Hua-Zhuo-Kai-Yu decoction inhibits apoptosis in nonalcoholic fatty liver disease
2021-12-04YuTingLiHuanTianCuiLuYangLuLuJinYuMingWangXueQianDongWeiBoWenHongWuWangZhaiYiZhang
Yu-Ting Li, Huan-Tian Cui, Lu Yang, Lu-Lu Jin, Yu-Ming Wang, Xue-Qian Dong, Wei-Bo Wen, Hong-Wu Wang,Zhai-Yi Zhang
1College of Traditional Chinese Medicine, Tianjin University of Traditional Chinese Medicine, Tianjin 301617, China;2Shandong Provincial Key Laboratory of Animal Cell and Developmental Biology, School of Life Sciences, Shandong University, Qingdao 250100, China; 3Graduate School, Tianjin University of Traditional Chinese Medicine, Tianjin 301617,China; 4Department of Endocrinology in Yunnan Provincial Hospital of Traditional Chinese Medicine, Kunming 650021,China; 5College of Integrated Chinese and Western Medicine, Tianjin University of Traditional Chinese Medicine, Tianjin 301617,China.
Abstract
Background:Hua-Zhuo-Kai-Yu decoction(HZKY)is an empirical formula in traditional Chinese medicine that is derived from the classic ancient prescription Da-Chai-Hu decoction.It has been demonstrated to have good clinical effects on nonalcoholic fatty liver disease (NAFLD).However, the mechanism by which HZKY acts on NAFLD remains unclear.In this study,network pharmacology was used to predict the potential targets of HZKY in NAFLD.Additionally, in vivo studies were conducted to validate the crucial pathways determined using network pharmacology.Methods: Active compounds in HZKY were screened using the Traditional Chinese Medicine Systems Pharmacology and Analysis Platform and Traditional Chinese Medicine Integrated Database, and the potential targets of compounds in HZKY were predicted using Traditional Chinese Medicine Systems Pharmacology and Analysis Platform, Traditional Chinese Medicine Integrated Database, Bioinformatics Analysis Tool for Molecular mechANism of Traditional Chinese Medicine, and PUBCHEM.In addition,targets involved in NAFLD were obtained from the GeneCards and Online Mendelian Inheritance in Man databases,and the potential targets of HZKY in NAFLD were identified based on the common potential targets between HZKY and NAFLD.Cytoscape 3.7.2 was used to visualize crosstalk and identify the key genes from the potential targets of HZKY in NAFLD.Kyoto Encyclopedia of Genes and Genomes analysis was conducted to predict the pathways by which HZKY acts on NAFLD.Rats were fed with a high-fat diet for 12 weeks to induce NAFLD and were then orally administered HZKY.Serum lipid levels and hematoxylin and eosin and oil red O staining results were assessed to determine the effects of HZKY in NALFD.Furthermore, the mechanisms of action of HZKY in NAFLD, as determined using network pharmacology, were validated based on the inhibition of apoptosis in the liver using Western blotting.Results:A total of 269 potential targets of 130 active compounds in HZKY were identified(oral bioavailability ≥30% and drug-like ≥0.18), and 62 targets were selected after being compared with the targets of NAFLD.Bcl-2-associated X protein (BAX), caspase3 (CASP3), and caspase9 (CASP9) were the key genes with the highest values of network connectivity.In addition, 45 Kyoto Encyclopedia of Genes and Genomes pathways,including apoptosis, fatty acid synthesis, and estrogen signaling, were enriched according to the selected genes of HZKY.In vivo studies showed that the serum levels of total cholesterol, triglyceride, and low-density lipoprotein cholesterol were significantly elevated and the serum level of high-density lipoprotein cholesterol was decreased in the model group compared with those in the control group(P <0.01 for all).The expressions of BAX,CASP9,and CASP3 were upregulated in the model group compared with those in the control group(P <0.05,P <0.01,and P <0.01, respectively), while HZKY treatment decreased the body weights and serum levels of total cholesterol,triglyceride, and low-density lipoprotein cholesterol and increased the serum levels of high-density lipoprotein cholesterol in NAFLD model rats(P <0.05,P <0.01,P <0.05,and P <0.05,respectively).Hematoxylin and eosin and oil red O staining indicated that HZKY treatment reduced steatosis in the hepatocytes.Moreover, HZKY treatment inhibited apoptosis in the liver by downregulating the expressions of BAX, CASP3, and CASP9 (P <0.05, P <0.01,and P <0.05,respectively).Conclusion:Our study demonstrates that HZKY improves NAFLD by inhibiting apoptosis in the liver by reducing the levels of BAX,CASP3,and CASP9.
