How light aff ect the magnetotactic behavior and reproduction of ellipsoidal multicellular magnetoglobules?*
2021-12-09XinxinQIANYicongZHAOClaireLiseSANTINIHongmiaoPANTianXIAOHaitaoCHENTaoSONGJinhuaLIFrancoisALBERTOSophieBRUSTLEINLongFeiWU
Xinxin QIAN , Yicong ZHAO , Claire-Lise SANTINI , Hongmiao PAN ,Tian XIAO , Haitao CHEN , Tao SONG , Jinhua LI , Francois ALBERTO ,Sophie BRUSTLEIN , Long-Fei WU ,**
1 State Key Laboratory of Microbial Resources, Institute of Microbiology, Chinese Academy of Sciences, Beijing 100101, China
2 Aix Marseille University, CNRS, LCB, Centuri, IM2B, IMM, Marseille 13009, France
3 International Associated Laboratory of Evolution and Development of Magnetotactic Multicellular Organisms (LIA-MagMC),CNRS-CAS, Marseille 13402, France
4 CAS Key Laboratory of Marine Ecology and Environmental Sciences, Institute of Oceanology, Chinese Academy of Sciences,Qingdao 266071, China
5 Institute of Electrical Engineering, Chinese Academy of Sciences, Beijing 100190, China
6 Key Laboratory of Earth and Planetary Physics, Institute of Geology and Geophysics, Chinese Academy of Sciences, Beijing 100029, China
7 Aix Marseille University, Centuri, Marseille 13009, France
Abstract Magnetotactic bacteria (MTB) synthesize intracellular magnetic organelles, magnetosomes,which consist of magnetic crystals that are enveloped in a membrane. Magnetosomes are organized into a chain(s) and confer on cells a magnetic dipolar moment. This magnetic property allows MTB cells to align and swim along geomagnetic f ield lines, a movement referred to as magnetotaxis. Some MTB species change their swim direction in response to illumination by UV, violet and blue light. Here we analyzed the polarity of morphology, magnetism, and motion in Mediterranean multicellular magnetotactic prokaryotes,also called, magnetoglobules or MMP. The magnetoglobules were assembled from 60-80 cells into an asymmetric ellipsoidal morphology with a relative narrow and large end. They swam dominantly northward,parallel to the direction of the magnetic f ield, with the narrow-end as the leading side. In response to a reversal in the direction of the magnetic f ield, they aligned quickly along the magnetic f ield lines and kept swimming northward. Interestingly, under constant illumination, 385-nm UV light, magnetoglobules changed their swimming direction southward anti-parallel to the direction of the magnetic f ield, with the large-end as the leading side. The change from a northward to southward direction occurred along with an increase of swimming speed. A minimum of 35-mW/cm 2 irradiance of UV light was suffi cient to trigger the swimming re-orientation. UV radiation also triggered the unidirectional division of magnetoglobules.Together these results revealed a coordination of the polarity of magnetoglobule morphology, magnetic moment, and swimming orientation, in response to magnetic and optical stimuli. The UV triggered the reversal of magnetotaxis and magnetoglobule division indicating the ecological signif icance of light for multicellular magnetotactic prokaryotes.
Keyword: photo-response; magnetic alignment; coordinated swimming
1 INTRODUCTION
Magnetotactic bacteria (MTB) are a group of heterogeneous Gram-negative bacteria that can produce single domain magnetite (Fe3O4) or greigite(Fe3S4) crystals in intracellular organelles called magnetosomes (Bazylinski and Frankel, 2004). The magnetosomes give bacteria the capacity of aligning to and swimming along geomagnetic f ield lines, this phenomenon is referred to as magnetotaxis. Anerobic or micro-aerobic MTB dwell at the oxic-anoxic interface (OAI) in chemically stratif ied sediments or water columns in freshwater and marine environments.
