Harmful algal bloom-forming dinof lagellate Prorocentrum donghaiense inhibits the growth and photosynthesis of seaweed Sargassum fusiformis embryos*
2021-12-09CaixiaWANGMinWANGBinbinCHENWenliQINLidongLINChuanjunDAIHengguoYURenhuiLIMinZHAOZenglingMA
Caixia WANG , Min WANG , , Binbin CHEN , Wenli QIN , Lidong LIN ,Chuanjun DAI , Hengguo YU , Renhui LI , Min ZHAO ,**, Zengling MA ,**
1 Zhejiang Provincial Key Laboratory for Subtropical Water Environment and Marine Biological Resources Protection, Wenzhou University, Wenzhou 325035, China
2 National and Local Joint Engineering Research Center of Ecological Treatment Technology for Urban Water Pollution, Wenzhou University, Wenzhou 325035, China
3 Dongtou Fisheries Science and Technology Research Institute, Wenzhou 325700, China
Abstract Harmful algal bloom (HAB) is an ecological disaster to local mariculture. At present, its impact on macrophytes has not been well studied. In this study, we cultivated sexually propagated embryos of S argassum fusiformis— an edible seaweed—in Prorocentrum donghaiense suspensions at diff erent cell densities (0, 0.50×10 5, 0.75×10 5, 1.00×10 5, and 1.50×10 5 cells/mL) for 10 days, during which growth and photosynthetic activities of the embryos were determined, and a monocultivation was set up for comparison.Results show that the relative growth rate and photosynthetic activities of the embryos co-cultivated with P. donghaiense were inhibited mostly and signif icantly in the cell densities of 0.75× 10 5, 1.00×10 5, and 1.50×10 5 cells/mL, and the inhibitory eff ects increased in overall with increased cell densities. The maximum relative electron transport rates (rETR max) and apparent photosynthetic effi ciency ( α) of co-cultivated embryos were all signif icantly lower than monocultivation ones on the 10 th day. Furthermore, the photosynthetic activity detected by chlorophyll- a f luorescence transient (i.e., OJIP), the electron transport among electron transfer accepters of PSII (photosystem II) and that from PSII to PSI (photosystem I) was restricted, which is probably responsible for the decreases of rETR max and α in the co-cultivated embryos. In addition, parts of the photosynthetic reaction centers of PSII in the co-cultivated embryos were inactivated. Therefore, P.donghaiense bloom could restrain the development and photosynthetic activities of S. fusiformis embryos,reduce the seedlings stock, and eventually hinder the development of S. fusiformis production industry.
Keyword: embryo; JIP-test; photosynthesis; Prorocentrum donghaiense; Sargassum fusiformis; harmful algal bloom
1 INTRODUCTION
Harmful algal bloom (HAB) is a result of eutrophication in water, which is considered one of the major marine environmental threats in the world(Anderson et al., 2012). In recent years, eutrophication in the estuary of Changjiang (Yangtze) River and coastal of the East China Sea (ECS) has caused a huge damage to local aquatic systems and other related sectors (He et al., 2013; Shen et al., 2019). Among HAB-forming species, dinof lagellates are accountable for more than 70% of HAB events that have been reported (Smayda, 1997). In addition, dinof lagellateProrocentrumdonghaienseis one of the most common HAB-forming species in the ECS due to its high biomass accumulation, being responsible for the large-scale (ca. 10 000 km2) bloom events and for marine ecosystem damage (Lu et al., 2005; Tang et al., 2006; Zhao et al., 2009). It frequently co-blooms with other toxic algae such asAlexandriumcatenellaandKareniamikimotoi, resulting in mass mortalities of farmed f ish and other marine fauna (Wang and Wu,2009; Shen et al., 2019).
On the other hand, global climate changes aggravated HABs in frequency, scale, and severity(Moore et al., 2015; Wells et al., 2015). Recent works reported that phytoplankton, including HAB-forming species, revealed a large latitudinal range shifts on average of over 400 km per decade (Glibert et al.,2014). Ocean acidif ication is another factor for altering the community structure of phytoplankton and for increased occurrence of dinof lagellateinduced HAB (Howes et al., 2015). Some dinof lagellate species are able to release toxic metabolites lethal to marine organisms and hazardous to public health (Van Dolah, 2000; Zingone and Enevoldsen, 2000; Shang et al., 2020). As a nontoxic species,P.donghaienseoften cause a series of HAB over large areas ( 1 000-10 000 km2) in a long time( > 30 d) (Lu et al., 2005; Glibert et al., 2012). During a HAB occurrence period,P.donghaiensecompetes with other marine organisms for oxygen and nutrient,risking to those co-existent creatures (Anderson et al.,2002; Lin et al., 2015).
Seaweed aquaculture provides important services for marine ecosystems, e.g., increasing overall biodiversity, sequestrating CO2, mitigating eutrophication, and thus suppressing HABs (Tang and Gobler, 2011; Yang et al., 2015). Mariculture of seaweeds has been developing rapidly in East Asian countries since the early 20thcentury by continuously rising demands for dietary supplements and healthy food (Tseng, 2001; Krumhansl and Scheibling, 2012;Ma et al., 2018). Natural macroalgae populations are vital components of coastal and estuarine ecosystems,have signif icantly declined in many parts of the world because of the increasing anthropogenic disturbances(Lotze et al., 2006; Sala and Knowlton, 2006; Branch et al., 2013). It have been found that HABs could cause population loss, seedling development delay,thalli bleaching, and photosynthetic rate decrease of natural and maricultured macroalgae (Kim et al.,2015; Ma et al., 2017, 2020). However, the physiological and developmental responses of seaweed to HAB have rarely been documented due to the “unpredictable” nature of algal blooms (Ma et al.,2017; Shang et al., 2020).

Fig.1 An aerial view of S. fusiformis farming in coastal areas of Dongtou District, Wenzhou City, Zhejiang Province (a) and the occurrence of P. donghaiense blooms in the aquaculture area (b)
Brown seaweedSargassumfusiformisis an edible marine vegetable consumed widely in the East Asian countries (Ma et al., 2018). In China, coastal waters off Dongtou District, Wenzhou City, Zhejiang Province are the major production region (Fig.1a). In recent years, farmers began to use zygote-derived seedlings to satisfy the market demands and to promote the commerce (Pang et al., 2006; Lin et al.,2020). To obtain the sexually propagated seedlings,some healthy and strong thalli were left at the aquaculture sites as parental strains during harvest waiting for the thalli to discharge germ cells (Pang et al., 2006; Lin et al., 2020). After fertilization, the eggs developed into oval zygotes within 18-h postfertilization and into embryos in 24-h post-fertilization(Pang et al., 2006; Lin et al., 2020). A recent study by Lin et al. (2020) found that some juvenile sporophytes of mariculturedS.fusiformiscould produce matured receptacles (from August to November) that could also discharge germ cells for producing sexually propagated embryos. On the other hand, from May to September, HABs formed by dinof lagellateP.donghaienseoccur frequently in the coastal waters of the Dongtou District (Shen et al., 2019). Small- to medium-sized HABs often break out in the aquaculture regions ofS.fusiformis(Fig.1b). In practice, farmers would use cotton strips with whichS.fusiformisembryos or seedlings could be attached and collected,and then transferred into a non-HAB region to avoid further damage.
