Qiming granule in treating type 2 diabetic kidney disease patients:study protocol for a randomized controlled trial
2021-12-04JianHuoJunGuoDuanXueJingLuFuWenZhangWenYuanJianJiaQingSuiJiaLingAiLiShaLiu
Jian Huo,Jun-Guo Duan,Xue-Jing Lu,Fu-Wen Zhang,Wen-Yuan Jian,Jia-Qing Sui,Jia-Ling Ai,Li-ShaLiu
1College of Ophthalmology, Chengdu University of Traditional Chinese Medicine, Chengdu 610075, China.2Chengdu Women's and Children's Central Hospital, School of Medicine, University of Electronic Science and Technology of China,Chengdu 611731,China.3Ineye Hospital,Chengdu 610084,China.
Abstract
Background: Diabetic kidney disease (DKD) is a chronic renal microvascular complication associated with abnormal glucose metabolism.According to traditional Chinese medicine (TCM) theory, Qi and Yin deficiency with blood stasis(the name of TCM symptoms,its main clinical features are fatigue,dry mouth,red or pale tongue,weak pulse, etc.) is the primary TCM syndrome of DKD, and Qiming granule (QMG) is suitable for the treatment of Qi and Yin deficiency with blood stasis syndrome.In view of this, we designed a randomized controlled trial to assess whether QMG is efficacious and safe in treating DKD patients.Methods:This protocol is for a randomized,double-blind, placebo-controlled, parallel group, six-centre clinical trial.A total of 180 participants will be randomized into the QMG group or placebo group, with a 1:1 ratio.The study will last for 50 weeks, including a 2-week run-in period, 24 weeks of intervention, and 24 weeks of follow-up.The experimental intervention will be QMG, and the control intervention will be a placebo.The primary outcome will be the 24h urinary albumin excretion ratio and the change in the albumin-to-creatinine ratio.The secondary outcome will be evaluation of renal function, fundus changes, management of blood lipids, TCM symptom improvement and safety assessments.Adverse events will be recorded during the trial.Discussion:This study is a randomized controlled trial to test the effectiveness and safety of QMG for DKD patients.The findings of this study will help to provide evidence-based recommendations in treating DKD patients.Trial registration: Chinese Clinical Trial Registry,ChiCTR-TRC-12002953.Registered 23 December 2012.
Key words:Diabetic kidney disease,Qiming granule,Traditional Chinese medicine,Randomized controlled trial
Background
Diabetic kidney disease (DKD) is a chronic renal microvascular complication associated with abnormal glucose metabolism [1], which has become a common cause of end-stage renal disease and is one of the main causes of death in patients with diabetes [2].Diabetic retinopathy (DR) often precedes DKD, and has been used by the National Kidney Foundation Kidney Disease Outcomes Quality Initiative Guidelines as a diagnostic base for DKD in type 2 diabetic patients[3].Based on its unique theoretical basis and treatment methods, traditional Chinese medicine (TCM) has the potential to prevent further development and deterioration of the disease and delay the progression of the disease in patient with DKD.
Treatment based on syndrome differentiation is the basic principle of understanding and treating diseases in TCM and a special research and treatment method of diseases in TCM [4].Qi and Yin deficiency with blood stasis (the name of TCM symptoms, its main clinical features are fatigue, dry mouth, red or pale tongue, weak pulse, etc.) is the primary TCM syndrome of DR and DKD [5, 6].An empirical formula of Chinese medicine, Qiming granule (QMG),is the first national approved drug of Chinese medicine for treating non-proliferative diabetic retinopathy(NPDR).QMG, made by 2 famous TCM Doctors Pin-Zheng Liao, the great master of national medicine,and Jun-Guo Duan and manufactured by Zhejiang Wansheng Pharmaceutical Co., Ltd.(Register No:Z20090036), is now suitable for the treatment of NPDR.In addition, based on syndrome differentiation in TCM, QMG is suitable for Qi and Yin deficiency with blood stasis syndrome[7-9].
QMG consists of 8 traditional plants, namely Huangqi (Astragalus membranaceus(Fisch.) Bge.),Gegen (Pueraria lobata(Willd.) Ohw), Dihuang(Rehmannia glutinosaLibosch.), Juemingzi (Cassia obtusifoliaL.), Gouqizi (Lycium barbarumL.),Chongweizi (Leonurus japonicusHoutt.), Puhuang(Typha angustifoliaL.) and Shuizhi (Whitmania pigraWhitman).Astragalus saponin and puerarin are the major components of QMG [10].In TCM, Huangqi(Astragalus membranaceus(Fisch.) Bge.) is viewed as an immune-modulating herb, and the main active component is astragalus saponin.Huangqi (Astragalus membranaceus(Fisch.) Bge.) could exert therapeutic effects in DKD patients that consist of reducing urine protein and improving renal function [11-13].In addition, puerarin is the primary active component of Gegen (Pueraria lobata(Willd.) Ohw) [14], which could attenuate early diabetic kidney injury by down-regulation of matrix metalloproteinase 9[15].