Interestingly, MTB in the Northern Hemisphere preferentially swim northward and parallel to the geomagnetic f ield lines (north-seeking, NS)(Blakemore, 1975). The MTB in the Southern Hemisphere swim in the direction of the magnetic south pole anti-parallel to the geomagnetic f ield lines(south-seeking, SS) (Blakemore et al., 1980). The current geomagnetic f ield is inclined downward in the Northern Hemisphere and upward in the Southern Hemisphere, and its inclination increases from the equator to the poles. Therefore, it is believed that the magnetotactic eff ect guides the cells in each hemisphere down to the lower oxygen content of the aquatic habitat, where they may stop swimming until the conditions change (Bazylinski and Frankel, 2004).This behavior has been referred to as magnetoaerotaxis. In previous studies we provided direct evidence to show that magnetotaxis is benef icial to magneto-cocci growth and even essential at low cell density (Zhang et al., 2014b).
MTBs display great morphologic diversity that includes coccoid to ovoid cells, rods, vibrios, and spirilla (Bazylinski et al., 2013). Additional to the most abundant unicellular MTB, a group of multicellular magnetotactic prokaryotes (MMPs in abbreviation,also called Magnetoglobules) in particular have attracted our attention because they display complex swimming behaviors such as photophobic response,bounce motion, axial magnetotaxis, and magnetic photokinesis (Qian et al., 2020). Two morphotypes of magnetoglobules have been reported. The spherical mulberry-like magnetoglobules (sMMPs) are typically 3-12 μm in diameter and comprise of 10-40 cells arranged with helical symmetry (Keim et al., 2004b;Zhou et al., 2013; Zhang et al., 2014a). The other morphotype of ellipsoidal pineapple-like magnetoglobules (eMMPs) have been found from the China Sea and the Mediterranean Sea. They are typically comprise of 28-101 cells arranged in interlaced circles with 8-20 μm in length and 5-15 μm in width (Zhou et al., 2012; Chen et al., 2015, 2016;Qian et al., 2020). Both morphotypes of magnetoglobules have peritrichous f lagella (Keim et al., 2004a; Zhang et al., 2014a; Chen et al., 2015; Qian et al., 2020) and are capable of producing magnetite and / or greigite magnetosomes (Zhou et al., 2011,2012, 2013; Zhang et al., 2014a; Chen et al., 2015;Abreu et al., 2018). The ellipsoidal, oval shaped magnetoglobules are easier to recognize (from the side determination) than spherical magnetoglobules when studying the relationship between magnetic polarity and swimming orientation as the narrow end indicates the north side of magnetoglobules.
Beside the aero-magnetotaxis observed for all MTB, the multicellular magnetoglobules exhibit two kinds of peculiar swimming behavior. The f irst is escape motility or ping-pong motion, which was recently described as bounce motion (Qian et al.,2020). Magnetoglobules swim northward until they are blocked at the north edge of the droplets, where some magnetoglobules then swim southward with an increase of swimming speed. At variable distances,magnetoglobules change swimming direction northward and return to the north edge (Rodgers et al., 1990; Simmons et al., 2004; Zhou et al., 2012,2013; Zhang et al., 2014a; Chen et al., 2015, 2016;Qian et al., 2020). When blocked by obstacles magnetoglobules display a similar bounce motion(Qian et al., 2020). In the second case, magnetoglobules accumulating at droplet edges swam in the opposite direction of magnetotaxis, towards the inner center of the droplet when they were illuminated by blue,violet, or UV light. This is named as photophobic response or is sometimes miscalled negative phototaxis (Sobrinho et al., 2011; Zhou et al., 2012,2013; Zhang et al., 2014a; Chen et al., 2015, 2016).Recently we observed magnetotactic photokinesis of ellipsoidal magnetoglobules. The north-seeking eMMPs under constant illumination by UV light might suddenly change their swimming direction southward (Qian et al., 2020). The relationship between magnetic polarity and swimming direction in response to light stimuli is poorly understood.Fluorescence confocal microscopy and ultra-thin section images revealed periphery-core unilateral constriction of constituent cells and unidirectional binary f ission of the ellipsoidal magnetoglobules(Qian et al., 2020). An “open space” was also found at one end of the ellipsoidal magnetoglobules. Is there a polarity that governs the division direction of the magnetoglobules? Here we carried out a detailed analysis of ellipsoidal magnetoglobule polarity and assessed the inf luence of light on swimming and division direction within the magnetic f ields. We also discussed the potential ecological signif icance of photosensitive magnetotaxis.
