Jian-Gan-Xiao-Zhi decoction ameliorates high-fat high-carbohydrate diet-induced non-alcoholic fatty liver disease and insulin resistance by regulating the AMPK/JNK pathway
2021-12-04XueHuaXieJiaBaoLiaoFangFangJieZhaoYongJunCaoHuanTianCuiHongWuWangZhaiYiZhangZhaoHuiSunYuanYinWeiBoWen
Xue-Hua Xie, Jia-Bao Liao, Fang Fang, Jie Zhao, Yong-Jun Cao, Huan-Tian Cui, Hong-Wu Wang, Zhai-Yi Zhang,Zhao-Hui Sun,Yuan Yin,Wei-Bo Wen*
1Department of Endocrinology, Yunnan Provincial Hospital of Traditional Chinese Medicine, Kunming 650021, China;2Department of Emergency, Jiaxing Hospital of Traditional Chinese Medicine, Jiaxing 314001, China; 3Department of Nephrology, Nantong Hospital of Traditional Chinese Medicine, Nantong 226001, China; 4College of Integrated Chinese and Western Medicine, Tianjin University of Traditional Chinese Medicine, Tianjin 301617, China;5College of Traditional Chinese Medicine, Tianjin University of Traditional Chinese Medicine, Tianjin 301617, China;6Department of Endocrinology,Suzhou Hospital of Traditional Chinese Medicine,Suzhou 215001,China.
Abstract
Background: Non-alcoholic fatty liver disease (NAFLD) can cause insulin resistance (IR) and diabetes.Our previous studies have demonstrated that Jian-Gan-Xiao-Zhi decoction (JGXZ) could be effective for the treatment of NAFLD and IR.However, the possible mechanism underlying the effects of JGXZ on NAFLD and IR remains unknown.Methods:Fifty rats received a high-fat high-carbohydrate (HFHC)diet for 12 weeks to induce NAFLD.After 4 weeks of HFHC treatment,rats were orally treated with JGXZ(8,16,and 32 g/kg weight)for 8 weeks.Ten rats in the control group received standard chow.In the positive control group, rats were orally treated with metformin (90 mg/kg weight) for 8 weeks.After JGXZ and metformin treatment, H&E staining was conducted on rat livers and serum biochemical markers, including alanine aminotransferase (ALT), aspartate aminotransferase(AST), triglyceride (TG), and total cholesterol (TC), were measured using test kits.Moreover, a fasting blood glucose test and an oral glucose tolerance test (OGTT) were conducted.Serum levels of insulin were determined using ELISA kit, and the homeostatic model assessment of insulin resistance (HOMA-IR) was calculated.The levels of total insulin receptor substrate-1 (IRS1),AMP-activated protein kinase-α (AMPKα)and c-Jun N-terminal kinase(JNK)as well as the levels of phosphorylation of IRS1(p-IRS1),phosphorylation of AMPK(p-AMPK)and phosphorylation of JNK (p-JNK)were measured using western blotting.Results:The body weights in JGXZ low-,middle-, and high-dose groups were lower than those in the model group (P <0.05, P <0.01, P <0.01,respectively).The serum levels of AST(P <0.05 in JGXZ middle-and high-dose groups),ALT(P <0.01 in JGXZ middle-dose group and P <0.05 in JGXZ high-dose group),TG(P <0.01 in JGXZ middle-and high-dose groups),and TC (P <0.01) upon JGXZ treatment were lower those than in NAFLD model rats.H&E staining showed that JGXZ treatment reduced steatosis of the hepatocytes in NAFLD model rats.JGXZ decreased the levels of fasting blood glucose (P <0.01), HOMA-IR (P <0.01),AUC (area under the curve) of the OGTT (P <0.05) and p-IRS1(P <0.01 in JGXZ middle-and high-dose groups,P <0.05 in JGXZ low-dose groups).Moreover,JGXZ regulated the hepatic AMPKα/JNK pathway in NAFLD model rats,which reflected the induction of p-AMPKα and inhibition of p-JNK.Conclusion: This study showed that JGXZ improved liver function and reduced steatosis of the hepatocytes in NAFLD model rats.Moreover,JGXZ improved IR in NAFLD model rats.The possible mechanism underlying the effects of JGXZ on NAFLD and IR involves the modulation of the AMPK/JNK pathway.