Based on the TCM theory, assuming that QMG could be used to treat DKD,we designed a randomized controlled trial to assess whether QMG is efficacious and safe in treating DKD patients.
Methods
Study design,setting and participants
This will be a randomized, double-blind,placebo-controlled,parallel group,multi-centre clinical trial.The study will last for 50 weeks, including a 2-week run-in period,24 weeks of intervention,and 24 weeks of follow-up.Investigators, the statistician and participants will be blinded (Figure 1).Six hospitals of China will participate in the study: The Affiliated Hospital of Chengdu University of TCM, Chengdu;The Affiliated Hospital of Nanjing University of TCM,Nanjing; The Six People's Hospital Affiliated with Shanghai Jiao Tong University, Shanghai; Beijing Tongren Hospital, Capital Medical University,Beijing;West China Hospital of Sichuan University, Chengdu;and The People's Hospital of Guangxi Zhuang Autonomous Region, Nanning.We plan to enrol 180 patients diagnosed with type 2 DKD.
Eligibility criteria
Diagnostic criteria.Type 2 diabetes will be defined by the American Diabetes Association criteria [16].DR diagnosis criteria will be based on the international clinical DR severity scale [17].The diagnosis of DKD will be based on the National Kidney Foundation Kidney Disease Outcomes Quality Initiative Guidelines [3].Estimated glomerular filtration rate(eGFR)will be calculated using the following equation[18]:186×serum Cr−1.154×age−0.203×0.742(if female)×1.233.
Diagnostic criteria of TCM of Qi and Yin deficiency with blood stasis syndrome will be based on the Guiding Principles of Clinical Research on New Drug of Traditional Chinese Medicine [19]: primary symptoms include turbid urine and blurred vision.Secondary symptoms include fatigued spirit and lack of strength, spontaneous sweating, swollen face and limbs; vexing heat in chest, palms and soles, thirsty,night sweats, constipation; soreness of the low back and knees, dizziness, tinnitus; red tongue with scant liquid, and thready or weak pulse.When participants have all the primary symptoms and meet at least one symptom in the four groups of secondary symptoms,the participants will be diagnosed with Qi and Yin deficiency with blood stasis syndrome
Inclusion criteria
1.Type 2 diabetes.
2.Diagnosis of DR(moderate to severe NPDR).
3.Diagnosis of DKD in phase II,III,IV.
4.Meet the diagnostic criteria of TCM syndromes.
5.Best corrected visual acuity (BCVA) ≥15 early treatment diabetic retinopathy study visual chart characters (tested at 4 meters, visual acuity is equivalent to a score of 20/125,decimal 0.16).
6.Hemoglobin A1c(HbA1c)≤9.0%.
7.Blood pressure ≤140/90 mmHg.
8.Age between 35 and 75 years.
9.Volunteer subjects sign the informed consent, the process of obtaining informed consent complies with good clinical practice regulations.
Exclusion criteria
1.Inspection confirmed that nephropathy is caused by systemic disease such as primary hypertension.
2.Proliferative DR patients.
3.Eyes having been treated with panretinal photocoagulation.
4.Refractive media turbidity difficult to evaluate fundus image.
5.Combined with severe heart disease (those with chronic heart failure New York grading standard of cardiac function above level 3, severe arrhythmia), abnormal liver function (alanine transaminase, aspartate aminotransferase ≥ 1.5 times the upper limit of normal value), and hematopoietic system disease(moderate or higher anemia).
6.Allergy to the known constituents of experimental drugs or physical allergies.
7.With urologic diseases like glomerulonephritis,idiopathic nephrotic syndrome.
8.With severe infections, diabetic ketoacidosis,hyperkalaemia.
9.With severe ocular diseases such as age-related macular degeneration, glaucoma and optic neuropathy.
10.Women preparing to be pregnant, pregnant or lactating.
11.Severe mental disorders.
12.Participants in other clinical trials in the past 3 months.
The eye diseases involved in the inclusion and exclusion criteria are in monocular units, that is, for patients in whom one eye meets the criteria, that eye can be used as the target eye to observe the object.If both eyes match, the researcher determines the target eye.