2 MATERIAL AND METHOD
2.1 Sample collection
Sediment samples of eMMPs were collected from the Mediterranean Sea, Southern France, Six-Four les Plages (43°07′N, 5°79′E). Freshly collected sediments were transferred into 1 000-mL plastic bottles, with approximately 500 mL of in-situ seawater. Bottles with sediments were transported to the laboratory and incubated at room temperature. MTBs were further enriched by magnetic collection (Qian et al., 2019,2020).
2.2 Characterization of swimming behavior
Magnetotaxis was analyzed in droplets or microchannels with the Magnetodrome System(MAG Instruments UG, Germany) as previously described (Zhang et al., 2017; Qian et al., 2020). In brief, the Magnetodrome System consists of a Zeiss Axio Observer A1 microscope with a set of four electromagnets generating homogenous magnetic f ields that guide the magnetoglobule swimming direction (Mag-Instruments, Germany). To characterize photosensitive magnetotactic behavior,we modif ied the Zeiss Axio-Observer A1 microscope by replacing the HBO 100 light source with a Zeiss Colibri 7 (Zeiss, SAS, Germany) or CHROLIS-C2 6-Wavelength High-Power LED Source (Thorlabs,SAS, France). There are two light paths. The transmission halogen light was used for imaging and observation, whereas the epi-illumination monochromatic LED light for triggering photophobic response or characterizing photokinesis. The swimming of magnetoglobules was maintained within the UV spot in meddle of the microchannels by application of an alternate uniform magnetic f ield(10 Gs, 0.2-0.4 Hz) in order to periodically reverse their swimming direction before they left the illumination area. To measure the light irradiance, a photodiode power sensor S120VC or S142c (Thorlabs SAS, France) was connected to a Powermeter PM100D (Thorlabs SAS, France). At the slide level with a 10× objective (Zeiss A-Plan 10×/0.25) the irradiances were measured as 110 mW/cm2(for 385-nm UV LED), 100 mW/cm2(for 430-nm violet LED), 80 mW/cm2(for 475-nm blue LED),40 mW/cm2(for 511-nm cyan LED), 20 mW/cm2(for 550-nm green LED), 12 mW/cm2(for 590-nm yellow LED) and 45 mW/cm2(for 630-nm red LED). The motility was recorded with Point_Grey_Grasshopper camera at 30 frames/s and analysis was conducted by MTrackJ Plugins of ImageJ choosing snap feature of bright centroid and snap range of 51×51 pixels(Meijering et al., 2012).
2.3 Microscopy analyses
For laser confocal analysis, MMPs were f irstly f ixed with 1% paraformaldehyde for 1.5 h at room temperature, and then stained membranes with 7.5-μg/mL FM4-64, and stained chromosomal DNA with 2-μg/mL DAPI. The stained cells were observed with the Olympus FV1000 microscope with a laser set at 405-nm excitation and 425-475-nm emission for DAPI and 543-nm excitation and 555-655-nm emission for FM4-64. Images were taken from a series of 0.17-μm intervals focal levels.For scanning electron microscopy (SEM)observation, the MTB cells were f ixed in 2.5%glutaraldehyde for 4 h at 4 °C, ethanol dehydrated,critical point-dried, coated with gold, and then observed using a Zeiss Ultra-55 f ield-emission gun SEM (Carl Zeiss, Germany) operating at 5 kV. SEM morphology analyses of magnetoglobules were carried out using a KVKV-2800B microscope as described by Chen et al. (2015).