Although HAB inhibits the seaweed farming,which has received much scientif ic attention (Yang et al., 2015; Inaba et al., 2017), the adverse impact of HAB-forming species on macroalgae growth remain poorly studied (Ma et al., 2017, 2020; Shang et al.,2020). A recent study shows that co-cultivation with toxin-producing dinof lagellateK.mikimotoiinhibited the development, pigment content, and photosynthetic activities ofS.fusiformisembryos but not the egg fertilization (Ma et al., 2020). Furthermore, the inhibitory eff ects are mainly caused by allelochemicals released fromK.mikimotoiin a cell density-dependent manner (Shang et al., 2020). As the most important bloom-forming contributor,P.donghaiensecould form dense blooms, although it is non-toxin species, it is regarded as a harmful species to mariculturedS.fusiformisbecause the blooms formed by this species that has been found able to aff ect the plankton community structure (Lin et al., 2014; Chai et al.,2020) and to inhibit the growth of co-cultured microalgae (Shen et al., 2015). Unfortunately, at present, our knowledge of the impact ofP.donghaienseblooms onS.fusiformisremains blank.
The sexually propagated embryos ofS.fusiformisare more vulnerable than its mature sporophytes (Ma et al., 2020; Shang et al., 2020), and serious consequences such as a def iciency of sexually propagated seedlings and a subsequent reduction of commercial production may happen when the embryos suff ered fromP.donghaiense-induced HAB.Therefore, we performed a series of laboratory experiments to investigate the eff ects ofP.donghaiensecell density gradients on the development and the photosynthetic ability ofS.fusiformisembryos. In addition, the analysis of the O-J-I-P polyphasic rising transient (i.e., JIP-test) was used to quantify the photosystems (PSII and PSI) during the co-cultivation with the HAB-forming species.
2 MATERIAL AND METHOD
2.1 Cultivation condition
The dinof lagellateP.donghaiensewas provided by Prof. Douding LU from the Second Institute of Oceanography, Ministry of Natural Resources of the People’s Republic of China (MNR) based in Hangzhou, China. The dinof lagellate was grown in sterile f/2 medium (Guillard, 1975) that prepared with seawater (salinity ca. 29) from mariculture region ofS.fusiformis. The cultivation was maintained at 22± 1 °C under cold white light of 100 μ mol photons/(m2·s) in 12-h꞉12-h light-dark photoperiod cycle. All cultivations were manually shaken f ive times per day until the cells reached the exponential growth phase during which cells were harvested and used for follow-up experiments.
MatureS.fusiformissporophytes were collected from mariculture f ield of the Dongtou Fisheries Science and Technology Research Institute in May 2019. Thalli were transported to laboratory in a cooler box within 2 h to avoid thermal and dehydration damage. Epiphytes were removed and the thalli were washed three times to clean other impurities.To get the fertilized eggs, female and male mature thalli at germ cell release were cultivated together in a weight ratio of 5 to 1. The thalli were incubated in a plastic basin containing f iltered natural seawater(salinity ca. 29) and kept in a illumination incubators(GXZ-300D, Ningbo, China) at 22± 1 °C under cold
white light of 100 μ mol photons/(m2·s) in 12-h꞉12-h light-dark regime. Seawater was aerated with f iltered ambient air (0.22 μm) at 0.5 L/min and the cultures were run for 24 h to ensure complete release and fertilization of eggs. Fertilized eggs were then harvested with a 270-mesh nylon f ilter (pore diameter=53 μm) and were re-suspended in seawater in beaker.
2.2 Co-cultivation of fertilized eggs (zygotes) of S. fusiformis and P. donghaiense
To investigate the eff ects ofP.donghaiensesuspensions on development and photosynthetic activities of the fertilized eggs, re-suspended fertilized eggs were sprinkled evenly on 6-well plates in density of ~30 eggs/cm2. After 24-h post-fertilization, zygotes developed into embryos and attached to the bottom of 6-well plates with their f ilamentous pseudo-roots. To create the diff erent treatments, sterile fresh f/2 medium andP.donghaiensesuspensions that concentrated by centrifugation were added to the wells with 6-mL suspension in each well. The f inal cell densities ofP.donghaiensein the wells were 0(the control), 0.50×1.05, 0.75×105, 1.00×105, and 1.50×105cells/mL in triplicate on 6-well plates. In addition, to eliminate the eff ects of nutrition limitation,a semi-continuous cultivation were conducted during the experiment, i.e., the cultivations were renewed to the initial cell density ofP.donghaienseevery other day by adding fresh f/2 medium to dilute the suspensions according to the actual cell densities.These mono- and co-cultivated embryos were grown at 22 °C under cold white light at 100 μ mol photons/(m2·s) in 12-h꞉12-h light-dark scheme for 10 days.The morphological changes of the embryos cultivated under each treatment were examined every other day and their photosynthetic activities detected by rapid light curves, and chlorophyll-a(Chl-a) f luorescence transient analysis (i.e., OJIP) were determined on the 10thday post-inoculation.
2.3 Observation of morphology and determination of growth of the embryos
The morphological changes of the embryos were examined with an ECLIPSE Ts2 inverted routine microscope (Nikon, Japan) and the digital images were captured with a digital microscope camera(Nikon DS-Ri2). To collect continuous information on the development of the embryos, the same locations of the wells were examined every other day. The lengths and widths of 150 embryos under each treatment, not including the rhizoid lengths, were measured with the Nikon software NIS Elements Ar 3.0 (Nikon, Japan). The embryo volumes were calculated as cylinders because of their cylindrical shape (Shang et al., 2020), and the relative growth rate (RGR, /d) was determined according to the equation of Glenn and Doty (1992): RGR=(lnV2-lnV1)/(t2-t1), whereV1andV2are the volumes at timet1andt2, respectively.
2.4 Determination of photosynthetic activity of S. fusiformis embryos
To obtain photosynthetic capacity information of the embryos cultivated in the suspensions at diff erent cell densities, the photosynthetic f luorescence parameters of the embryos were detected using a Multi-color-PAM f luorometer (Heinz Walz GmbH,Eff eltrich, Germany). Before measurements, embryos were washed more than three times with f iltered seawater (0.45 μm) to completely removeP.donghaiensecells attached. Embryos were then scraped from the bottom of the wells and were resuspended in f iltered seawater, of which 3-mL sample was used to determine the f luorescence parameters.For measurements of all the photosynthetic parameters, each sample was acclimated to darkness for 15 min to ensure all reaction centers were in open state, and non-photochemical dissipation of excitation energy was in minimum. The minimum f luorescence(Fo) was excited by a very low intensity of measuring light; the maximum f luorescence (Fm) was excited by a saturating f lash in a fixed emission wavelength at 480 nm. The variable f luorescence (Fv) was def ined asFv=Fm-Foand theFv/Fmwas calculated as per Kitajima and Butler (1975):Fv/Fm=(Fm-Fo)/Fm.