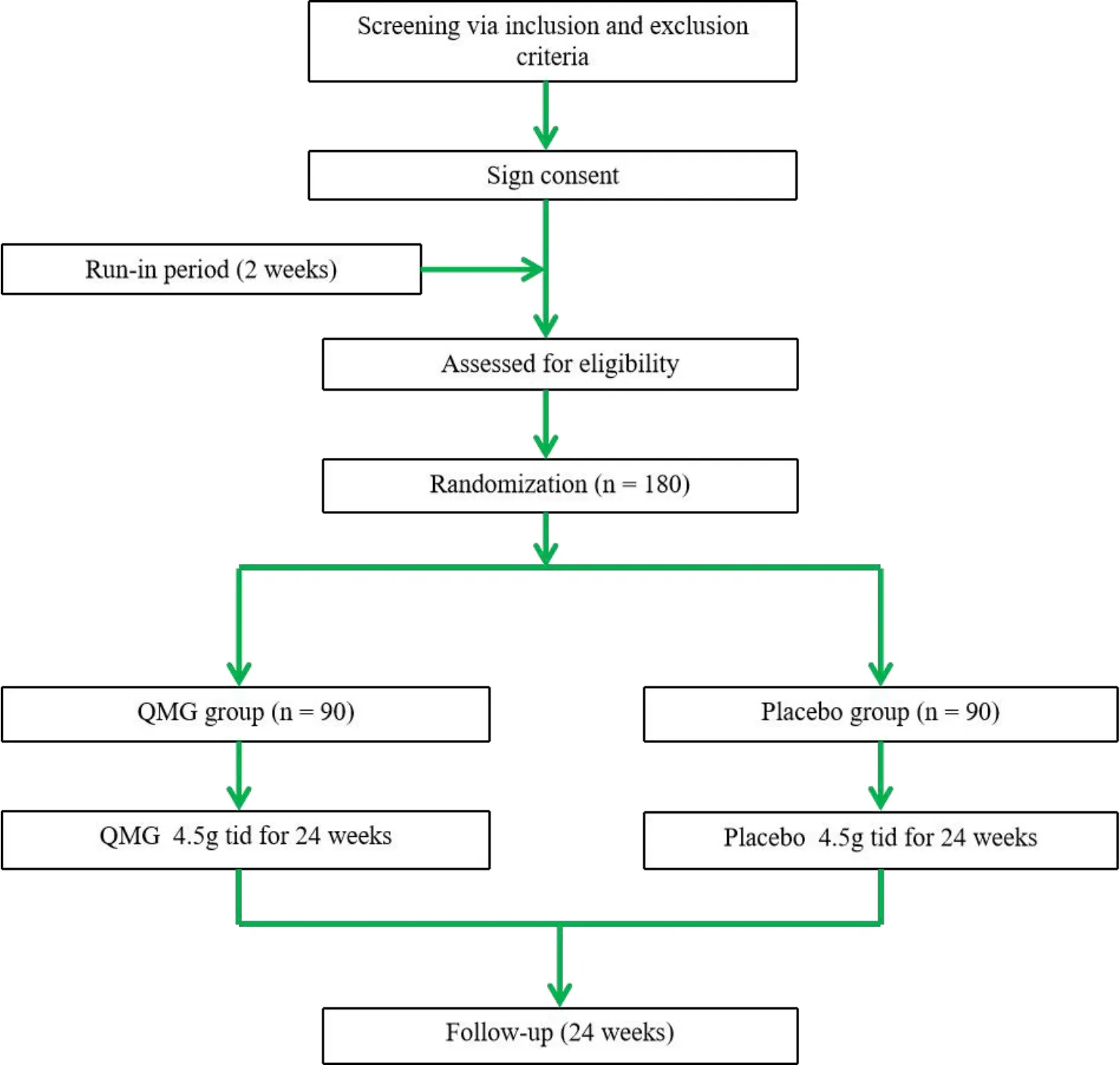
Figure 1 Flow chart of clinical trial.QMG,Qiming granule.
Withdrawal criteria
1.Serious adverse events, serious complications and special physiological changes are not appropriate for further trials.
2.Poor adherence of subjects, affecting effectiveness and safety judgement.
3.For whatever reason, the subject is unwilling or unable to continue the clinical study and the subject submits a request to withdraw from the study.
4.Subjects do not explicitly withdraw from the study, but are no longer receiving medication and testing and are lost to follow-up.