3 RESULT
3.1 Basic swimming behavior of ellipsoidal magnetoglobules
The ellipsoidal magnetoglobules isolated from the Mediterranean Sea sediments possess multiples chains of bullet-shaped magnetosomes that organize along the long body axis (Fig.1a). The resulting magnetic dipolar moment confers on the magnetoglobules a north-seeking magnetotaxis with a velocity of 207.3±60.4 μm/s (n=10). While translocating along the long body axis, the magnetoglobules also rotated around this axis with a frequency of 3.6 turn/s (Fig.1b;Supplementary Information Movie S1). Therefore,most f lagellum-propelled bacteria magnetoglobules displayed a helical trajectory of swimming.
3.2 Polarities of ellipsoidal magnetoglobules
3.2.1 Morphology polarity
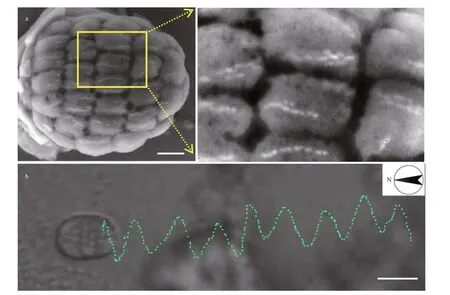
Fig.1 Swimming behavior of ellipsoidal Mediterranean magnetoglobules
Fluorescence confocal microscopy inspection revealed that Mediterranean ellipsoidal magnetoglobules are assembled from 60-80 cells into a one-layer, hollow architecture (Fig.2a; Qian et al.,2020). They had an oval morphology; one end was wider than the other was. Phase contrast images consistently showed the oval morphology (Fig.2b).Due to the relatively big size of magnetoglobules,6-10 μm, the wide end can be distinguished from the narrow end under optical microscope especially when using magnification objectives 40× or higher. A ratio of the wide curvature radius over the narrow curvature radius was about 1.27 for the image in Fig.2b.
3.2.2 Magnetic polarity and swimming orientation
Because of the multiple magnetosome chains magnetoglobules have an intrinsic magnetic polarity,which allows them to align along the magnetic f ield lines. To characterize the photosensitive magnetotaxis of magnetoglobules, two light-sources were used, i.e.,a transmission halogen light for observation and imaging, and an epi-illumination (from bottom to top)with Colibri 7 or CHROLIS-C2 LED sources on a def ined area for analyzing the photo-eff ect on swimming behavior. The swimming of the magnetoglobules was maintained within the UV illumination zone by applying a uniform magnetic f ield (10 Gs) where the direction was alternately switched (0.4 Hz). When distinguishable the narrow end of the oval magnetoglobules often corresponded to the body north pole of the entity and a static magnetoglobule realigned, in response of the reversal of the f ield, within 4-5 frames at 30 frames/s (Fig.2,panel c2-c7; Supplementary Information Movie S2).Under UV illumination, magnetoglobules maintained for a while, the north-seeking motility and could change their swimming direction to south-seeking after a variable time of UV illumination (1 to 13 s).When UV illumination was combined with the reversal of the magnetic f ield direction,magnetoglobules re-aligned their swimming direction to the novel north (Supplementary Information Movie S3). Interestingly, the north-seeking magnetoglobules(Fig.2, panel d1, d11-d12, red spots) could change,within approximate 2 frames, their swimming direction southwards (Fig.2, panel d13-d14, green spots). The time required for the reversal of the swimming direction was less than half of that for magnetic realignment (Fig.2, panel d23-d29, magenta spots; Supplementary Information Movie S4). In addition, the southward swimming displayed an increase of speed and remained south-seeking for quite a long time (Fig.2, panel d16-d46;Supplementary Information Movies S3 and S4). The change from northward to southward swimming might be the result of a reversal in the magnetosome magnetic polarity and subsequent realignment of magnetoglobules, or a change of swimming direction without magnetic realignment. The relatively big size and ellipsoidal morphology of the magnetoglobules enabled us to examine both possibilities. In fact, the magnetoglobules stayed alignment in the f ield but swarm southward (Fig.2, panel d13 to d15;Supplementary Information Movie S4). Therefore,the UV irradiation might trigger the reversal of swimming from north-seeking to south-seeking through coordinated control of f lagellar rotation.