Keywords: Jian-Gan-Xiao-Zhi decoction, Non-alcoholic fatty liver disease, Insulin resistance, AMPK/JNK pathway
Background
With changes in lifestyles and dietary habits in recent years, the morbidity of chronic metabolic diseases,such as obesity, non-alcoholic fatty liver disease(NAFLD) and diabetes, is increasing [1, 2].Excessive lipid content in the liver can induce hepatic steatosis and trigger NAFLD.In addition, long term NAFLD can cause insulin resistance (IR) and diabetes [3, 4].Insulin signal transduction in hepatocytes can be impaired after exposure to excessive lipid content and,ultimately,cause IR and diabetes[5,6].
Traditional Chinese medicine (TCM) has been used for the treatment of NAFLD for thousands of years.Studies show the significant effects of various TCM formulas on NAFLD.The Hugan Qingzhi tablet, a Chinese patent medicine, may treat NAFLD by inhibiting the activation of NF-κB and reducing oxidative stress [7].A classical prescription of TCM,Sini powder, alleviates stress-induced NAFLD by improving the blood lipid content and inflammatory response [8].A Chinese patent medicine, Jiang Zhi granules, decreases body mass index and serum levels of triglyceride (TG) and total cholesterol (TC) in patients with NAFLD[9].
Jian-Gan-Xiao-Zhi decoction (JGXZ) is a formula of TCM established by Dr.Wen Weibo in the Yunnan Provincial Hospital of TCM, China.It is an adaptation of a classical prescription of TCM, Jiajian Huangqin decoction, which is recorded in the ancient book of TCM named Make Huoren, written by Yuqiong Xie in 1748 C.E.(Qing dynasty of China).The original prescription was composed of Huangqin (Scutellaria Baicalensis Georgi), Huanglian (Coptis Chinensis),Danggui (Angelica Sinensis), Zhiqiao (Rhizoma Pinelliae), Binglang (Areca catechu Linn), Qingpi(Vatica mangachapoi Blauco), Zexie (Rhizoma Alismatis), Shanzha (Crataegus Pinnatifida), Huaihua(Flos Sophorae), Baishao (Radix Paeoniae Alba), and Gancao(Glycyrrhiza Uralensis Fisch).
Our clinical studies show that JGXZ can improve blood lipid levels and liver function and decrease fasting blood glucose levels in patients with NAFLD[10, 11].Moreover,in vivostudies show that JGXZ can decrease hepatic steatosis in NAFLD model rats[12].However, the mechanisms underlying the effects of JGXZ on NAFLD and IR remain unknown.
In this study, we investigated the therapeutic effects of JGXZ on a rat model of NAFLD and IR.Moreover,the relative mechanisms underlying the effects of JGXZ on NAFLD and IR were studied with regard to the AMP-activated protein kinase-α (AMPKα)/c-Jun N-terminal kinase(JNK)pathway.
Materials and methods
Reagents
BCA protein assay, alanine aminotransferase (ALT),aspartate aminotransferase(AST),TG,and TC test kits were purchased from the Nanjing Jiancheng Biological Engineering Institute (Nanjing, China).Primary antibodies against rat GAPDH (5174; 1:1000),AMPKα (2752S; 1:1000), phosphorylated-AMPKα(p-AMPKα) (5831; 1:1000), JNK (9252; 1:1000),phosphorylated-JNK (p-JNK) (9255; 1:1000), insulin receptor substrate-1 (IRS1) (2390; 1:1000), and phosphorylated-IRS1 (p-IRS1) (2381; 1:1000), and the corresponding secondary antibodies were obtained from Cell Signaling Technology, Inc., (Boston, USA).The rat insulin ELISA test kit was purchased from Bluegene Bioscience Co.,Ltd.(Shanghai,China).
Animals
Male Sprague-Dawley rats, 190-210 g and 6-7 weeks old, were purchased from Weitonglihua Animal Company (Beijing, China).All animal experiments were conducted according to the National Institutes of Health Regulations and approved by the Institutional Animal Care and Use Committee at Yunnan University of TCM (SYXK2017-0006).During the acclimatization and study periods, all animals were maintained in specific pathogen-free conditions(temperature:21±2 ℃,humidity: 45±10%,and a 12 h light/dark cycle)with food and water ad libitum.
Induction of the NAFLD rat model
The NAFLD model was established as previously described [13, 14].Briefly, the rats received a high-fat high-carbohydrate (HFHC) diet, which contained 17.7% sucrose, 17.7% fructose, 19.4% protein, and 40% fat, for 12 weeks.To assess the induction of NAFLD in the rats, body weight and serum levels of ALT, AST, TG, and TC were measured.The livers of the rats were stained with H&E to observe pathological changes in the liver.