Randomization and blinding
Central block randomization method will be applied in this study.Eligible participants will be randomly assigned to the QMG group or placebo group at a 1:1 allocation.Random numbers will be generated by an independent third party (the Brightech Clinical Information Management System (Brightech CIMS),Chengdu BM Clinical Researches Ltd,www.bmclinsys.com).Then randomization codes made by the third party will be labelled to the packages of the QMG and placebo by the manufacturer.Participants will be assigned to receive QMG or placebo in this sequence.The randomization codes and group allocation will be stored by the principle investigator and the third party.All participants and researchers will be unaware of the drug allocation.Both QMG and placebo are supplied by Zhejiang Wansheng Pharmaceutical Co., Ltd., Zhejiang, China.The internal and external packages, colour, taste,dosage form and size of the QMG and placebo are identical.
Intervention
Trial medicine treatment.Eligible participants in each centre will be randomized to either the QMG or placebo group, receiving either QMG or placebo 4.5 g 3 times daily for 24 weeks.
Basic treatment.Establish unified basic treatment principles based on the 2012 American Diabetes Association Practice Guidelines:
1.Participants will receive health education, such as proper diet and exercise instructions.
2.Control blood glucose: biguanide drugs(preferably metformin) are used in participants with obesity and/or) eGFR above 60 mL/min/1.73 m2; glinides drugs (preferably repaglinide) are used in participants with non-obesity and/or eGFR below 60 mL/min/1.73 m2.When the blood sugar control is not ideal, choose 2 oral drugs or supplement insulin.HbA1c should be controlled below 9.0%.
3.Control blood pressure: angiotensin-converting enzyme inhibitors/angiotensin receptor blockers will be the recommended drugs.For participants whose blood pressure cannot be controlled at 140/90 mmHg by angiotensin-converting enzyme inhibitors/angiotensin receptor blockers,non-dihydropyridine calcium channel blockers,diuretics and/or beta blockers will be added.Blood pressure in type 2 diabetic patients should be controlled at ≤ 140/90 mmHg.
4.Control blood lipids: choose statins or fibrates based on lipid profile (atorvastatin, simvastatin,fenofibrate).
Outcome measurements
During the intervention period, participants need to visit every 4 weeks, and during the follow-up period,visits will be required at the 28th, 36thand 48thweeks(Table 1).
Primary outcome measures.The primary outcome measures are the 24h urinary albumin excretion ratio and the albumin-to-creatinine ratio.The change in the urinary albumin excretion ratio and albumin-to-creatinine ratio from baseline to 24 weeks will be compared between the QMG and placebo group.
Secondary outcome measures.Secondary outcome measures are as follows:
1.Serum creatinine, eGFR, 24h urinary protein, and fasting plasma glucose will be assessed every 4 weeks, while HbA1c will be assessed at weeks 12 and 24.
2.The change in area of the retinal haemorrhage,retinal hard exudation and soft exudation, retinal thickness and BCVA.All of these will be measured at weeks 12 and 24, except for BCVA,which will be measured every 4 weeks.
3.TCM symptoms score (Table 2) will be measured every 4 weeks.
4.Change in blood lipid profiles will be assessed at baseline, at week 12, and at week 24.It will include total cholesterol, triglycerides,high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol(LDL-C).
5.Safety assessment will be accomplished at the 12thand 24thweeks, which will include the results of routine blood and urine, alanine transaminase,aspartate aminotransferase, serum total bilirubin and electrocardiogram.Furthermore, urine-human chorionicgonadotropin must be conducted before enrolling a female patient of childbearing age.
Efficacy criteria
The efficacy criteria is based on the evaluation of the efficacy of the European angiotensin-converting enzyme inhibition in progressive renal insufficiency study [20] and the criteria developed by the Diabetes Committee of the Chinese Society of Traditional Chinese Medicine[21].
Clinical control: 24h ultrasonic assisted extraction(UAE) <30 mg, normal renal function; excellence effective: 24h UAE reduction ≥50% before treatment,normal renal function; effective: 24h UAE reduction ≥20% before treatment, the renal function is normal or almost normal(with a difference of less than 15%from the normal value);invalid:failure to reach the target.
Adverse events
Any adverse events that occurred during the study should be filled in the case report form, and follow-up investigations should be made to record the treatment process and results in detail until the laboratory tests return to normal and symptoms and signs disappear.Follow-up methods can be based on the severity of adverse reactions, such as inpatient, outpatient, home visit, telephone, communication and other forms.Serious adverse events should be immediately recorded in the "Serious Adverse Event Report Form"and must be reported to the Drug Safety Supervision Department of the State Drug Administration, the Provincial Drug Administration, the Research Unit Ethics Committee and Research Unit within 24 hours.