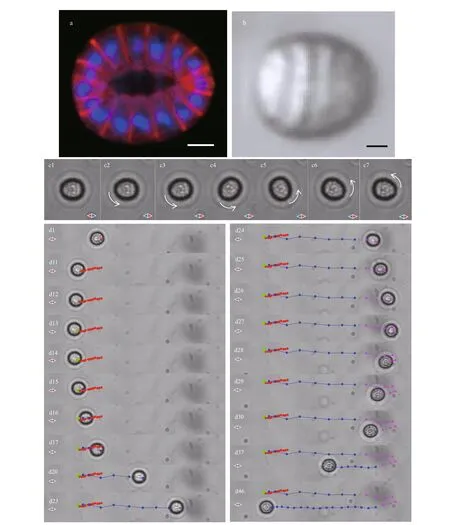
Fig.2 Polarity of ellipsoidal magnetoglobules
We analyzed the wavelength and power required for changing the swimming orientation from northseeking to south-seeking. Minimal 35-mW/cm2UV(385 nm) irradiance was required for triggering the reversion of swimming orientation. From an average of 40 analyses each for more than 50 collected samples the proportion of magnetoglobules reacting to UV illumination ranged 60%-95% depending on sampling seasons and places, storage times of samples in laboratory and observation duration under the microscope. Notably magnetoglobules were microaerobic, sensitive to oxygen and lost progressively the motility during the observation. The violet light(430 nm) at maximal irradiance of 100 mW/cm2could lead to the reversal of less than 5% magnetoglobules whereas illumination by blue light (475 nm,80 mW/cm2), cyan (511 nm, 40 mW/cm2), green(550 nm, 20 mW/cm2), yellow (590 nm, 12-mW/cm2LED), and red (630 nm, 45 mW/cm2) had no eff ect on the magnetotactic swimming of the magnetoglobules,perhaps because of insuffi cient photon energy and light intensity.
3.3 UV light triggered magnetoglobule division
As previously reported the ellipsoidal magnetoglobules divided unidirectionally from one side across the whole body to the other side during reproduction (Qian et al., 2020). Fluorescence confocal microscopy showed the division initiated at a position close to the narrow end of ellipsoidal magnetoglobules (Fig.3a). This position corresponded to an indentation site found at the narrow end on the SEM images of magnetoglobules (Fig.3b), which could suggest that the division may start at the narrow north end of the ellipsoidal magnetoglobules.Unidirectional division led to two off spring with similar ellipsoidal morphology (Fig.3c).Sea sediment samples were collected and stored in plastic sampling bottles, in the laboratory in dim light,at room temperature. During the f irst 3 days, the number of magnetoglobules decreased, and then increased, reaching their maximal in about 7 days.The maximum size and division of magnetoglobules were observed in the 7-14-day samples. Interestingly,when magnetoglobules under this state were observed under the microscope, UV radiation initiated their unidirectional division, which was completed between 100 to 300 s (Fig.3d). Therefore, UV irradiation not only results in the change in magnetotaxis direction but also triggers magnetoglobule division.