Key words:Network pharmacology,Hua-Zhuo-Kai-Yu decoction,Nonalcoholic fatty liver disease,Apoptosis
Backgro und
Nonalcoholic fatty liver disease(NAFLD)is caused by metabolic liver injury accompanied by extensive hepatic steatosis.Epidemiological studies have indicated that the morbidity of NAFLD is approximately 6.3%-45% in adults and 15% in developed areas such as Beijing, Shanghai, and Guangzhou in China [1, 2].Long-term NAFLD can cause chronic metabolic diseases such as diabetes,liver fibrosis, liver cirrhosis, and hepatocellular carcinoma [3, 4].Currently, clinical treatment for NAFLD includes administration of insulin sensitizers,antioxidants, and lipid-lowering drugs.However, these drugs have been shown to have multiple side effects.Lipid-lowering drugs can trigger the dysfunction of hepatic enzymes [5].Insulin sensitizers and antioxidants have been shown to induce water-sodium retention and increase the risk of cardiac diseases[6].
Traditional Chinese medicine (TCM) has been demonstrated to have better treatment effects on NAFLD.Ling-Gui-Zhu-Gan decoction, which is a classic ancient TCM prescription, can attenuate high-fat diet (HFD)-induced NAFLD by improving insulin resistance (IR) and regulating lipid metabolism-related pathways [7].The empirical formula of the TCM Huo-Xue-Xiao-Zhi decoction can decrease tumor necrosis factor-α, transforming growth factor, and free fatty acid levels and improve hepatic function in patients with NAFLD [8].Additionally, the empirical formula of the TCM Yi-Qi-Huo-Xue-Qu-Tan can modulate the dysfunction of lipid metabolism in these patients[9].
The classic ancient prescription Da-Chai-Hu (DCH)decoction (Chaihu (Bupleuri Radix), Huangqin(Scutellariae Radix), Shaoyao (Paeoniae Radix Alba),Banxia (Pinelliae Rhizoma), Shengjiang (Zingiberis Rhizoma Recens),Zhishi(Aurantii Fructus Immaturus),Dazao (Jujubae Fructus)) is described in the ancient book on Chinese medicine titledShanghan Zabing Lun(Treatise on Cold Damage Diseases,25 C.E.-220 C.E.), written by Zhang Zhongjing.According to the book,DCH is used to treat diseases of the liver,gallbladder, and pancreas.Recent studies have also demonstrated the hepatoprotective, cholagogue,anti-inflammatory, and lipid-lowering effects of DCH[10, 11].In addition, clinical studies have shown that DCH improves dyslipidemia in patients with NAFLD[12].Hua-Zhuo-Kai-Yu (HZKY) decoction, derived from a modification of DCH, was established by Doctor Wang Hongwu at the Tianjin University of Traditional Chinese Medicine.It has shown obvious clinical effects on NAFLD.However, the mechanisms of action of HZKY in NAFLD remain unknown.
Network pharmacology, developed by Hopkins AL in England, is the study of the relationships among drugs, diseases, genes, and targets based on the combination of subject theory, including systematic biology, pharmacology, and multiple “omics” studies such as genomics and proteomics [13-16].The application of network pharmacology in studying the mechanisms of TCM is in line with the characteristics of TCM, including a systematic and holistic perspective with multiple components.It is developing into an effective tool for identifying the overall mechanisms of TCM drugs[17].In this study,network pharmacology was used to predict the potential targets of HZKY in NAFLD.Furthermore, rats were fed with HFD for 12 weeks to induce NAFLD and were then administered HZKY orally.Serum lipid levels and hematoxylin and eosin (H&E) and oil red O staining results were assessed to observe the effects of HZKY in NALFD.Moreover, the mechanisms of action of HZKY in NAFLD, as determined using network pharmacology, were validated using Western blotting,which showed apoptosis inhibition in the liver.
