeffi cients in the test index in the thalli of S. horneri The biomass and size of f loating harmful algal blooms increased because of apposite temperature and nutrient richness (Shi et al., 2015; Li et al., 2017;Wu et al., 2018). Temperature is an important factor for aff ecting the scale, f loating paths, and distribution area of harmful algal blooms (Fan et al., 2014; Song et al., 2015). Nutrient also inf luenced the forming of harmful algal blooms. Excess inorganic nutrients could be absorbed and utilized byU.prolifera(Li et al., 2019). Higher phosphate levels could increase the relative growth rate and the nitrate uptake rate ofSargassummuticum(Xu et al., 2017). Furthermore,Zhang et al. (2020) conf irmed that DIN and DOP were main nutrients for algae. The increasingly serious eutrophication in the estuaries along the coast of Jiangsu Province is probably the key factor for the occurrence of harmful algal blooms (Zhang et al.,2020). There was signif icant negative correlation among salinity, Chla, and Car inU.prolifera, while signif icant positive correlation was observed between temperature andY(NO) inS.horneri(Tables 5-6).These results may mean that salinity had a greater inf luence onU.prolifera, while temperature had a greater inf luence onS.horneri. The result of signif icant correlation among DIN, DOP, andY(NPQ)may mean stronger ability to utilize DIN and DOP forU.prolifera(Tables 5-6). UlvaproliferaandS.hornerihave diff erent environmental responding strategies. CEF and antioxidant system play more important roles in protectingU.prolifera, while NPQ is more important for adapting to the environment inS.horneri.Meanwhile,U.proliferahas a stronger ability to utilize nutrients and rapidly increase its biomass under suitable condition compared toS.horneri. All data generated and/or analyzed during this study are available from the corresponding author upon request on reasonable request.3.4 Analysis of the antioxidant system
3.5 Results of environmental parameters

4 DISCUSSION
4.1 Comparison of the environmental responding strategies in U. prolifera and S. horneri

4.2 Relationship between environmental factors and responding strategies in macroalgae
5 CONCLUSION
6 DATA AVAILABILITY STATEMENT
杂志排行
Journal of Oceanology and Limnology的其它文章
- MamZ protein plays an essential role in magnetosome maturation process of Magnetospirillum gryphiswaldense MSR-1*
- Magnetotactic bacteria from the human gut microbiome associated with orientation and navigation regions of the brain*
- How light aff ect the magnetotactic behavior and reproduction of ellipsoidal multicellular magnetoglobules?*
- Biocompatibility of marine magnetotactic ovoid strain MO-1 for in vivo application*
- Determination of the heating effi ciency of magnetotactic bacteria in alternating magnetic f ield*
- An approach to determine coeffi cients of logarithmic velocity vertical prof ile in the bottom boundary layer*