Rapid light curves, i.e., the relative electron transport rate (rETR) versus irradiance (I) curves were built under gradient photosynthetically active radiation (PAR) levels (each measurement lasted for 20 s). The parameters of the rETR vs.Icurves were analyzed as per Eilers and Peeters (1988): rETR=I/(aI2+bI+c), in whicha,b, andcare adjustment parameters. The effi ciency of electron transport (α),the saturating light intensity for the photosynthesis(Ik), and the maximum relative electron transport rate(rETRmax), are expressed as functions of the parametersa,b, andc, i.e.,α=1/c,Ik=(c/a)1/2, and rETRmax=1/[b+2(ac)1/2].
2.5 Chlorophyll- a fluorescence transient analysis of S. fusiformis embryos
The chlorophyll-af luorescence (i.e., OJIP)transient, known commonly as the Kautsky curve and shown by photosynthetic organisms under diff erent treatments, can provide detailed information of the structure, conformation, and function of the photosynthetic apparatus, especially the PSII (Strasser et al., 2004). The transients (10 μ s) were detected using Multi-color-PAM f luorometer (Heinz Walz GmbH, Eff eltrich, Germany) after 15-min darkness acclimation, and analyzed in the JIP-test theory and application to reveal information about the f luxes of photons, excitons, electrons, and further metabolic events (Appenroth et al., 2001; Strasser et al., 2004).The Chl-af luorescence curves includes the following points: (1) the f luorescence in 50 μ s, calledFo,represents the O step of the f luorescence curve, during which all the reaction centers (RCs) were open; (2)afterFo, the photons were transported to primary quinone electron acceptor (QA) in 2 ms, when the f luorescence was called J step in f luorescence curve;(3) in the process of photons transported from QAto QB(the secondary quinone electron acceptor) in 30 ms, when the f luorescence was called I step; (4)lastly, a maximum transient in the curve was obtained,it was called P step and at this time the QAwas fully reduced.
VJ, the relative variable f luorescence intensity at the J step, andMo, the initial slope of the OJIP curve,were calculated asVJ=(F2ms-Fo)/(Fm-Fo) andMo=4×(F300ms-Fo)/(Fm-Fo).F2msandF300msrepresented the f luorescence values at 2 and 300 ms, respectively.The quantum effi ciencies of electron transport chain(ETC) included parametersφPo,ψo, andφEo:φPorepresent the trapped electrons per absorption, i.e.maximum quantum yield for photochemistry (Fv/Fm);ψomeans the effi ciency that a trapped excition could move an electron into the ETC beyond the primary acceptor plastoquinones (QA), i.e. the yield of electron transport of trapped excition; andφEomeans the quantum yield of electron transport. These parameters were calculated by the following equations:φPo=(1-Fo)/Fm,ψo=1-VJ, andφEo=(1-Fo/Fm)×ψo.
The specif ic energy f luxes ratios in electron transport chain included ABS/RC, TRo/RC, ETo/RC,and DIo/RC, representing the energy f luxes ratio, the trapped energy f lux ratio, the electron transport f lux,and the dissipated energy f lux ratio per reaction center, respectively. These ratios were calculated by the following equations: ABS/RC=Mo×(1/VJ)×(1/φPo),TRo/RC=Mo×(1/VJ), ETo/RC=Mo×(1-VJ)×φPo, and DIo/RC=(ABS/RC)-(TRo/RC).
Furthermore, the energy f lux ratios were represented in terms of “per absorption (/ABS)”,including RC/ABS, TRo/ABS, ETo/ABS, and DIo/ABS, representing the reaction center of PSII, the trapped energy f lux ratio, the electron transport f lux,and the dissipated energy f lux ratio per absorption,respectively. They were derived from the energy f luxes ratio representing in terms of “per active reaction center of PSII (/RC)”.
The performance index (PI) is an indicator to vitality, and associated with three independent parts contributing to the photosynthesis: the amount of photosynthetic reaction centers of PSII (RC/ABS);the contribution of the light reactions to primary photochemistry, i.e., the performance due to the trapping probability (PTR); and the contribution of dark reactions, i.e., the performance due to the conversion of excitation energy to electron transport(PET) (Thach et al., 2007). PI can be calculated as per Strasser et al. (2000): PI=(1-Fo/Fm)/(Mo/VJ)×(Fm-Fo)/Fo×(1-VJ)/VJ.
2.6 Determination of the cell density, nutrient, and pH of the P. donghaiense suspensions during the mono- and co-cultivations
The cell number ofP.donghaiensesuspensions was counted under optical microscope (BX43,Olympus, Japan), and the cell density was determined using the cell numbers in 0.1-mL suspension. In addition, to address the concentration change of nutrients among suspensions during the co-cultivation,the total dissolved nitrogen (TN) and total dissolved phosphorus (TP) were determined every other day.The suspensions were filtered with 0.45-μm cellulose acetate membranes immediately after sampling and the filtrates were used for TN and TP determination.The TN concentration was measured in the hightemperature catalytic oxidation (HTCO) method using automatic TOC-VCPH analyzer (Shimadzu,Japan). The TP concentration was analyzed in phosphomolybdate-blue spectrophotometry. All the TN and TP concentration were measured in triplicate for each treatment. The pH values of the suspensions during the co-cultivation period were measured using a HACH Hq40d multi pH meter.
2.7 Statistical analysis
All data are expressed as the mean and standard deviation. After the testing for normal distribution and homogeneity of variance, one-way analysis of variance (ANOVA) with post-hoc tests (Tukey) were conducted to reveal diff erences among treatments,and the signif icance level was set atP=0.05. Statistical analysis was performed using SPSS V.16.0 for Windows (SPSS Inc., Chicago, IL, USA), and all charts were generated using Origin 9.0 (OriginLab,USA).
3 RESULT
3.1 Eff ect of P. donghaiense cell density on growth of S. fusiformis embryos
The zygotes (fertilized cells) developed into embryos in 24-h post-inoculation by continuous cell division, during which the embryos attached to the well bottom with their f ilamentous pseudo-roots. In addition, the morphology of the embryos changed from being elliptical to cylindrical in shape by gradually elongating along the long axis, and the broader axis was widened to form a thallus at last(Fig.2). No signif icant morphological changes of the embryos could be observed between the mono- and co-cultivations except for the sizes (Fig.2). When volumes of the embryos were determined as cylinders,the sizes of the embryos co-cultivated withP.donghaiensein the suspensions at 1.00× 105and 1.50× 105cells/mL began to decrease signif icantly(P<0.05) below the monocultivated ones on the 10thday post-inoculation.