Materials and Methods
Network Pharmacology Study
Identification of the main active compounds.HZKY is composed of Chaihu 12 g (Bupleuri Radix),Huangqin 9 g (Scutellariae Radix), Shaoyao 9 g(Paeoniae Radix Rubra), Zhishi 9 g (Aurantii Fructus Immaturus), Banxia 9 g (Pinelliae Rhizoma), Dahuang 6 g (Rhei Radix et Rhizoma), Bohe 9 g (Menthae Haplocalycis Herba), Heye 9 g (Nelumbinis Folium),and Peilan 9 g (Eupatorii Herba).The main compounds in HZKY were screened using the Traditional Chinese Medicine Systems Pharmacology and Analysis Platform (TCMSP)(http://tcmspw.com/tcmsp.php) and Traditional Chinese Medicine Integrated Database (TCMID)(http://www.megabionet.org/tcmid).The repeated compounds were counted only once.
Because of the oral administration of HZKY for the treatment of NAFLD, oral bioavailability (OB) was regarded as the pharmacokinetic parameter for HZKY to be considered in the network pharmacology evaluations.The compounds in HZKY (OB ≥30%),which were considered to be absorbed, metabolized,and functioning in the human body,were chosen as the candidate compounds [18].Then, the structural and physiochemical properties of candidate compounds in HZKY were identified using the DrugBank(https://www.drugbank.ca/) database.Compounds that were drug-like (DL) ≥0.18 were identified as the main active compounds of HZKY[19,20].
Potential target prediction of active compounds.The potential targets for active compounds in HZKY were screened using TCMSP, TCMID, Bioinformatics Analysis Tool for Molecular mechANism of Traditional Chinese Medicine (BATMAN-TCM), and PUBCHEM (https://pubchem.ncbi.nlm.nih.gov)databases.The repeated targets were counted only once.
Potential targets for prediction of the disease.Targets related to NAFLD were obtained from GeneCards (https://www.genecards.org/) and online Mendelian Inheritance in Man (OMIM)(https://www.omim.org/) databases.The protein data bank IDs for each target were imported into the Uniprot database (http://www.uniprot.org/) for standardization,and the repeated targets were removed.In addition, the potential targets of HZKY in NAFLD were identified based on the common potential targets between HZKY and NAFLD.
Biological function and signaling pathway analysis.The Kyoto Encyclopedia of Genes and Genomes(KEGG) database was used to integrate the information and functions of potential targets.Briefly,the potential targets were analyzed and enriched in the KEGG database (https://www.kegg.jp/).KEGG terms withP<0.05 and q <0.05 were screened.
Establishment and analysis of the compound-target-pathway network.The relationships among compounds,targets,and pathways of HZKY in NAFLD were visualized using the compound-target-pathway network using Cytoscape 3.7.2 [21].According to the compound-target-pathway network, compounds, targets, and pathways were visualized using different colors and were combined based on their potential interactions.
Experimental Validation
Reagents.HFD (17.7% sucrose, 17.7% fructose,19.4% protein, and 40% fat) was purchased from Beijing HFK Bioscience Co., Ltd.(Beijing, China).A BCA protein assay kit and triglyceride (TG), total cholesterol (TC), low-density lipoprotein cholesterol(LDL-C), and high-density lipoprotein cholesterol(HDL-C) test kits were purchased from Nanjing Jiancheng Biological Engineering Institute (Nanjing,China).Oil red O staining kit was obtained from Solarbio Biotechnology Co., Ltd.(Beijing, China).Primary antibodies against rat caspase-3 (CASP3;ab13847; 1:500), caspase-9 (CASP9; ab184786;1:1000), BAX (Bcl-2 associated X protein; ab32503;1:10000), and β-ACTIN (ab179467 1:5000) were purchased from Abcam, Inc.(Shanghai, China), and the corresponding secondary antibodies were obtained from Abcam,Inc.(Shanghai,China).
Animals.Male Sprague-Dawley (SD) rats weighing 190-210 g, were purchased from Weitonglihua Animal Co.,Ltd.(Beijing,China).All animals were housed for 1 week in a temperature- and humidity-controlled environment (12-h light/dark cycle, 21 ± 2℃, and a relative humidity of 45 ± 10%) in groups of five rats/cage and were allowed feed and water ad libitum.