Preparation of JGXZ and metformin
Instant granules of this TCM formula were purchased from the Yunnan Provincial Hospital of TCM(Kunming, China).Each JGXZ granule contained the same amount of each drug as in the JGXZ formula:15 g Danshen (Salvia Miltiorrhiza Bunge), 6 g Sanqi(Panax Notoginseng), 15 g Ezhu (Curcuma Zedoaria),20 g Shanzha (Crataegus pinnatifida), 20 g Huangqi(Astragalus Membranaceus), 10 g Qingpi (Vatica Mangachapoi Blanco), 20 g Chishao (Radix Paeoniae Rubra),12 g Jianghuang(Curcuma Longa),15 g Zexie(Rhizoma Alismatis), 15 g Juhua (Dendranthema Morifolium), 15 g Heye (Folium Nelumbinis), and 6 g Gancao (Glycyrrhiza Uralensis Fisch).Metformin(CAS: 1115-70-4) was obtained from Solarbio Biotechnology Co.Ltd.(Beijing, China).Metformin and the JGXZ granules were diluted in saline for gavage.The concentration of metformin was 9 mg/mL,and the concentration of JGXZ was 0.8 g/mL, 1.6 g/mL, and 3.2 g/mL for the low-, middle-, and high-doses,respectively.
Animal grouping
After acclimatization for 1 week, 60 rats were randomized into six groups: control, model, positive control, JGXZ low-dose, JGXZ middle-dose, and JGXZ high-dose.Rats in the control group received standard chow, which contained 59.4% total carbohydrate, 20% protein, and 4.8% fat.Rats in the model, positive control, JGXZ low-dose, JGXZ middle-dose, and JGXZ high-dose groups received the HFHC diet to induce NAFLD.Moreover, rats in the positive control, JGXZ low-dose, JGXZ middle-dose,and JGXZ high-dose groups received a gavage of metformin (90 mg/kg weight) and JGXZ (8, 16, 32 g/kg weight), respectively, once per day for 8 weeks after the HFHC diet treatment for 4 weeks.The 16 g/kg dose in the JGXZ middle-dose group was equivalent to the adult daily dose of JGXZ, which was calculated using the following formula: equivalent dosage (g/kg) = human dose of crude herbs in clinic(g/kg) × 6.3.Rats in the control and model groups received the gavage of same volume of saline solution once per day for 8 weeks after the HFHC diet treatment for 4 weeks.
Fasting blood glucose assay and oral glucose tolerance test(OGTT)
The levels of fasting blood glucose were investigated using an Accu-check glucometer every 2 weeks.The OGTT was conducted after JGXZ treatment as previously described [15, 16].Briefly, rats were fasted for 24 h, and the baseline blood glucose level was detected.Then, rats were orally treated with 40%D-glucose solution (20 g/kg), and the levels of blood glucose were investigated at 30, 60, and 120 min after D-glucose solution administration.Results of the OGTT were expressed as the integrated area under the curve(AUC)over a period of 0-120 min.
Serum biochemical markers assay
After JGXZ treatment, rats were anesthetized by intraperitoneal injection with 10% chloral hydrate solution (0.3 mL/100 g weight).Then, blood was collected from the inner canthus using a capillary glass tube and centrifuged at 3000 rpm for 15 min to obtain serum.Serum levels of ALT, AST, TG, and TC were measured according to the manufacturer's instructions from Nanjing Jiancheng Biological Engineering Institute(Nanjing,China).
Briefly,a 5 μL serum sample was incubated with 20 μL of ALT or AST matrix solution at 37 ℃for 30 min,followed by incubation with 20 μL of 2,4-dinitrophenylhydrazine at 37 ℃for 20 min.Then,200 μL of stop buffer(NaOH solution, 0.4 mol/L) was added to each well; subsequently, the absorbance was detected using a microplate reader at 510 nm.The obtained optical density (OD) value was recorded as OD1.Meanwhile, 20 μL of ALT or AST matrix solution was incubated at 37 ℃for 30 min, followed by incubation with a 5 μL serum sample, 20 μL of 2,4-dinitrophenylhydrazine, and 200 μL of NaOH solution,which was detected and recorded as OD2.The absolute absorbance of each sample was recorded as OD0and was calculated using the following formula:OD0= OD1- OD2.The activities of ALT and AST in serum were expressed as U/L and calculated according to the standard curve of ALT and AST at 0,2,4,6,and 8 μL of sodium pyruvate standard (2 μmol/mL).The standard curve was calculated based on the relative values of sodium pyruvate standard dilutions.