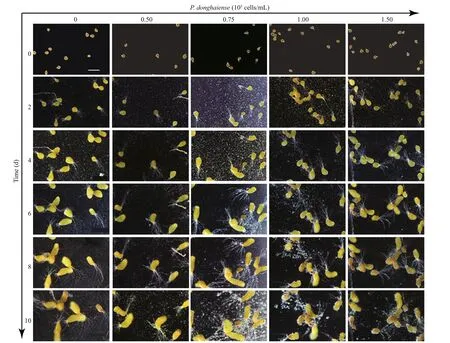
Fig.2 Morphological changes of S. fusiformis embryos cultivated in P. donghaiense suspensions at diff erent cell densities with inoculation time lasting from Day 0 to Day 10
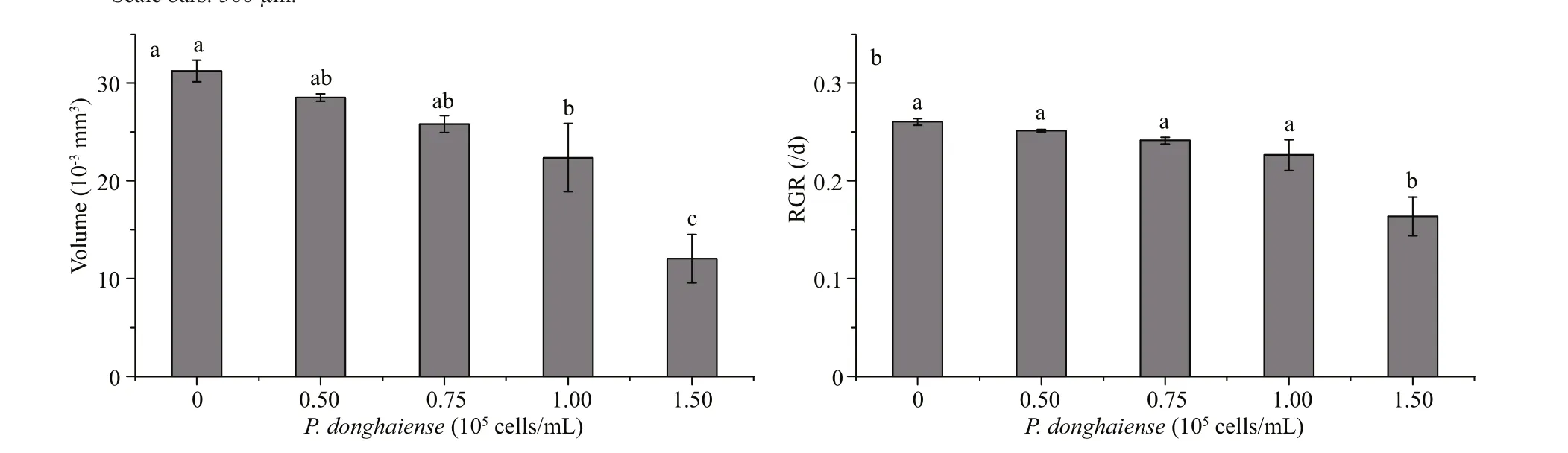
Fig.3 Changes in volumes (a) and relative growth rate (b) of S. fusiformis embryos cultivated in P. donghaiense suspensions in diff erent cell densities
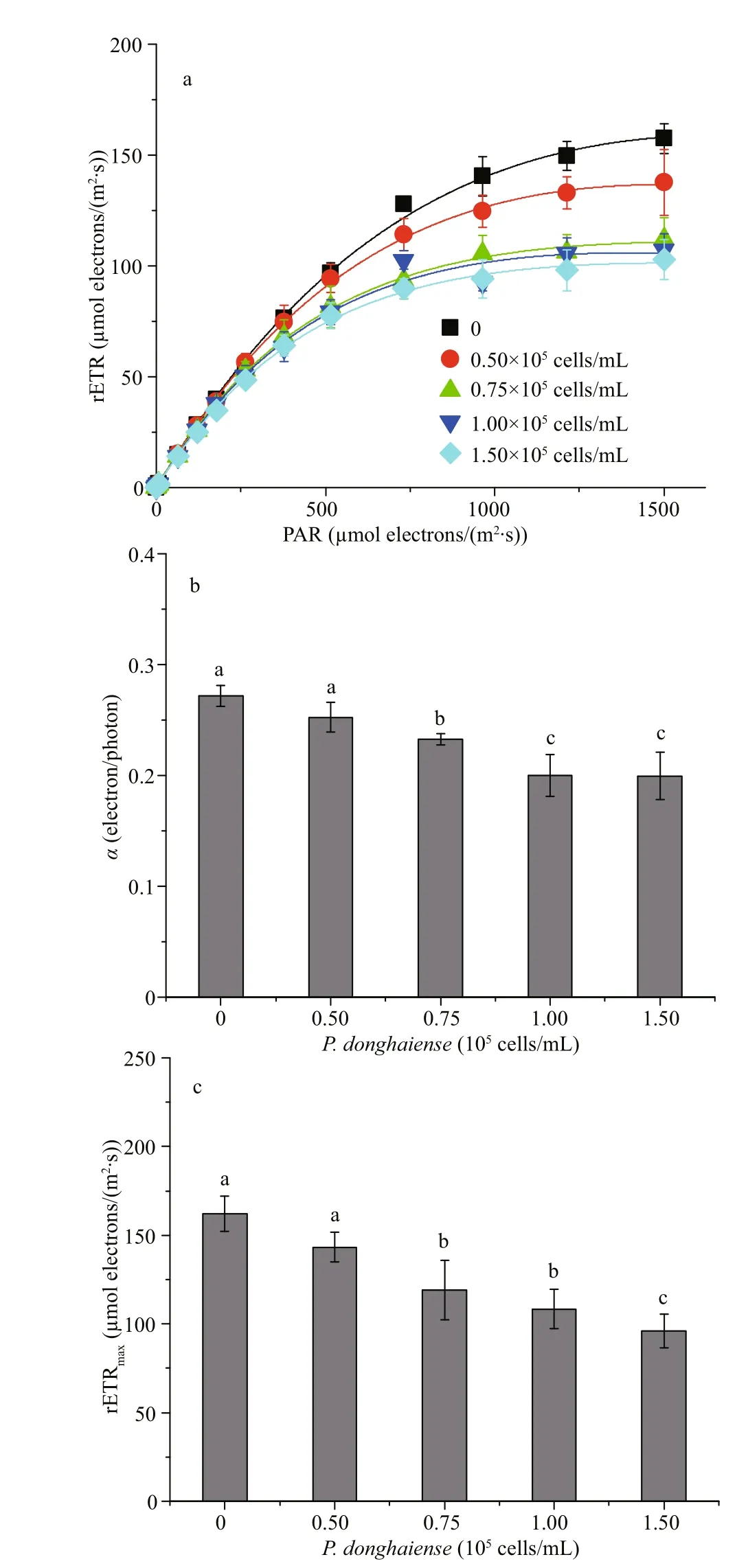
Fig.4 Variation in relative electron transport rate (rETR;a), apparent photosynthetic effi ciency ( α; b), and the maximal electron transport rate (rETR max; c) of cocultivated S. fuisiforms embryos with P. donghaiense
On the 10thday post-inoculation, the volume of the monocultivated embryos was 31.23× 10-3mm3while those cultivated inP.donghaiensesuspensions at 0.50× 105, 0.75× 105, 1.00× 105, and 1.50× 105cells/mL were 28.54× 10-3, 25.80× 10-3, 22.36× 10-3, and 12.05× 10-3mm3, respectively (Fig.3a). In addition,the embryo size decreased with increased cell density ofP.donghaiense. The corresponding average RGR of the embryos during the cultivation period was 0.26/d for the mono-cultivation, and 0.25, 0.24, 0.23,and 0.16/d for the co-cultivation, respectively(Fig.3b). Meanwhile, after 10-day co-cultivation at diff erent cell densities mentioned above, the embryo volume was inhibited from that of the control by 8.61%, 17.39%, 28.40%, and 61.42%, and the RGR by 3.45%, 7.35%, 13.06%, and 37.08%, respectively.