Induction of the NAFLD model rat.The NAFLD model was induced as described previously [22].Briefly, the rats were fed with HFD diet for 12 weeks.Their body weights and serum TG, TC, LDL-C, and HDL-C levels were measured to evaluate the induction of NAFLD.In addition, H&E and oil red O staining were used to observe the pathological changes in the rat livers.All animal experiments were approved by the Institutional Animal Care and Use Committee of Tianjin University of Traditional Chinese Medicine(SYXK2019-0006) and were performed in accordance with the National Institutes of Health “Guide for the Care and Use of LaboratoryAnimals”.
Preparation of the HZKY Test Solution.The HZKY decoction was provided by the First Affiliated Hospital of Tianjin University of Traditional Chinese Medicine(Tianjin, China).The herbs included in the HZKY decoction were identified by the pharmacist in the pharmacy of the First Affiliated Hospital of Tianjin University of Traditional Chinese Medicine.The water extract of HZKY was concentrated at the laboratory of Tianjin University of Traditional Chinese Medicine.Briefly, the herbs were immersed in water for 30 min(the surface of the water covered the herbs by 2-3 cm)and boiled at 100°C for 20 min using a gallipot.The liquid was then filtered through gauze.The same volume of water and herbs were boiled for another 15 min and filtered through gauze.The two filtrates were mixed to obtain the water extract of HZKY [23].The water extract of HZKY was concentrated to a density of 0.85 g crude herb/mL under decompressed distillation using a DZF-6050 vacuum drying box(Beijing SanBo Technology Co., Ltd., Beijing, China)and SENCO R rotating evaporator (Beijing Biological Innovation Technology Co.,Ltd.,Beijing,China).
Animal grouping.After 1 week of adaptive feeding,30 rats were randomly classified into three groups:control, model, and HZKY groups.Rats in the control group were provided standard laboratory chow containing 59.4% total carbohydrate, 20% protein, and 4.8% fat.An NAFLD model rat was induced using HFD in the model and HZKY groups.Moreover, the HZKY group received an oral gavage of HZKY(8.5 g crude herb/kg rat weight) once daily for 8 weeks after 4 weeks of HFD feeding.The dosage of 8.5 g crude herb/kg in the HZKY group was equivalent to the daily dosage of HZKY in human adults, which was calculated using the following formula: equivalent dosage (g/kg) = clinical dosage of crude herbs in humans (g/kg) × 6.25.Meanwhile, the control and model group rats were given oral gavage of same volume of saline once daily for 8 weeks after 4 weeks of feeding.
Serum biochemical marker assays.Following HZKY treatment, rats were anesthetized (chloral hydrate intraperitoneal administration,0.3 mL/100 g rat weight)after a 5-h period of fasting, and blood was harvested from the inner canthus using a capillary glass tube.Serum was obtained by centrifugation with a SW 60 Ti rotor (Beckman Coulter Inc., Palo Alto, CA) at 3000 rpm for 15 min.Serum levels of TG, TC, LDL-C, and HDL-C were measured according to the manufacturer's instructions (Nanjing Jiancheng Biological Engineering Institute, Nanjing, China), and the absorbance was detected using a Varioskan Flash microplate reader(Thermo Fisher Scientific,USA).
In a 96-well plate, 2.5 μL of distilled water,TG and TC standards,and serum samples were mixed with 250 μL of working solution and incubated at 37°C for 10 min.The absorbance value of each pore was measured using a microplate reader at 510 nm.The derived optical density (OD) value of distilled water, TG and TC standards, and serum samples were denoted by OD1, OD2, and OD3, respectively.The TG and TC levels were calculated using the following formulae:
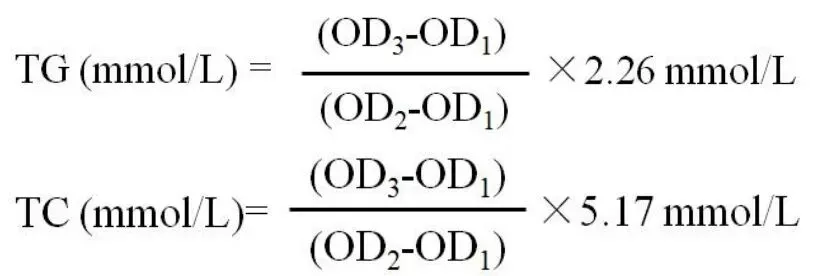
In accordance with the instructions for LDL-C and HDL-C, 2.5 μL of distilled water, LDL-C and HDL-C standards,and serum samples were incubated with 180 μL of reagent R1 supplied by kit at 37°C for 5 min in 96-well plates.After determining the absorbance value of a microplate reader at 546 nm, the OD values of distilled water, standards, and serum samples were recorded as OD1, OD2and OD3, respectively.Then, 60 μL of reagent R2 was added into each well, repeating the previous step, and the mixed solution was incubated at 37℃for 5 min.The absorbance value was detected by microplate reader at 546 nm.The OD values of distilled water,standards,and serum samples were recorded as OD1', OD2', and OD3', respectively.The LDL-C and HDL-C levels were obtained using the following formulae:
H&E staining.Rats were sacrificed after blood collection.The liver was immediately removed and fixed with 10%formalin,dehydrated,and embedded in paraffin wax.It was then cut into 5-µm sections using a slicing machine (RM2125, Leica, Buffalo Grove,USA) at the laboratory at Tianjin University of Traditional Chinese Medicine.Sections were stained with H&E as described previously [24].Briefly, after dewaxing, rehydration, staining, dehydration,transparency, and sealing, the pathological changes in the liver were visualized under a light microscope(BX50,Olympus America,Melville,NY,USA).