To calculate TG and TC content, 2.5 μL of serum sample, distilled water, and TG/TC standard was incubated with 250 μL of working solution at 37°C for 10 min.The absorbance value was detected using a microplate reader at 510 nm; the obtained OD values of the serum sample, distilled water, and TG/TC standard were recorded as OD1, OD2, and OD3,respectively.The levels of TG and TC in serum were expressed as mmol/L and calculated using the following formulas: TG levels (mmol/L) =(OD1-OD2)/(OD3-OD2) × 2.26 mmol/L, TC levels(mmol/L)=(OD1-OD2)/(OD3-OD2)×5.17 mmol/L.
Determination of serum insulin concentrations and homeostatic model assessment of insulin resistance(HOMA-IR)
Serum insulin concentrations were determined using a rat insulin ELISA kit, following the manufacturer's instructions, from Bluegene Bioscience Company(Shanghai, China).Briefly, 100 μL of serum sample,standards, and PBS were added to the test wells,followed by incubation with 50 μL of conjugate, for 1 h at 37 ℃.Then, the incubation mixture was removed from each well.Each well was washed with 350 μL of wash solution five times.After washing, each well was incubated with 50 μL of substrate A and 50 μL of substrate B for 15 min at 37 ℃;subsequently,50 μL of stop solution was added to each well.The absorbance was detected using a microplate reader at 450 nm to obtain the OD for each well.The standard curve was calculated according to the concentrations of the standards and the OD in the standard wells.The level of insulin was calculated according to the standard curve.
The severity of IR was determined by the HOMA-IR according to the following formula [17]:HOMA-IR = fasting serum insulin (μIU/mL) × fasting serum glucose(mmol/L)/22.5.
Histology
Rat livers were removed and fixed in 10% formalin after JGXZ treatment.The livers were dehydrated,embedded in paraffin, and cut into 5-µm sections.Sections were stained with H&E as described previously [18].Briefly, liver sections were deparaffinized in xylene, rehydrated in a reverse-gradient series of ethyl alcohol, and stained with hematoxylin for 3 min and eosin for 1 min.
Western blot analysis
Protein samples were isolated from rat livers after JGXZ treatment, as described previously [19].Then,the protein was quantified and normalized using the BCA protein assay.For western blotting, protein samples were separated by 8-12% SDS-PAGE and transferred onto a nitrocellulose membrane.The membrane was blocked using BSA and incubated with primary antibody (rabbit anti-p-IRS1 1:1000, rabbit anti-IRS1 1:1000, rabbit anti-p-AMPKα 1:1000, rabbit anti-AMPKα 1:1000, rabbit anti-p-JNK 1:1000, rabbit anti-JNK 1:1000, rabbit anti-GAPDH 1:1000) at 4 °C overnight.After incubation of primary antibody for 1 h,the membrane was washed with TBST, followed by secondary antibody probing (HRP-conjugate goat anti-rabbit IgG 1:4000).The blots were visualized using chemiluminescence (Odyssey CL, LI-COR,USA).The intensity of the bands was quantified using Image J software(NIH,Bethesda,MD,USA).
Statistics
All data are expressed as the mean ± standard deviation (mean ± SD) and were analyzed using one way analysis of variance.Statistical di erences between the experimental groups and control were examined using SPSS version 20.0 (SPSS, Inc.,Chicago, IL, USA), andP<0.05 was considered statistically significant.Curve-fitting was carried out using GraphPad Prism 5 (Graphpad Software, Inc., La Jolla,USA).
Results
Effects of JGXZ on NAFLD model rats
Body weight is lower in the model group than in the control group (P<0.01).Additionally, body weight is lower in the JGXZ low-, middle-, and high-dose groups than those in the model group (P<0.05,P<0.01,andP<0.01,respectively); moreover,it is lower in the metformin-treated group than that in the model group (P<0.01).In addition,the serum levels of ALT,AST, TG, and TC in NAFLD model rats are higher than those in the control group rats (P<0.01).The levels of ALT(P<0.01 andP<0.05 in JGXZ middleand high-dose groups, respectively),AST (P<0.05 in JGXZ middle-and high-dose groups),TG (P<0.01 in JGXZ middle- and high-dose groups), and TC (P<0.01) in the JGXZ treatment groups are significantly lower than those in the model group.The serum levels of AST (P<0.05), TG (P<0.01), and TC (P<0.01)are lower in the metformin-treated groups than those in the model group (Table 1).H&E staining reveals enlarged hepatocytes with severe microvesicular steatosis in the model group, whereas liver sections in the JGXZ-treated rats have only moderate steatosis(Figure 1).