3.2 Eff ect of P. donghaiense cell density on photosynthetic activity of S. fusiformis embryos
The rapid light curves (RLCs) of theS.fusiformisembryos co-cultivated withP.donghaienseare shown in Fig.4a, and the critical photosynthetic parametersαand rETRmaxderived from the RLCs are shown in Fig.4b-c. Compared with the control, the cocultivation resulted in signif icant (P<0.05) inhibition onαand rETRmaxof the embryos. To be compared,αof the monocultivated embryos was 0.27 electron/photon, which was greater than those of the cocultivated in cell densities of 0.50× 105, 0.75× 105,1.00× 105, and 1.50× 105cells/mL at 0.25, 0.23, 0.20,and 0.20 electron/photon (Fig.4b), or in percentage of decrease by 7.21%, 14.52%, 26.47%, and 26.65%,respectively. In addition, the rETRmaxvalue of embryos was 162.24 μmol electrons/(m2·s) in the mono-cultivation, and 143.33, 119.08, 108.41, and 95.84 μmol electrons/(m2·s) in the co-cultivation(Fig.4c), respectively, or in percentage of decrease by 11.66%, 26.60%, 33.18%, and 40.87%, respectively.
3.3 Eff ect of P. donghaiense cell density on fast chlorophyll- a f luorescence of S. fusiforms embryos
The fast chlorophyll-af luorescence induction kinetics of the mono- and co-cultivatedS.fusiformisembryos are shown in Fig.5a. To visualize the changes of Chl-af luorescence, the averages of the raw f luorescence kinetics plotted on a logarithmic time scale from 50 μ s to 1 s are shown in Fig.5b. The Chlaf luorescence curves started from the initial f luorescenceFo(50 μ s) in intensity and f inally increased to the peakFm, and between them, two intermediate stepsFJ(about 2 ms) andFI(about 30 ms) could be observed (Fig.5a). In the raw f luorescence kinetics, the averageFoandFmintensities of the co-cultivated embryos decreased with increased cell densities ofP.donghaiense(Fig.5a). However,when the OJIP-curves were plotted with variable f luorescence (Vt), theVJandVIintensities of the cocultivated embryos increased with the increase in cell density ofP.donghaienseand were signif icantlygreater than that of the monocultivation (Fig.5b),although the value ofFmintensity in the raw f luorescence kinetics was lower than that of the monocultivation (Fig.5a).

Table 1 Comparison in some chlorophyll- a f luorescence parameters determined from S. fusiformis embryos cultivated in P. donghaiense suspension at diff erent cell densities
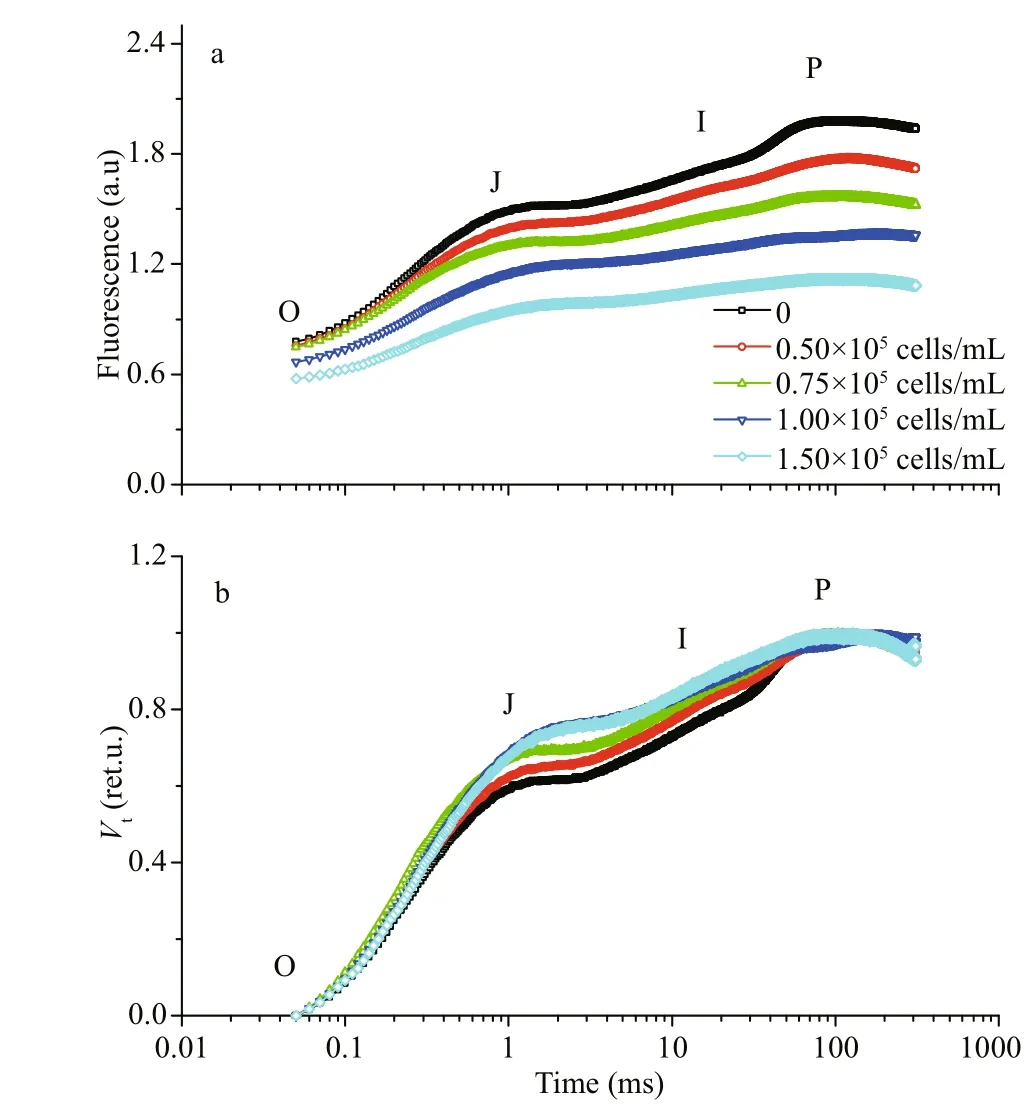
Fig.5 The original OJIP-curves of S. fusiformis embryos cultivated in P. donghaiense suspensions at cell density gradients for 10 days (a) and the normalized OJIP-curves based on the variable f luorescence curves (b)
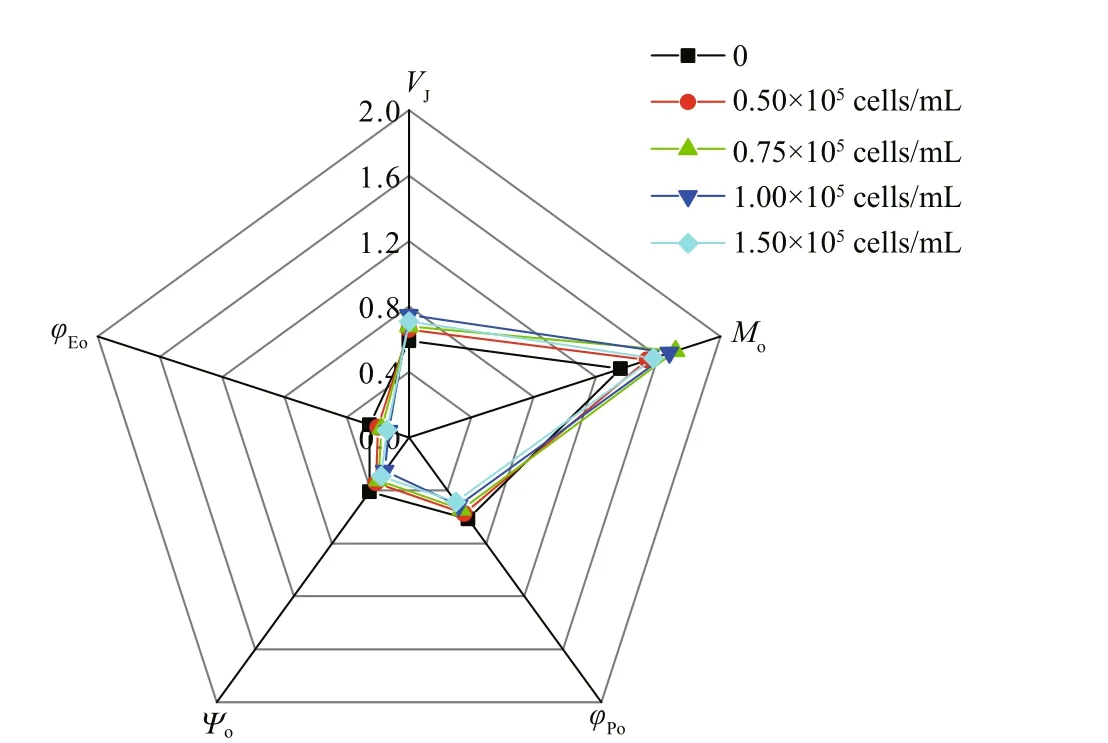
Fig.6 The radar plot of selected JIP-test parameters derived from the chlorophyll- a f luorescence transients ( V J,M o, φ po, ψ o, φ Eo)