Oil red O staining.Fresh liver tissue was rapidly frozen in liquid nitrogen and sectioned using a frozen slicer (CM3050S, Leica, Buffalo Grove, USA) to obtain coronal cryostat sections (10-μm thick).The sections were then dried at room temperature for 5 min.Next, the sections were stained with oil red O according to the manufacturer's instructions (Solarbio Biotechnology Co., Ltd., Beijing, China).Briefly, the sections were stained with oil red O lipid staining working buffer for 45 min after fixing with 5%formaldehyde solution, washed with 60% isopropanol five times, and subsequently washed with distilled water for 2 min.Sections were stained again with hematoxylin for 2 min, washed with distilled water for 5 min, and dried and sealed with glycerine.They were then immediately photographed under a light microscope (BX50, Olympus America, Melville, NY,USA).
Western blotting analysis.The liver samples were homogenized with whole lysis buffer to extract proteins from the liver.Then,a BCA protein assay was used to quantify and standardize the proteins.Electrophoresis (8-12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis) was used to isolate the proteins, which were transferred onto a polyvinylidene difluoride membrane for Western blotting.The membrane was then sealed with 5% milk and incubated overnight with primary antibodies(rabbit anti-CASP1,1:500;rabbit anti-CASP9, 1:1000;rabbit anti-BAX, 1:10000; and rabbit anti-β-ACTIN,1:10000) at 4℃.After incubation for 1 hour, TBST was used to clean the membrane, and the secondary antibody probe (HRP combined with goat anti-rabbit IgG, 1:4000) was used for detection.Blotting was observed using chemiluminescence (TanonTMHigh-sig ECL, Li COR, USA).The strip strength was quantitatively analyzed using Image J software (NIH,Bethesda,MD,USA).
Data Analysis.All values were reported as the mean±standard deviation (mean ± SD).SPSS software version 20.0 was used for single factor analysis of variance, and the statistical significance standard wasP<0.05.
Results
Results of Network Pharmacology
Main active compounds in HZKY.The 924 compounds present in HZKY, including 65 from Zhishi (Aurantii Fructus Immaturus), 60 from Peilan(Eupatorii Herba), 58 from Huangqin (Scutellariae Radix), 93 from Heye (Nelumbinis Folium), 92 from Dahuang (Rhei Radix et Rhizoma), 75 from Shaoyao(Paeoniae Radix Rubra), 288 from Chaihu (Bupleuri Radix), 77 from Bohe (Menthae Haplocalycis Herba),and 116 from Banxia (Pinelliae Rhizoma), were screened using the TCMSP and TCMID.A total of 130 active compounds, including 22 from Zhishi (Aurantii Fructus Immaturus),11 from Peilan(Eupatorii Herba),24 from Huangqin (Scutellariae Radix), 15 from Heye(Nelumbinis Folium), 16 from Dahuang (Rhei Radix et Rhizoma), 27 from Shaoyao (Paeoniae Radix Rubra),17 from Chaihu (Bupleuri Radix), 10 from Bohe(Menthae Haplocalycis Herba), and 36 from Banxia(Pinelliae Rhizoma), were identified with OB ≥ 30%and DL ≥ 0.18.The structure and physiochemical properties of the compounds are listed in Table 1.