JGXZ improved IR in NAFLD model rats
All rats exhibited similar levels of blood glucose before the HFHC diet treatment.After 12 weeks of HFHC diet treatment, the levels of fasting blood glucose are significantly higher in the model group than that in the control (P<0.01).In addition, the levels of fasting blood glucose in the positive control,JGXZ low-, middle-, and high-dose groups (P<0.05)are lower after 12 weeks of HFHC diet treatment than those in the model group(Figure 2).The serum insulin concentration(P<0.01)and HOMA-IR(P<0.01)are higher in the model group than those in the control.The levels of serum insulin (P<0.01) and HOMA-IR(P<0.01) upon JGXZ and metformin treatments are lower than those in the model group(Table 2).
OGTT was conducted at the end of week 12.The blood glucose level in the model group increases more rapidly (within 60 min), following glucose loading,than that in the control group, and continues to increase slightly for a further 60 min before declining.The AUC in the model group tends to be higher than that in the control group(P<0.01).The blood glucose levels in the positive control, JGXZ low-, middle-, and high-dose groups progressively increase,similar to that in the normal control, up to 1 h, and, then, drop gradually(Figure 3a).The AUC of the positive control,JGXZ low-, middle-, and high-dose groups are significantly lower than that in the model group (P<0.01 for positive control,P<0.05 for JGXZ low-,middle-and high-dose groups)(Figure 3b).
We also investigated the phosphorylation of the IRS1 in the liver by observing the ratio of p-IRS1/IRS1 in the liver.The phosphorylation of IRS1 is higher in the model group than that in the control group(P<0.01),whereas the phosphorylation of IRS1 in NAFLD model rats with JGXZ and metformin treatments are lower than that in the model group (P<0.05 in positive control,P<0.05 in JGXZ low-,P<0.01 in JGXZ middle- and high- groups) (Figure 4a,4b).