4 DISCUSSION
Here we report the UV-triggered reversal of swimming direction in MTB. Not all MTB change their magnetotactic swimming direction in response to light illumination. In addition, the swimming behavior of photo-responsive magnetotaxis shows species specif icity. The marineMagnetococcusmarinusMC-1 strain observed in a capillary tube, swim away from high [O2] toward low [O2] in the same direction as magnetotaxis when illuminated with short-wavelength light (≤500 nm) (Frankel et al., 1997). MarineMagnetospirasp. strain QH-2 (Zhu et al., 2010) and multicellular magnetoglobules (Chen et al., 2015)swim away from the edge of droplets in the opposite direction of magnetotaxis in response to illumination by blue (450-480 nm), violet (400-410 nm), and ultraviolet light (330-385 nm). The freshwaterMagnetospirillummagneticumAMB-1 exhibits positive phototaxis behavior, i.e. towards the light source, that is independent of wavelength and magnetotaxis (Li et al., 2017; Chen et al., 2020a).Photoreceptors have been identif ied only in the group of magnetospirillum among MTB. The extensively studied model strain isM.magneticumAMB-1 that has a cysteine-less light-oxygen-voltage (LOV)photoreceptor and bacteriophytochrome (MmBphPs)(Chen et al., 2020a). Biochemistry and genetic analyses have revealed that red light, but not blue light, upregulated expression of genes involved in magnetosome synthesis and anti-oxidative stress,decreased intracellular reactive oxygen species (ROS)concentration, increased cell growth and phototaxis behavior of AMB-1 (Chen et al., 2020a, b). Ellipsoidal magnetoglobules showed a conspicuous photokinesis behavior, they change the magnetotaxis direction with an increase of swimming speed upon constant illumination by short wavelength lights. Photokinesis behavior has been reported for spherical magnetoglobulesCandidatusMagnetoglobuls multicellularis (de Azevedo et al., 2013; de Azevedo and Acosta-Avalos, 2015). In these studies,monochromatic LED lamp of blue (469 nm), green(517 nm), yellow (596 nm) or red (628 nm) was used for imaging and studying the eff ect of light on swimming speed. The spherical magnetoglobules were analyzed at edge of droplets (de Azevedo et al.,2013; de Azevedo and Acosta-Avalos, 2015). In this area magnetoglobules display ping-pong or bounce motion, which consists of an original magnetotactic swimming with constant velocity to the edge,accumulation at the edge, and then a sudden swimming away from the edge against the original magnetotaxis direction with an increase of speed to variable distance and back again to the edge (De Lins et al., 1990;Rodgers et al., 1990; Lins et al., 1999; Keim et al.,2004a; Greenberg et al., 2005; Zhou et al., 2012; Chen et al., 2015; Qian et al., 2020). In the studies of spherical magnetoglobules photokinesis how the two distinct velocities of the ping-pong motion were considered is unclear. To avoid this edge eff ect, we studied the ellipsoidal magnetoglobules in microchannel and restricted their swimming in an area by application of an alternate uniform magnetic f ield.

Fig.3 UV triggered division of magnetoglobules

Fig.4 Intracellular level of ROS might aff ect the swimmingdirection of magnetoglobules
What could be the mechanism that governs photosensitive magnetotaxis? At present magnetoglobules cannot be cultivated in laboratories.Environmental samples exhibit variable swimming velocities depending on the sample origin, collection season, time of enrichment in the laboratory and the duration of microscopic observation. Experimental data for photosensitive magnetotaxis show f luctuations due to the variation of cellular properties. Thought experiments can be proposed to speculate the mechanism of magnetic photokinesis and help guide future experimental analysis. UV light is harmful for cells because the photons of short wavelength are highly energetic and can break covalent bonds creating deleterious ROS that chemically alter and denature macromolecules. Magnetosomes are involved in the removal of ROS (Chen et al., 2011; Wang and Zhang,2017). Therefore, the intracellular ROS concentration is controlled by the dynamic equilibrium between UV resulted production and magnetosome catalyzed reduction (Fig.4). Once the accumulation reaches a certain threshold, the concentration of ROS would steer the magnetoglobules changing the swimming direction to escape from the deleterious light. The reaction time of 1 to 13 s for magnetoglobules to change their swimming direction from north-seeking to south-seeking might indicate the physiological variation of the magnetoglobules. Application of magnetic f ield may aff ect intracellular ROS level depending on f ield type, intensity and frequency and model organisms analyzed (Wang and Zhang, 2017).Reversal of the magnetic f ield interacts with the magnetosomes and applies a magnetic torque, which could aff ect the velocity of ROS removal by magnetosomes. Therefore, the intracellular ROS concentration could quickly reach the toxic threshold,which is as indicated by the very narrow time delay,around 0.5 s, for the magnetoglobules to change their magnetotaxis direction after the reversal of the direction of the magnetic f ield. An increase of swimming speed might require more energy.Photoreceptor rhodopsin can convert photons into electrochemical potential of H+or Na+that can be used for driving f lagellar motors. Metagenomic analysis can provide answers for this hypothesis. The ultimate solution is success in cultivating magnetoglobules,which would enable us to study the mechanisms for magnetic photokinesis at a molecular, biochemistry and cell biology level. Metagenomic analysis will provide metabolism information that is useful in the cultivation of magnetoglobules.
