Preoperative intravitreal Ranibizumab with panretinal photocoagulation followed by conventional trabeculectomy without drainage device for neovascular glaucoma
2021-12-06,
,
Abstract
KEYWORDS:neovascular glaucoma; Ranibizumab; panretinal photocoagulation; trabeculectomy
INTRODUCTION
Surgical management of neovascular glaucoma (NVG) has always been challenging because it is associated with a higher failure rate and more complications than that of primary glaucoma[1]. Glaucoma drainage devices are usually considered the first treatment option for NVG[2]. However, patients with NVG are at greater risk for surgical failure after glaucoma valve surgery compared with controls[3]. In addition, compared to other types of glaucoma, NVG eyes also seem to be at higher risk for tube shunt exposure[4]. Moreover, there is no large randomized trial to prove glaucoma drainage device better than conventional trabeculectomy in NVG treatment or vice versa[5]. Therefore, the selection of the surgery type depends on surgeon’s judgment and consideration of patient variables[6]. The stage in which NVG is diagnosed may have an impact on the treatment outcome and the angle anatomy ranging from complete open to focal or complete synechial closure is an important factor in determining the selection of surgery type[7]. Whether conventional trabeculectomy without drainage device is effective in treating NVG and which stage of NVG is the optimal indication for this procedure are still uncertain.
In this study, we evaluated the efficacy and safety of preoperative intravitreal injection of ranibizumab (IVR) coupled with panretinal photocoagulation (PRP) and combined with conventional trabeculectomy without drainage device in patients with NVG. We also assessed the difference in efficacy of this procedure between eyes with peripheral anterior synechiae (PAS)≤50% and those with PAS >50%.
SUBJECTS AND METHODS
EthicalApprovalThe study complied with the principles of the Declaration of Helsinki and was approved by Xi’an Aier Ancient City Eye Hospital and Xianyang Aier Eye Hospital Ethics Committees. All patients received a detailed explanation of the treatment and signed informed consent.
StudyDesignandMethodsThe charts of 27 patients with NVG (27 eyes) who received preoperative IVR and PRP treatment combined with conventional trabeculectomy without drainage device at Xi’an Aier Ancient City Eye Hospital and Xianyang Aier Eye Hospital between August 2015 and November 2018 were reviewed. Signed informed consent was obtained from all patients after discussing the potential treatment benefits and risks. NVG was diagnosed by the presence of neovascularization of the anterior segment (NVA) and intraocular pressure(IOP)>21 mmHg with maximal tolerated topical and systemic antiglaucoma drugs. The exclusion criteria were optical media opacity (e.g.corneal edema, cataract, and vitreous hemorrhage) that made PRP impossible, retinal diseases [e.g.severe proliferative diabetic retinopathy (PDR) and tractional retinal detachment] requiring a concomitant vitrectomy surgery, and previous glaucoma filtering surgery.
Intravitreal injections were performed under topical anesthesia in a sterile operating room. After prepping the skin, 0.5 mg (0.05 mL) ranibizumab was injected into the vitreous cavity through the pars plana (3.5-4 mm posterior to the limbus) with a 27G needle. The injection site was compressed slightly for 60s after needle withdrawal. A paracentesis was performed to ensure that the IOP was within normal limits. The patients received topical antibiotics, corticosteroids, and IOP-lowering medicines after injection.
Five to seven days after IVR, PRP was applied in 2-3 sessions with the following parameters: 250-400 mW power, 200 ms pulse duration, and 200-500 μm spot size. We attempted to cover the whole peripheral retina by applying 800-1000 shots per session.
Conventional rabeculectomy without drainage was performed 2.4±0.8 (1-4)d after PRP. A fornix-based conjunctival flap was made in the superotemporal quadrant, followed by a limbal-based 3 mm×4 mm rectangular half-thickness scleral flap. An MMC-soaked sponge (0.4 mg/mL) was inserted under the conjunctival and scleral flaps for 1-2min. The area was then thoroughly irrigated with sterile saline (200 mL). Trabeculectomy (1 mm×1.5 mm) and peripheral iridectomy (1 mm × 1 mm) were performed. The scleral and conjunctival flaps were closed with 10-0 nylon sutures. Topical steroids, antibiotics, and cycloplegics were applied and tapered off over 4-6wk postoperatively.