3.4 Eff ect of P. donghaiense cell density on the behavior of PSII in S. fusiforms embryos
The behavior of the photosynthetic parameters that characterize the PSII functioning varied with theP.donghaiensecell densities in the co-cultivation(Fig.6). All the parameters that derived from the OJIP-curves of the co-cultivated embryos diff ered signif icantly (P<0.05) from those of the monocultivation (Fig.6; Table 1). For instance, the value ofMothat ref lects the accumulation rates of inactive RCs in embryos, increased with the increase in the cell density. In addition, all the values ofMoin co-cultivated embryos were signif icantly (P<0.05)higher than those of the monocultivation (Fig.6; Table 1). Meanwhile, the values ofVJin the co-cultivated embryos also increased with increased cell density ofP.donghaiense, which indicated an increase in the proportions of closed RCs at J step during the cocultivation (Fig.6; Table 1). In addition, compared with monocultivated ones, all the photosynthetic parameters (φPo,φo, andφEo) of co-cultivated embryos signif icantly (P<0.05) decreased (Fig.6; Table 1).

Fig.7 Changes in PI and its components of S. fusiformis embryos cultivated in P. donghaiense suspensions at diff erent cell densities (a); the ratios relative to the monocultivated embryos (b)
Performance index (PI) was decreased in all cocultivated embryos and the inhibitory eff ects increased with increased cell density ofP.donghaiense.Compared with the control, co-cultivation withP.donghaiensein cell densities of 0.50× 105, 0.75× 105,1.00× 105, and 1.50× 105cells/mL inhibited the PI by 33.29%, 60.83%, 72.97%, and 70.77% (Fig.7a & b),respectively. Furthermore, among the parameters,PTR, RC/ABS, andPETcontributed to the reduction of PI by 14.26%-40.92%, 7.56%-22.53%, and 21.79%-40.05% (Fig.7b), respectively. In addition, the density of PSII reaction center (RC/ABS), maximum quantum yield (TRo/ABS), and the yield of electron transport(ETo/ABS) of the embryos cultivated inP.donghaiensesuspensions at 0.75× 105, 1.00× 105, and 1.50× 105cells/mL decreased signif icantly (P<0.05) compared with those of the monocultivated embryos. It indicated that co-cultivation withP.donghaiensehad a variety of inhibitory eff ects on PSII of the embryos, including inhibition on the average absorption per reaction centers, the dark reactions in electron transport (PET),and the light-dependent reactions (PTR) (Fig.8a). The ETo/RC showed a similar trend to that of the f luorescent transient per absorption (/ABS), whereas the TRo/RC have no signif icant diff erence between control and treated embryos, in general. Meanwhile,the ABS/RC of the co-cultivated embryos, which characterizing the average antenna size, increased signif icantly (P<0.05) compared with those the monocultivated (Fig.8b).