MTB dwell in chemically stratif ied sediments or water columns. They shuttle within and between layers to obtain appropriate nutrients. The nutrient layers exhibit daily vertical displacement in correlation with changing sunlight, temperature, and tide. Shapiro et al.(2011) have proposed that the behavior of photophobic activate magnetoglobules to optimize its position and to adapt to circadian variation of chemical gradients and light intensity. Genes related to circadian rhythm control were found in spherical (Abreu et al., 2013)and ellipsoidal (Qian et al., 2020) magnetoglobules.Interestingly, Abreu et al. (2013) reported thatCa. M.multicellularis moved downward at night and the number of dividing microorganisms during this period was higher. Here, we found that UV light triggered division of ellipsoidal magnetoglobules. There is no explanation for this diff erence at present. Cultures of magnetoglobules could provide detailed analysis on the eff ect of illumination rhythms on the life cycle and displacement of magnetoglobules.
Photophobic reactions of MTB to UV, violet and blue light is generally studied under f luorescence microscope with a mercury light source that is 10 to 100 times brighter than the monochromatic LED sources. The weaker irradiance provided by the LED source might explain why only UV and violet light in this study could trigger the magnetic photokinesis response. In order to characterize the magnetic photokinesis mechanism concerning the minimal photon energy and numbers of photons required we are designing and constructing a specif ic microscope for future investigations. It is based on an optical tweezer mounted with homogenous magnetic f ields.MTB will be trapped with less harmful infrared light at 976 nm. A second monochromatic beam at 385 nm(photon energy at 3.23 eV), 420 nm (2.96 eV), 475 nm(2.61 eV) or 525 nm (2.36 eV) at diff erent density will be used to illuminate the trapped MTB. The moving direction and force of illuminated MTB can then be characterized. The results obtained should contribute to a comprehensive understanding of the mechanism of the MTB reaction to light.
5 CONCLUSION
Light aff ects magnetotactic behavior and magnetoglobule reproduction. Therefore, the impact of sunlight and circadian rhythm on the contribution of magnetoglobules to the biogeochemical cycles of iron and sulfur should be investigated.
6 DATA AVAILABILITY STATEMENT
The datasets generated during and/or analyzed during the current study are available from the corresponding author on request.
7 ACKNOWLEDGMENT
We are grateful to Mr. H. LE GUENNO for assistance in f luorescence confocal microscopy analysis and Mr. P. KRYCZKA for assistance in Magnetodrome software development.
杂志排行
Journal of Oceanology and Limnology的其它文章
- MamZ protein plays an essential role in magnetosome maturation process of Magnetospirillum gryphiswaldense MSR-1*
- Observations on a magnetotactic bacteria-grazing ciliate in sediment from the intertidal zone of Huiquan Bay, China*
- Biocompatibility of marine magnetotactic ovoid strain MO-1 for in vivo application*
- Determination of the heating effi ciency of magnetotactic bacteria in alternating magnetic f ield*
- An approach to determine coeffi cients of logarithmic velocity vertical prof ile in the bottom boundary layer*
- Three-dimensional mesoscale eddy identif ication and tracking algorithm based on pressure anomalies