Ophthalmic examinations were conducted on 1d after IVR and when the PRP was performed. After trabeculectomy, the patients were followed up after 1d and 1wk, as well as after 1, 3, and 6mo, and every 6mo thereafter. The main outcome measures were postoperative IOP control and the incidence of complications. Success was defined as an IOP of 6-21 mmHg without the need for additional glaucoma surgeries and the occurrence of vision-threatening complications. The surgery was considered either a complete success (without anti-glaucoma medications) or a qualified success (with antiglaucoma medications) when IOP < 21 mmHg was achieved. Surgical failure was defined as IOP≥21 mmHg even with the administration of anti-glaucoma medicines at two consecutive follow-up visits, presence of phthisis bulbi, the need for additional surgical procedures, or the loss of light perception. The intra-postoperative complications, along with the therapeutic or surgical treatments, were determined by reviewing patient charts. Best-corrected visual acuity (BCVA) was converted into the logarithm of the minimal angle of resolution (LogMAR) format. Counting fingers, hand motion, and light perception were assigned values 2, 2.6, and 3, respectively. An increase of 0.3 or more in LogMAR was defined as a marked decrease in BCVA. The NV of the iris and anterior chamber angle was observed by slip-lamp and gonioscopy.

RESULTS
The demographic and baseline clinical characteristics of the patients are presented in Table 1. Gonioscopy was performed after reducing the cornea edema by topical hypertonic saline and IOP-lowering medicines. Although the view to the angle was somewhat obscured, NVA and PAS caused by the contraction of the fibrovascular membrane were observed in all eyes. Among them,PAS ≤50% was present in 11 eyes (41%) and PAS >50% in 16 eyes (59%). At baseline, the IOP was 45.7±5.1 mmHg, and the number of IOP-lowering medications was 2.7±0.4. The baseline BCVA was 2.42±0.68. Twenty-three eyes (85.2%) presented severe corneal edema and anterior chamber inflammation. One day after IVR, a rapid