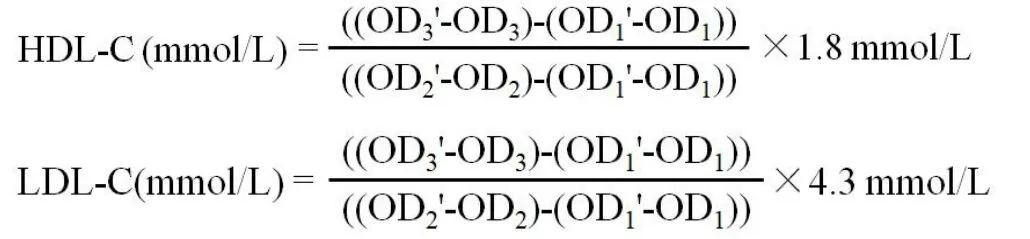
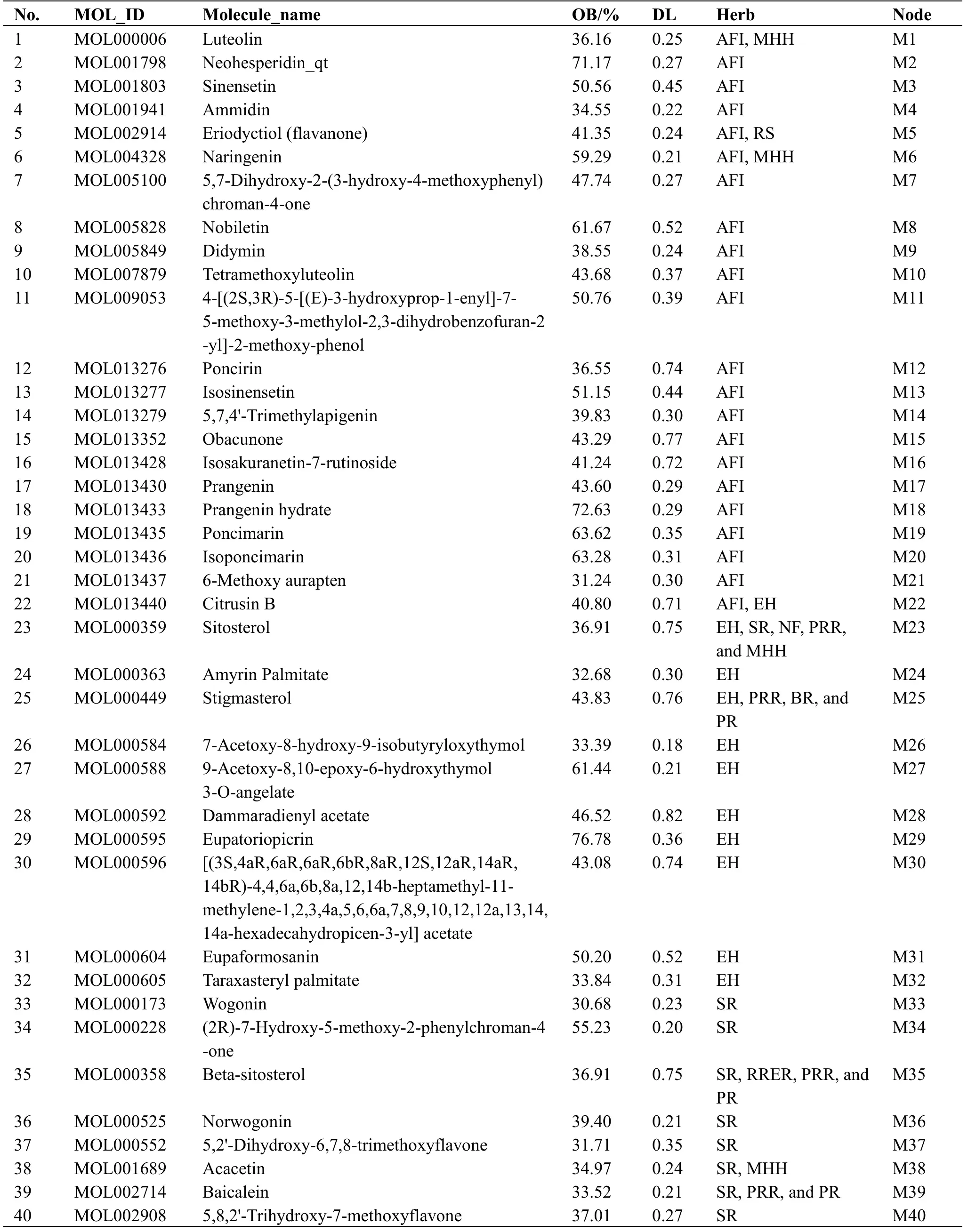
Table 1 List of active candidate components in HZKY