JGXZ affected AMPK/JNK pathway in NAFLD model rats
Studies demonstrate that the AMPK/JNK pathway is closely related to lipid metabolism during the progression of NAFLD [20, 21].Therefore, we investigated whether JGXZ could improve NAFLD and IR by modulating the AMPK/JNK pathway.The phosphorylation of AMPKα in the liver is lower in the model group than that in the control group (P<0.01).The phosphorylation of AMPKα is significantly higher in JGXZ middle-and high-dose groups than that in the model group (P<0.01, Figure 4a, 4c).In addition, the phosphorylation of JNK in the liver is higher in themodel group than that in the control group (P<0.01).The phosphorylation of JNK is significantly lower in the positive control, JGXZ low-, middle-, and high-dose groups than that in the model group (P<0.01, Figure 4a, 4d).Metformin induces the phosphorylation of AMPK in NAFLD models [22].However,no significant differences are observed in the phosphorylation of AMPKα, whereas the phosphorylation of JNK is lower in the positive control group than in the model group, which indicate that metformin does not induce the phosphorylation of AMPKα but inhibits the phosphorylation of JNK in our study.The mechanisms underlying the effects of metformin on NAFLD and IR may be related to its modulatory effects on the gut microbiota, which have been reported recently[23-25].
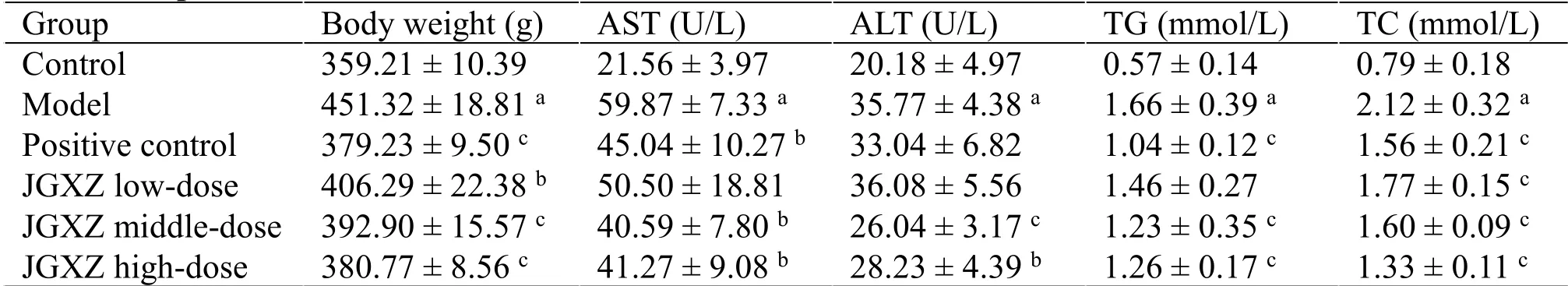
Table 1 JGXZ treatment decreased body weight and improved serum liver function biochemical markers and blood lipid levels in NAFLD model rats

Figure 1 JGXZ treatment improved hepatic steatosis in NAFLD model rats
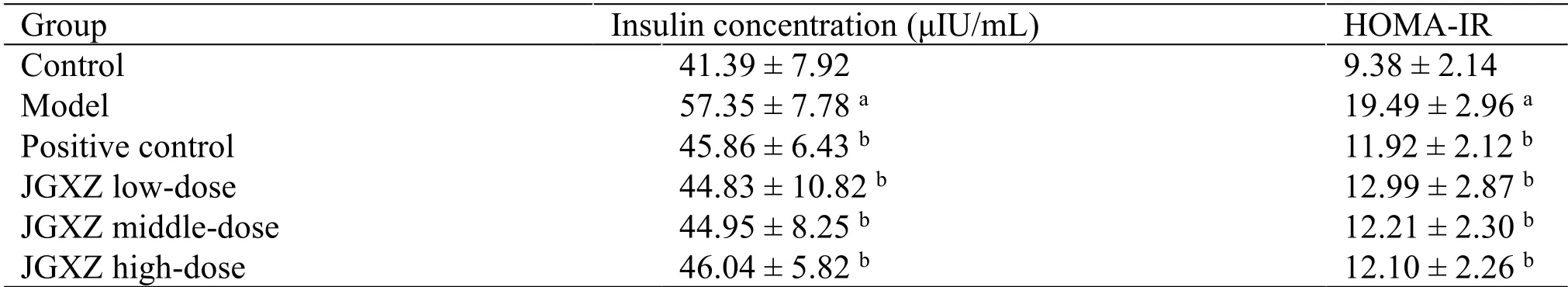