involution of NVA was seen in all eyes with significantly decreased IOP and slightly improved BCVA. Five to seven days after IVR (at the first PRP), the IOP was under control with substantially decreased NVA. The BCVA was improved with diminished corneal edema and anterior chamber inflammation. At the time of trabeculectomy, although NVA markedly regressed in all cases, was still visible in eight eyes (30%), but without intraoperative bleeding complications.
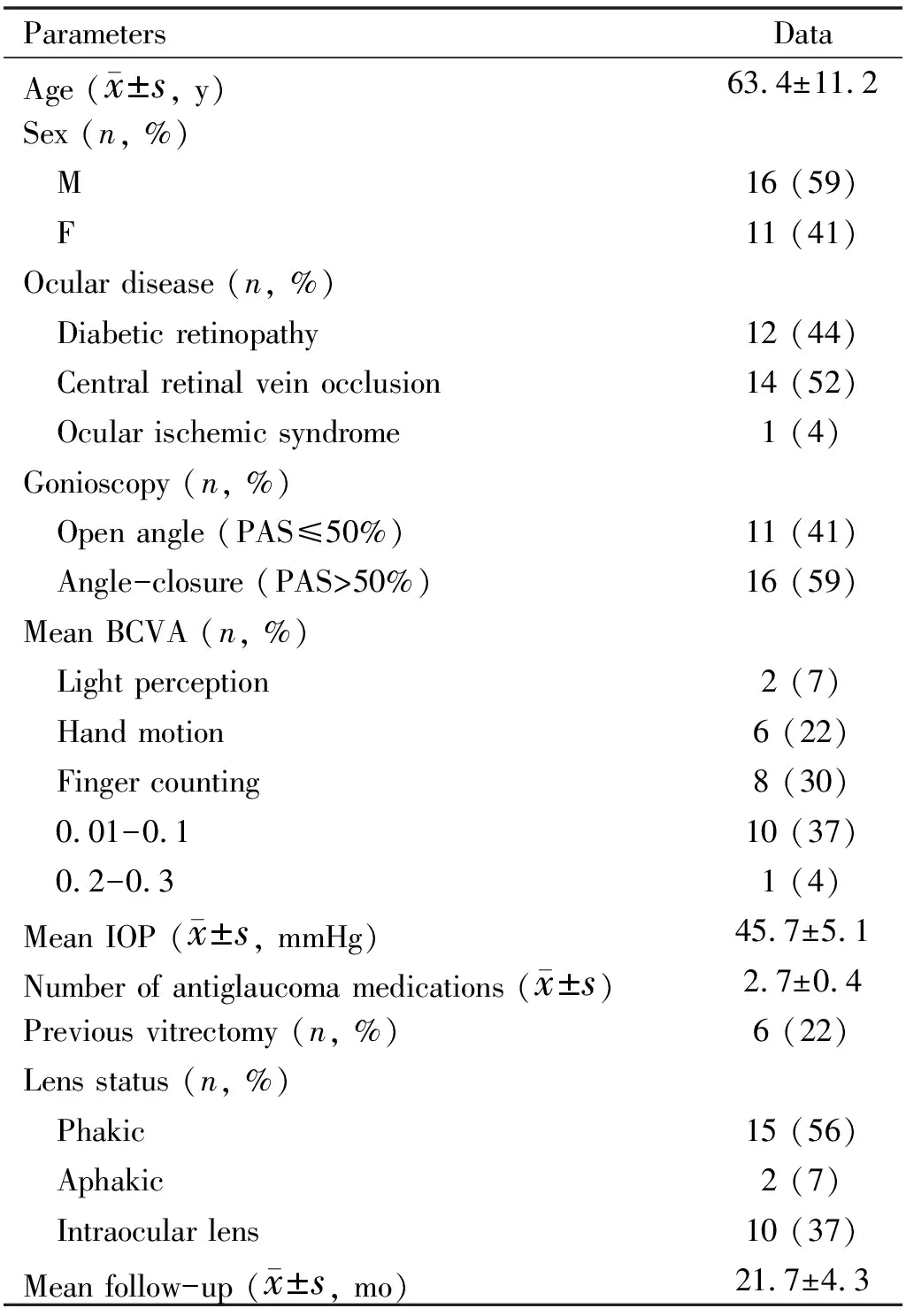
Table 1 Baseline characteristics of the patients included in the study

Table 2 Presentation and complications at baseline and follow-up time points
The post-trabeculectomy follow-up periods for all patients were at least 18 (mean: 21.7±4.3)mo. NV recurrence was not observed in any of the eyes. After conventional trabeculectomy without drainage device, the IOP was significantly decreased and the BCVA was slightly improved, compared with that of the baseline value. The mean IOP and BCVA of baseline, 1d after IVR, at the first PRP, at the time of trabeculectomy, and all follow-up time points are presented in Figure 1 and Figure 2. Without any anti-glaucoma medications, all eyes had an IOP <21 mmHg on 1d and at 1wk postoperatively (complete success rate, 100%). The success rates after 1, 3, and 6mo, and at the last visit were 100% (complete: 76.9%, qualified: 23.1%), 96.3% (complete: 69.1%, qualified: 27.2%), 92.6% (complete: 55.5%, qualified: 37.1%), and 88.9% (complete: 48.2%, qualified: 40.7%), respectively. Eyes with PAS ≤50% have a higher success rate than those with PAS >50% at 3, 6mo postoperatively and at the last visit (P<0.05). The comparison of the success rate in PAS ≤50% eyes with that in PAS >50% eyes at follow-up time points are presented in Figure 3. The number of IOP-lowering medications after trabeculectomy was 0.8±0.6, which was significantly lower than the baseline value (P=0.014). Three eyes (11.1%) were subjected to additional anti-glaucoma surgery (cyclophotocoagulation in 2 eyes and glaucoma drainage devices in 1 eye) due to an uncontrolled high IOP.