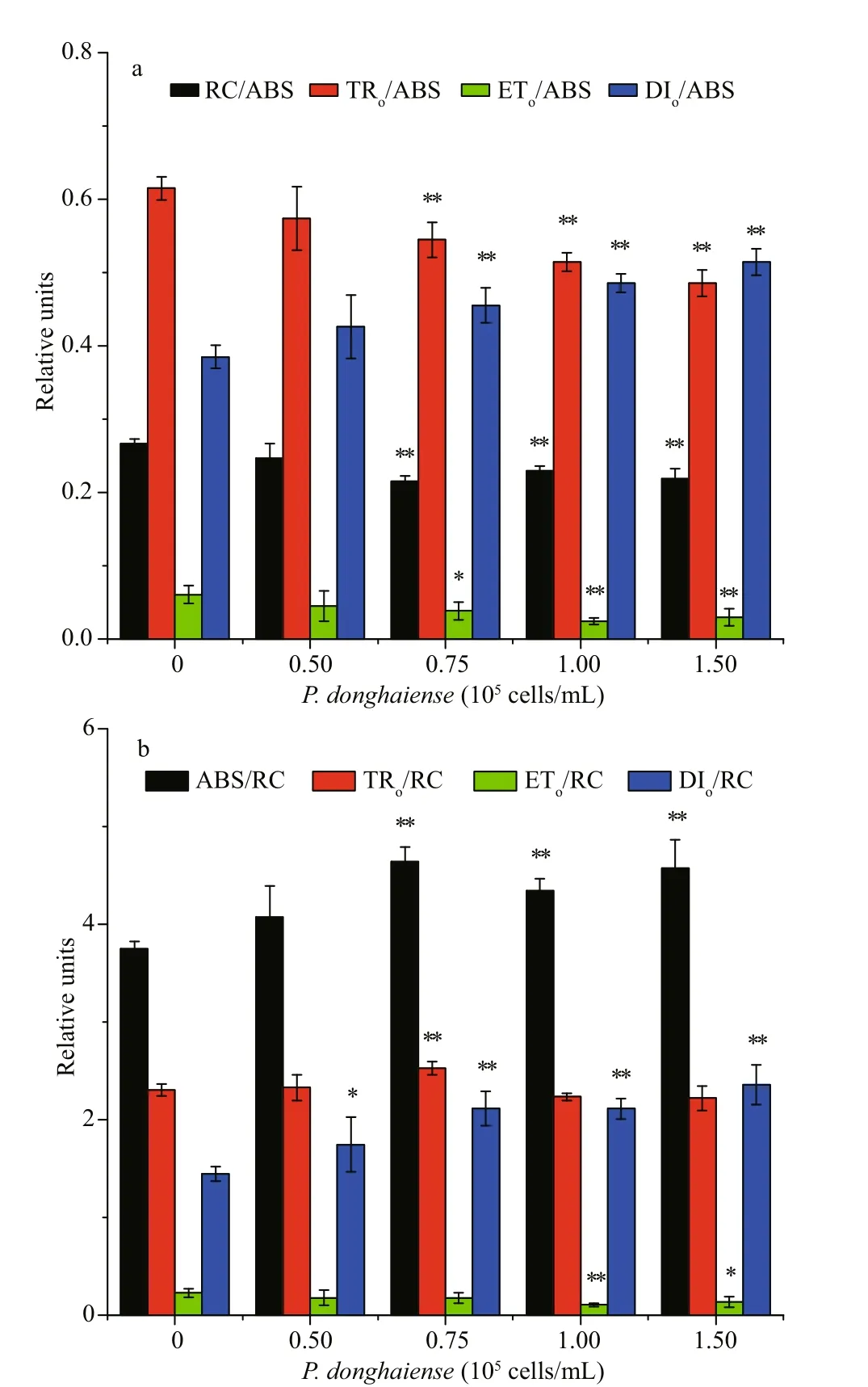
Fig.8 The fast chlorophyll transients energy f lux ratios per RC (a) and per ABS (b) of S. fusiformis embryos cultivated in P. donghaiense suspensions at diff erent cell densities
3.5 Changes in nutrients and pH of P. donghaiense suspensions during the mono- and co-cultivations
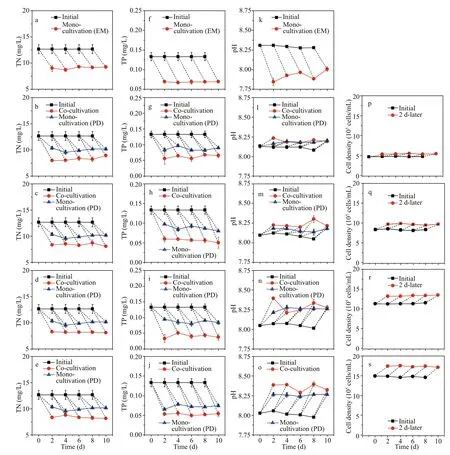
Fig.9 Variations of diff erent parameters in the mono- and the co-cultivations
During the co-cultivation, the TN and TP concentrations of all cultivations are shown in Fig.9a-e & f-j. At the inoculation time, the TN and TP concentrations of all cultivations were 12.67 and 0.13 mg/L, respectively. Two days after inoculation,TN concentrations in the monocultivation decreased by 28.38%, and so did in the co-cultivation atP.donghaiensecell densities of 0.50× 105, 0.75× 105,1.00× 105, and 1.50× 105cells/mL by 34.48%, 33.91%,35.25%, and 33.95%, respectively (Fig.9a-e).Correspondingly, the TP concentration decreased by 48.90% in the monocultivation, and 54.00%, 58.10%,69.30%, and 60.70%, respectively in the cocultivation (Fig.9f-j). In addition, both TN and TP concentrations in mono- and co-cultivations changed periodically in nearly the same trends during the whole period.
During the inoculation, the pH value of the f/2 medium in mono-cultivation was 8.29, while those of the co-cultivation media, i.e., theP.donghaiensesuspensions in cell density of 0.50× 105, 0.75× 105,1.00× 105, and 1.50× 105cells/mL were 8.12, 8.09,8.05, and 8.02 (Fig.9k-o), respectively. Two days after inoculation, the pH value of the f/2 medium decreased to 7.92, and those of the co-cultivation at the cell density gradient increased to 8.17, 8.23, 8.29,and 8.36, respectively (Fig.9k-o). Furthermore, pH values of all the media varied periodically in nearly the same trends throughout the whole experimental time. ForP.donghaiensesuspensions in cocultivation, the cell densities in all set gradients increased about 20% within every 2 days in the semicontinuous culture mode (Fig.9p-s).
4 DISCUSSION
During a bloom, the recorded cell densities ofProrocentrumspecies usually varied in a range of 104-106cells/mL (Lu et al., 2005). In this study, the cell density ofP.donghaioenseranged 0.50× 105-1.50× 105cells/mL, close to the normal cell densities during blooms but lower than the highest cell density(3.6× 108cells/mL) reported by Lu et al. (2005),implying that embryos in mariculture sites might have experienced the HABs caused byP.donghaioenseat similar cell densities, because they have occurred frequently in the coastal waters of Dongtou District(Shen et al., 2019). The exuded compounds ofP.donghaioensecould alter the physiology of its competitors by changing their lipid synthesis, cell membrane integrity, and photosynthesis (Poulin et al.,2018). In addition, in a recent research, we found that the inhibitory eff ects ofK.mikimotoion theS.fusiformisembryos followed a cell densitydependent manner, and they were mainly caused by allelochemicals (Shang et al., 2020). In this study, the inhibitory eff ects ofP.donghaienseon the development (Fig.2) and photosynthesis (Fig.4b-c)ofS.fusiformisembryos were observed when the cell densities were at 1.00× 105and 1.50× 105cells/mL,and the inhibitory eff ect increase was observed at higher cell densities, suggesting the inhibitory eff ects also followed a cell density-dependent manner.