Table 1 Schedule of enrollment,allocation,visits,and assessments

Table 2 TCM symptom scoring standard

Table 2 TCM symptom scoring standard(continued)
Quality control
A total of 6 units participated in this multicenter clinical study, organization management and quality control are the keys to the success of the project research.Therefore,the research will strictly screen the participating units and participants; a project executive committee, data security monitoring committee,monitoring working group and other organizations;before the start of the study, further improve the research design, strengthen training, focus on the process, dynamic and real-time collection of research data,statistical experts participate in the whole process but work independently.
Sample size calculation
Based on previous similar herbal studies in DKD [22,23] and the findings of QMG in DR [7], we assume that the excellence rate in the QMG group is 40.2%and 18.5% in the placebo group.The sample size is calculated as follows: n = (Ua+Ub)2(1 + 1/k)P(1 −P)/(P1-P0)2.With a significance level of 0.05 and power of 0.9, k = 1, a sample size of 75 in each group will be required.Assuming a dropout rate of 20%, 180 participants will be required for the 2 groups.
Statistical analysis
This study will use SAS 9.4 and SPSS 17.0 software for statistical analysis.For the statistical analysis of the main variables, the data of the full analysis set and per protocol set will be selected for analysis.Safety will be evaluated by safety set, which including those who have received at least one treatment after randomization and have data of safety assessment.Statistical significance will be defined asP< 0.05.The mean ± standard deviation will be used for continuous variables, paired sample t tests will be used to perform comparisons in each group,and two-sample t tests will be used to perform comparisons between the 2 groups.Categorical variables will be expressed as number and percentage and will be analysed by the Cochran-Mantel-Haenszel test.The incidence of adverse events will be analysed by the chi-squared test or Fisher's exact test.
Discussion
In clinical practice, diabetic microvascular complications such as DR and DKD are often seen at the same time.However, previous studies have mostly focused on a single disease, and clinical evaluation studies of TCM intervention in the presence of DR and DKD have not yet been conducted.According to the clinical characteristics of DR and DKD which are often combined at the same time and are mutually risky as well as Qi and Yin deficiency is the key pathogenesis of DR and DKD, we have established a treatment idea for simultaneous TCM intervention in patients with DR and DKD.
QMG, a Chinese patent drug, is the first national approved drug of Chinese medicine for treating NPDR.In a 3-month, randomized, controlled, double-blind,multicenter clinical trial, with 212 eyes from 212 patients with NPDR (QMG group, 107 patients;doxium-controlled group, 105 patients), QMG was more effective than doxium with respect to the excellence rate and total effective rate of the TCM.Therefore,the QMG may be superior to doxium tablets in treating NPDR [7].A favorable effect of QMG in the treatment of DR that was observed by analyzing fluorescence fundus angiography after 3 months of medication in a multicenter randomized controlled trial study (360 DR patients) was achieved via improving retinal blood circulation, indicating that QMG might alleviate retinal hypoxia and ischemia by increasing retinal blood flow and improving blood circulation[24].
QMG is recommended for treating symptoms including blurred vision, dry eyes, fatigued spirit and lack of strength, vexing heat in the chest, palms and soles, spontaneous sweating and night sweating, thirst,constipation, soreness of the low back and knees,dizziness, and tinnitus.DKD patients with Qi and Yin deficiency with blood stasis syndrome may share the same TCM syndrome.Based on the TCM thought of dialectical treatment, we speculate that QMG for the treatment of DR might be equally effective for DKD.Thus, we have designed this trial of QMG in DKD patients with Qi and Yin deficiency with blood stasis syndrome to test this hypothesis.The findings of this study will also provide sound evidence to assess the efficacy and safety of QMG as a complementary and alternative treatment option for DKD patients.
Trial status
The trial is currently recruiting participants.
Ethics approval and consent to participate
Our study is granted by the Sichuan Regional Ethics Review Committee on Traditional Chinese Medicine(Approval No.2012KL-001).Subjects are all obliged to sign the informed consent to confirm their participation.
杂志排行
Traditional Medicine Research的其它文章
- The dynamic changes and mechanisms of Rehmanniae radix processing based on Maillard reaction
- Investigation of in vitro antioxidant activity of dihydromyricetin and flavonoids rich extract from vine tea(Ampelopsis grossedentata)
- Advances in anti-inflammatory and immunoregulatory mechanisms of sinomenine
- Hua-Zhuo-Kai-Yu decoction inhibits apoptosis in nonalcoholic fatty liver disease
- Bibliometric analysis of acupuncture research through the Web of Science database from 1990 to 2019
- Jian-Gan-Xiao-Zhi decoction ameliorates high-fat high-carbohydrate diet-induced non-alcoholic fatty liver disease and insulin resistance by regulating the AMPK/JNK pathway