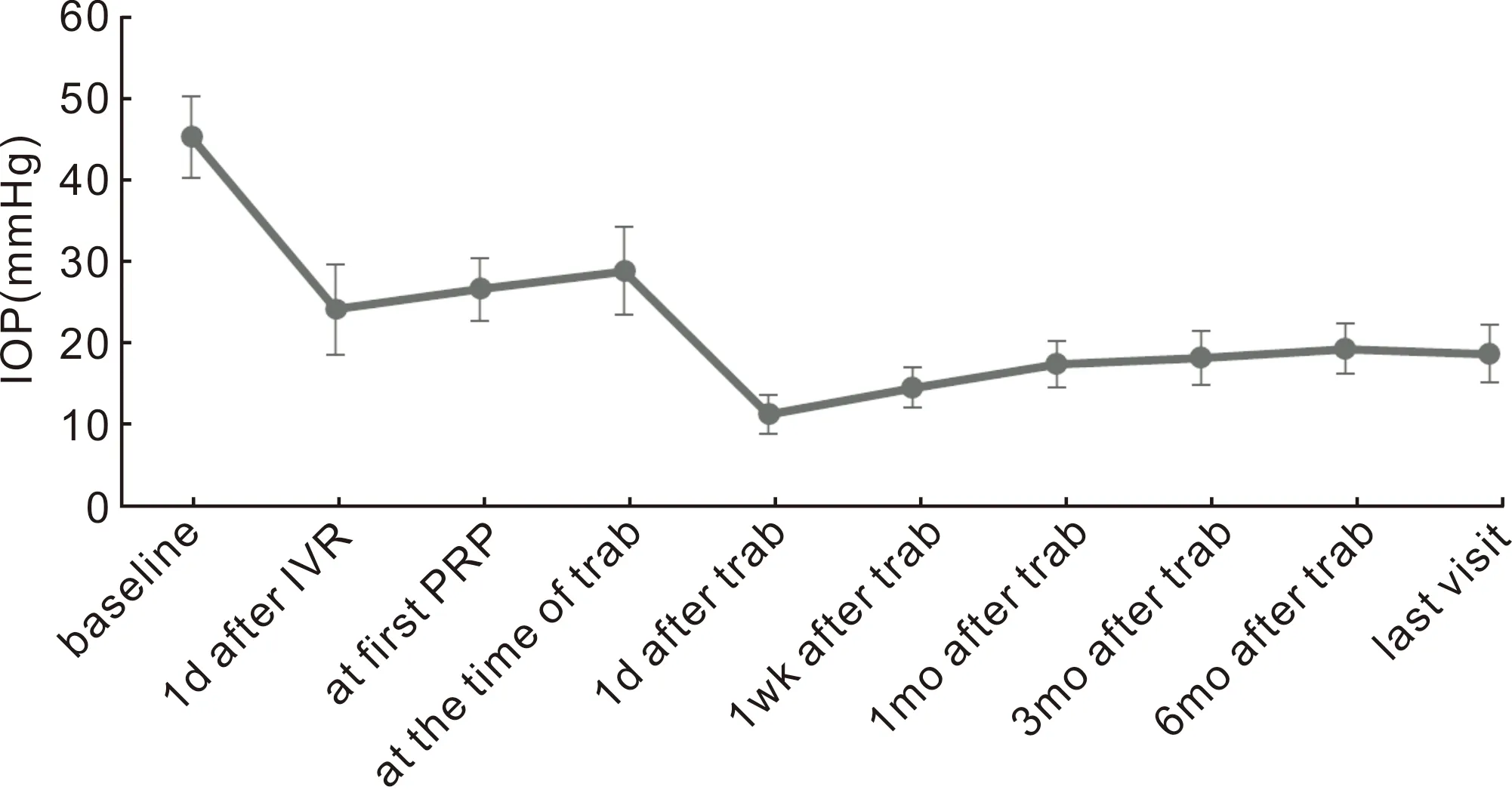
Figure 1 Mean IOP was significantly decreased compared to baseline during the treatment process and at all follow-up time points(P<0.01).

Figure 2 Mean BCVA was improved compared to baseline during the treatment process and at all follow-up time points(P<0.05).

Figure 3 Success rate in eyes with PAS ≤ 50% compared to that in eyes with PAS > 50% at all follow-up time points.
Five eyes (18.5%) received additional retinal photocoagulation based on fundus fluorescein angiography examination during the follow-up examinations.
Mild-to-moderate hyphema during or after the IVR procedure was observed, which resolved spontaneously within 1wk. Immediate IOP spikes after injection were not seen in any eye. There was no intraoperative complication in trabeculectomy. During the early postoperative period (within 2wk), the main complications were mild, self-limited hyphema and spontaneously resolved or medication controlled shallow anterior chamber with hypotony (IOP≤5 mmHg). The most common complication encountered during the late postoperative period (after 2wk) was an encapsulated bleb, which needed a needling revision (mean times 1.1±0.7) combined with a subconjunctival injection of anti-metabolites. Severe intraoperative or postoperative complications, such as choroidal detachment, suprachoroidal hemorrhage, endophthalmitis, vitreous hemorrhage, bleb leak, marked decrease in BCVA, and phthisis bulbi, were not observed. The presentation and complications at baseline and follow-up time points are presented in Table 2.