Performance index (PI) was considered a multiparametric expression of the functional and structural criteria of PSII (Strasser et al., 2000, 2004), which sharply decreased when the cell density ofP.donghaiensearrived at 0.50× 105cells/mL(Fig.7a-b). Moreover, it has been known to be more sensitive to environmental change and correlated well with plant vitality (Hermans et al., 2003). The inhibition on PI parameters indicated that RCs of the co-cultivated embryos were inactivated and the photosynthetic capacities were inhibited. In chlorophyll f luorescence curves, the f luorescence amplitude in O-J phase represents the energy used to reduce QA(quinone acceptor) to QA- . The increases in the f luorescence amplitude inVJstep and O-J phase were observed in the co-cultivated embryos (Fig.5b),which ref lected that the electron transport was stocked in QA- because of the photosynthetic elements damage(Kalaji et al., 2012; Zivcak et al., 2014). The following J-I phase (thermal phase) ref lected the reduction of the plastoquinone (PQ) pool by reduced QA(Schansker et al., 2011; Vredenberg, 2015). Thus the amplitudes in J-I phase ref lected the electron transported from QA-to QB(the secondary quinone electron acceptor), and theVIf luorescence ref lected the reduction of the PQ pool (Schreiber, 1998; Zivcak et al., 2015). The increases of f luorescence inVIand J-I phase of the co-cultivated embryos (Fig.5b) indicated the limitation of electron transport between QAand other electron accepters (QB, PQ pool), and then the limitation of the electron transport to terminal electron acceptor (PSI reaction center).
The electron transport parametersφPo,ψo, andφEoof the co-cultivated embryos inP.donghaiensesuspensions at all the cell densities decreased signif icantly (P<0.05), indicating that the overall quantum effi ciency of their photochemical reactors were inhibited, which diff ered from those of the monocultivated embryos (Fig.6; Table 1). The energy f lux ratios in the process of electron transport provide information of the structure and function of photosynthetic apparatus (Liu et al., 2017; Samborska et al., 2018). In addition, the value of ABS/RC represents the absorption energy per active RC(Appenroth et al., 2001), and the antenna size of the light-harvesting complexes (LHC) per PSII RC complex. In this study, the ABS/RC of the cocultivated embryos increased signif icantly (P<0.05)above those of the monocultivated ones (Fig.8),indicating that the antenna size of RCs was enlarged and a part of the RCs was inactivated, by which the excitation energy was transferred from the inactive centers to other PSII active units, converted to heat dissipation, and f inally increased the DIo/RC and DIo/ABS (Fig.8a-b).
In this study, the values of ETo/RC, TRo/ABS, and ETo/ABS of the co-cultivated embryos were signif icantly (P<0.05) less than the monocultivated ones’ (Fig.8a-b). Among these ratios, the trapped energy (TRo) was used to restore the QAto QA- , and then the electron transport (ETo), indicating that the photosynthetic electron transport was limited and the energy was used for the reduction of PSII acceptors(QA, QB, and PQ pool), thus resulting in the decrease of ETo(Fig.6; Table 1). The decreases of TRo/ABS,ETo/RC, and ETo/ABS were consistent with the results of JIP-test, showing that the inhibitory eff ects ofP.donghaienseonS.fusiformisembryos were resulted from a comprehensive damage on their photosynthetic systems. Furthermore, the parameters RC/ABS,PTR, andPETwere constitutes of the PI,among which the dark reaction (PET) contributed largely (21.79%-52.28%) to the PI decline (Fig.7a-b),thus the dark reaction of photochemistry was inhibited mostly.
The main mechanism of the inhibitory eff ects between algae was due probably to strong competition for nutrient and the excretion of allelopathic substances (Yang et al., 2015; Ma et al., 2017; Shang et al., 2020). In this study, f/2 media used for the cultivations were rich in N and P nearly 30 times of the natural seawater’s, and the media forS.fusiformismonocultivation andK.mikimotoi-S.fusiformiscocultivation were renewed every day during the experiment. In addition, in both cultivations, the TN changed from 12.67 to 8.46 mg/L and the TP from 0.13 to 0.06 mg/L, showing same trends in every 2 days (Fig.9a-e, f-j). Therefore, nutrients in all the cultivations were suffi cient for the demand of seedlings growth, thus the inhibitory or even lethal eff ects by nutrient def iciency were unlikely. Moreover,the high pH value has also been taken into account in the allelopathic eff ects of some autotrophs (Schmidt and Hansen, 2001; Lundholm et al., 2005). Whereas the pH values of both the cultivations ranged from 7.84 to 8.40, and that of theP.donghaioensesuspension of the co-cultivation was greater than that of the f/2 media in the monocultivation (Fig.9k-o),suggesting that the inhibition eff ects on embryos were irrelevant to the pH. Therefore, it is reasonable to assume that inhibition to the growth and photosynthesis of embryos was caused by allelochemicals that exuded fromP.donghaioense.
In the nature, interaction between HAB-forming species by cell contact was thought the key way of promoting the bloom formation (Yamasaki et al.,2007). Uchida et al. (1999) reported that the interaction betweenK.mikimotoiandHeterocapsacircularisquamadepended on the initial cell densities of the two species. In addition, the growth inhibition and the formation of morphologically abnormal cells ofAkashiwosanguineawere induced in a concentration-dependent manner by allelochemicals released fromH.akashiwo(Qiu et al., 2012). In a recent study, it was found that the suppression on pigment contents and photosynthetic activities ofS.fusiformisembryos byK.mikimotoiwere also induced in a cell density-dependent manner (Shang et al.,2020). It has been reported that the secondary substance released fromP.donghaiensesuppressed the growth of other microalga in bi-algal cultivations(Wang and Tang, 2008; Shen et al., 2015). In China,current mariculture ofS.fusiformisdepends almost entirely on sexually propagated seedlings (Lin et al.,2020), and thus any adverse eff ects on the development and growth of the embryos could seriously threaten the seedling stock. In this study, exposure ofS.fusiformisembryos to denseP.donghaiensesuspensions led to signif icant inhibition to the growth and photosynthetic activities. In addition, the inhibitory eff ects ofP.donghaiensesuspensions onS.fusiformisembryos followed a cell densitydependent manner. Therefore, during the seedlingbreeding period, the HABs formed by dinof lagellateP.donghaienseat high cell densities inS.fusiformisfarming region could seriously threaten the seedling stock, which would eventually hinder its farming industry.
5 CONCLUSION
The inhibitory eff ects ofP.donghaiensesuspensions on growth and photosynthetic activities ofS.fusiformisembryos increased with increased cell densitiesin the suspensions. In addition, the JIP-test analysis showed that the decreases of the maximum relative electron transport rates (rETRmax) and the apparent photosynthetic effi ciency (α) in co-cultivated embryos were due to the limitation of electron transport between electron transfer accepters and the inactivation of partial reaction centers, which promoted the conversion of excitation energy to heat dissipation. These results indicate that dense algal bloom formed byP.donghaienseis a threat toS.fusiformisfarming industry.
6 DATA AVAILABILITY STATEMENT
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
杂志排行
Journal of Oceanology and Limnology的其它文章
- MamZ protein plays an essential role in magnetosome maturation process of Magnetospirillum gryphiswaldense MSR-1*
- Magnetotactic bacteria from the human gut microbiome associated with orientation and navigation regions of the brain*
- How light aff ect the magnetotactic behavior and reproduction of ellipsoidal multicellular magnetoglobules?*
- Biocompatibility of marine magnetotactic ovoid strain MO-1 for in vivo application*
- Determination of the heating effi ciency of magnetotactic bacteria in alternating magnetic f ield*
- An approach to determine coeffi cients of logarithmic velocity vertical prof ile in the bottom boundary layer*