Table 2 JGXZ treatment improved serum insulin concentration and insulin resistance index (HOMA-IR) in NAFLD model rats
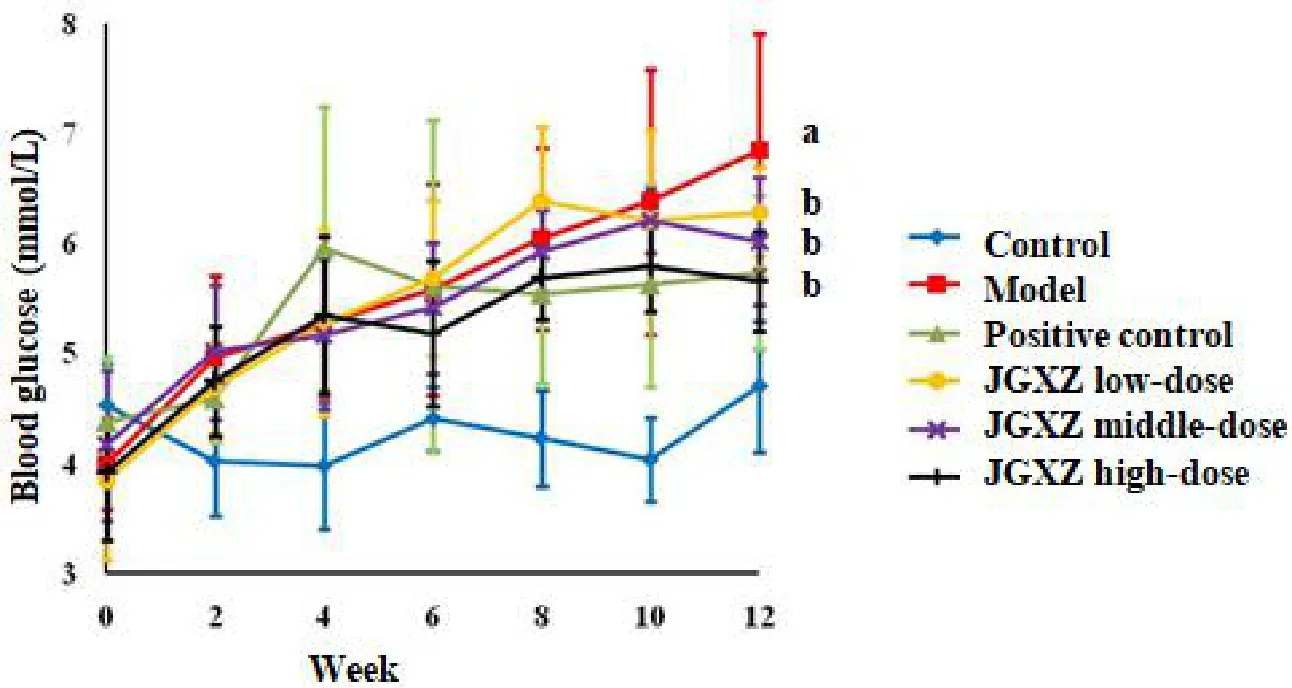
Figure 2 Rats received a HFHC diet to induce NAFLD
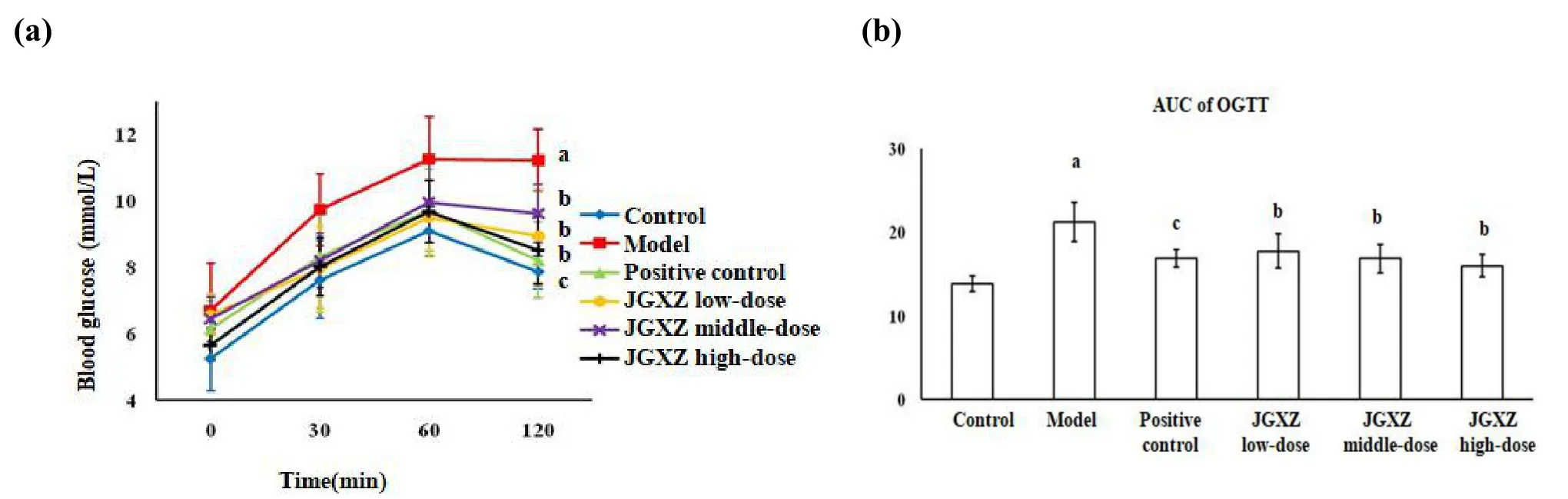
Figure 3 OGTT was conducted to measure the IR in NAFLD model rats after JGXZ treatment,JGXZ treatment improved the IR in NAFLD model rats
Discussion
In this study,we established a NAFLD rat model using a HFHC diet.In agreement with the results reported in previous studies [26], our results showed that the body weight and serum levels of AST, ALT, TG, and TC increased in rats that received the HFHC diet.Moreover, rats in the model group exhibited enlarged hepatocytes with severe microvesicular steatosis,which indicated that the rats had NAFLD.JGXZ had a remarkable effect on NAFLD, which resulted in the improvement of hepatic injury and lipid accumulation in NAFLD model rats.Metformin was used as the positive control in our study.There was no significant difference in liver function and lipid content between JGXZ and metformin-treated rats.Pharmacological studies demonstrate that many herbs and their ingredients in JGXZ are effective for the treatment of NAFLD and IR.Danshen (Salvia Miltiorrhiza Bunge)aqueous extract modulates lipid metabolism and inhibits oxidative stress in NAFLD model rats [27].Alisol A 24-acetate in Zexie (Rhizoma Alismatis)stimulates autophagy to ameliorate NAFLD via the AMPK/mTOR pathway [28].Astragalus polysaccharides in Huangqi (Astragalus Membranaceus) attenuate IR by regulating microRNA-203-3p in the liver [29].Further studies should be carried out to investigate the effects of the herbal ingredients and drug pairs in JGXZ on NAFLD to compare the therapeutic effects of the herbs and ingredients in JGXZ to the prescription as a whole.