DISCUSSION
Treatment of the underlying disease responsible for the development of NVA at the early stage and control of elevated IOP once established are the two key aspects to manage NVG[8]. PRP is considered the standard treatment for ischemic retinal disease and retinal NV[9]. Timely PRP can reduce not only the risk of NVA and NVG development, but also lead to vascular regression and preservation of the angle anatomy[10]. However, prompt treatment with PRP in NVG is relatively difficult because of the optical media opacity (corneal edema and anterior chamber inflammation) and ocular pain caused by acute IOP elevation[11]. In our study, PRP was performed 5-7d after the IVR. At the first PRP, the IOP significantly decreased from 45.7±5.1 (baseline) to 26.8±3.9 mmHg with a detectable reduction in NVA and an alleviation in media opacity and ocular pain. It demonstrated that IVR could rapidly induce NV involution and resolve inflammation, which provided more time to effectively perform the PRP.
The stage in which NVG is diagnosed may have an impact on the treatment selection and outcome[12]. Initial trials of anti-VEGF injections reported a substantial regression of NVA, and IOP control when the angle remains open[13-14]. However, the anti-VEGF efficacy declines gradually over time, and it is associated with NVA recurrence as well as IOP elevation[15]. When PAS is extensive and the secondary angle closure occurs, anti-VEGF cannot effectively control IOP and definitive surgery is necessary[16].
Glaucoma valve implantation is considered the first treatment option for NVG, however, the outcomes are still unfavorable[17]. For instance, Tangetal[18]showed that the success rate of NVG treatment with intravitreal ranibizumab combined with Ahmed glaucoma valve implantation was 73.7% at 6mo and 72.2% at 12mo compared with 71.4% and 68.4%, respectively, with Ahmed glaucoma valve implantation alone. Hernandez-Oteyzaetal[19]reported a success rate of 60% with Ahmed valve surgery at 1y of follow-up in patients with NVG. Yalvacetal[20]reported 63.2% and 56.2% success rates at 1 and 2y after Ahmed glaucoma valve implantation, respectively. Moreover, there is no evidence of improved surgical outcomes with glaucoma drainage devices as opposed to trabeculectomy. Shenetal[3]reported success rates of 70% and 65% at 1y and 60% and 55% at 2y after Ahmed glaucoma valve implantation and trabeculectomy with mitomycin C, respectively. Comparing to glaucoma drainage device surgery, conventional trabeculectomy is more economical.
The success rate of conventional trabeculectomy in patients with NVG is still lower even with the use of anti-metabolites[21]. The high failure rates are attributed to scarred and inflamed conjunctiva, occlusion of the sclerotomy with neovascular membrane, and the recurrence of new vessel postoperatively[22]. In our study, although the success rate decreased gradually after surgery, it was still 92.6% at 6mo and 88.9% at the last visit. And beyond that, the number of IOP-lowering medications used after operation was significantly below the baseline and no NVA recurrence was observed during the follow-up. This result demonstrated that preoperative IVR combined with PRP are effective adjunct procedures to improve conventional trabeculectomy success rate by suppressing NVA persistently. In addition, mild-to-moderate impairment of the anterior chamber angle (eyes with PAS ≤50% accounted for 40.7%) may be another determinant of the high success rate. Our study also showed that eyes with PAS ≤50% have a higher success rate than those with PAS >50% at 3, 6mo postoperatively and at the last visit. This results indicated that conventional trabeculectomy without drainage device may be more suitable for patients with open angle or focal synechial closure.
Postoperative hyphema is a common complication in patients with NVG and is associated with higher rates of trabeculectomy failure[23]. In our study, mild, self-limited hyphema was only observed in three eyes (11.1%), which suggested that preoperative IVR can significantly decrease the frequency and severity of postoperative hyphema, thereby improving the success rate of trabeculectomy.
NVG prognosis is relatively poor because of the increased risk for postoperatvie complications, and some patients will lose vision because of uncontrolled IOP or destructive surgery[24]. In our study, we observed an improvement in BCVA at all follow-up time points, compared with that at baseline. A marked BCVA decrease and severe intra- or postoperative complications were not observed, corroborating the safety of this treatment.
The limitations of this retrospective study include bias in the selection of patients (patients with severe optical media opacity or severe retinal disease requiring vitrectomy surgery are excluded from the study), small number of patients, and lack of control group, restricting the generalization of our results. Furthermore, short follow-up periods limited our ability to evaluate the final treatment effect because the success rate of NVG typically decreases over time postoperatively. Hence, a larger prospective study with longer follow-up periods should generate critical evidence for evaluating this NVG treatment strategy.
In conclusion, our study showed that IVR, coupled with PRP and combined with conventional trabeculectomy without drainage device, is an effective and safe NVG treatment strategy, especially for eyes with PAS ≤50%. Preoperative IVR and PRP appear to be beneficial by improving the trabeculectomy success rate in NVG.