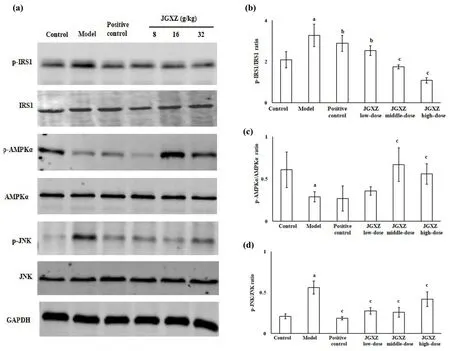
Figure 4 JGXZ treatment regulated the phosphorylation of IRS1,AMPKα and JNK in NAFLD model rats
Based on the close relationship between IR and NAFLD, we studied whether JGXZ could reduce IR in NAFLD model rats.IR is accompanied by an increase in blood glucose, HOMA-IR, and AUC in OGTT [30].IRS1 is an important factor during the signal transduction of insulin.IRS1 is a substrate of the insulin receptor kinase and activated by the phosphorylation of serine sites [31].Increased expression of p-IRS1 decreases the sensitivity of insulin transduction [32].The inhibition of IRS1 phosphorylation represents a therapeutic target to improve IR [33].In line with our previous clinical studies [10], this study revealed that JGXZ decreased the levels of fasting blood glucose, HOMA-IR, and AUC and inhibited the phosphorylation of IRS1 in NAFLD model rats, which indicated a significant effect of JGXZ on IR.No significant differences in IR indexes were observed between JGXZ and metformin-treated rats.
Considering the significance of the AMPK/JNK pathway in NAFLD and IR, we studied the effects of JGXZ on the AMPK/JNK pathway.JNK is a highly conserved serine/threonine kinase in the mitogen-activated protein kinase superfamily.JNK is phosphorylated and activated by high levels of glucose and lipid accumulation [34].Activation of JNK induces the phosphorylation of IRS1 to trigger IR[22].The phosphorylation of JNK increases in IR and NAFLD [35].AMPK, a heterotrimer complex that comprises one catalytic subunit (AMPKα) and two regulatory subunits (AMPKβ and AMPKγ), is crucial for regulating cellular energy metabolic processes[36].AMPK is activated by the phosphorylation of an amino acid residue (T172) on the AMPKα subunit [26].Activation of AMPK inhibits JNK activation, which inhibits the activation of IRS1, reducing IR and lipid accumulation.This study showed that JGXZ regulated the hepatic AMPK/JNK pathway in NAFLD model rats, which reflected the induction of AMPK phosphorylation and inhibition of JNK phosphorylation.
Our results also showed that metformin did not induce the phosphorylation of AMPK but inhibited the phosphorylation of JNK; these results do not agree with those of previous studies stating that metformin can activate AMPK[37].Metformin is commonly used to improve IR and maintain the homeostasis of blood lipids.The mechanisms underlying the effects of metformin on IR mainly involve the enhancement of the sensitivity to insulin and the inhibition of the glycogenolysis process [37].Recent studies show that the modulation of the gut microbiota is an important mechanism by which metformin acts on IR and NAFLD [23].Metformin decreases the abundance ofBacteroides fragilisin patients with NAFLD and IR[24].In addition, the gut microbiota trigger glucose intolerance and IR by activating TLR4-associated endoplasmic reticulum stress and JNK [25].The reason metformin did not induce AMPK phosphorylation but inhibited JNK phosphorylation in our study might be related to its interactions with the gut microbiota.However, the detailed mechanisms underlying the effects of metformin on NAFLD need to be studied further.
Conclusion
This study showed that JGXZ improved liver function and reduced steatosis of the hepatocytes in NAFLD model rats.Moreover, JGXZ improved IR in NAFLD model rats.The possible mechanism of JGXZ on NAFLD and IR was by modulating the AMPK/JNK pathway.
杂志排行
Traditional Medicine Research的其它文章
- The dynamic changes and mechanisms of Rehmanniae radix processing based on Maillard reaction
- Investigation of in vitro antioxidant activity of dihydromyricetin and flavonoids rich extract from vine tea(Ampelopsis grossedentata)
- Advances in anti-inflammatory and immunoregulatory mechanisms of sinomenine
- Hua-Zhuo-Kai-Yu decoction inhibits apoptosis in nonalcoholic fatty liver disease
- Qiming granule in treating type 2 diabetic kidney disease patients:study protocol for a randomized controlled trial
- Bibliometric analysis of acupuncture research through the Web of Science database from 1990 to 2019