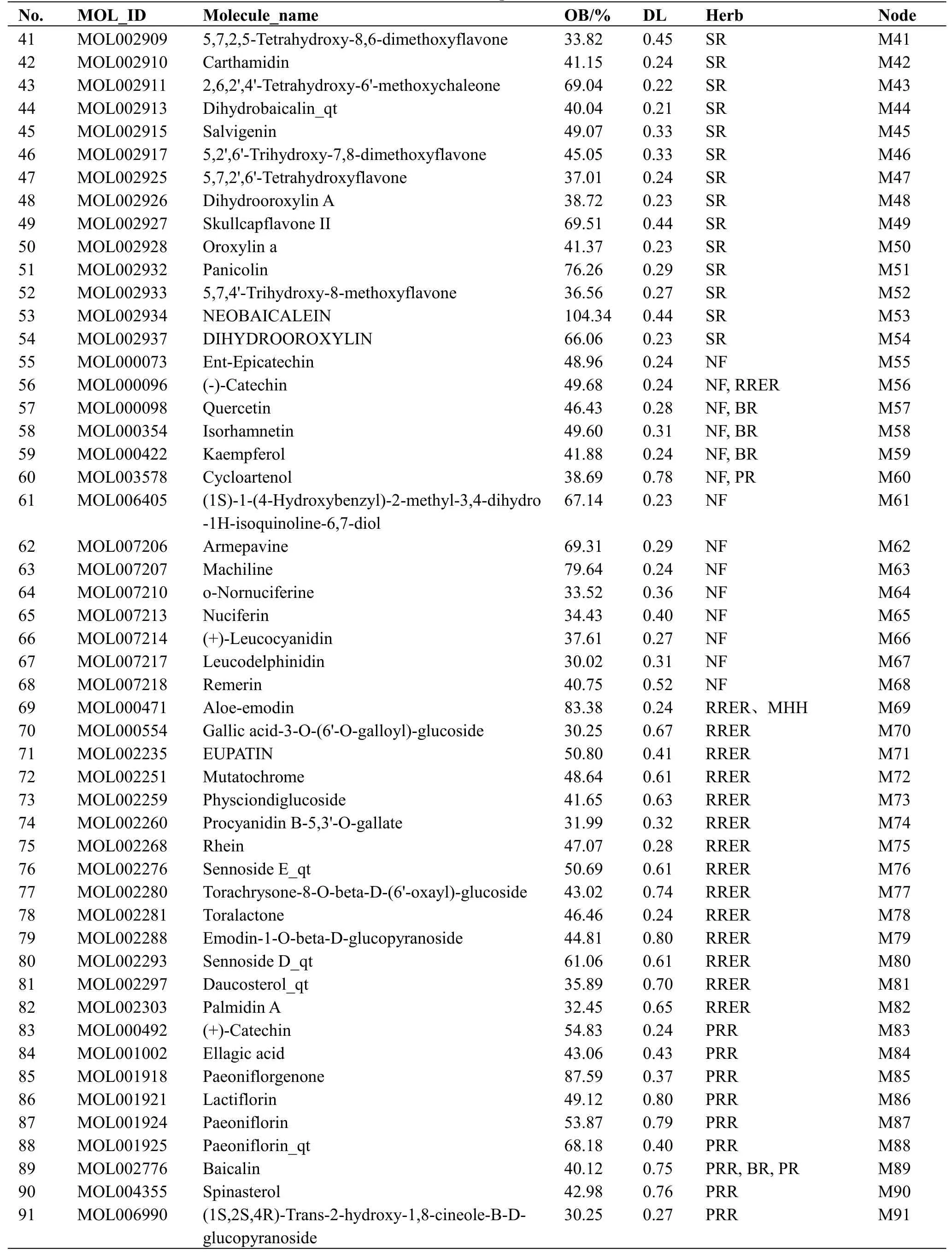
Table 1 List of active candidate components in HZKY(Continued)
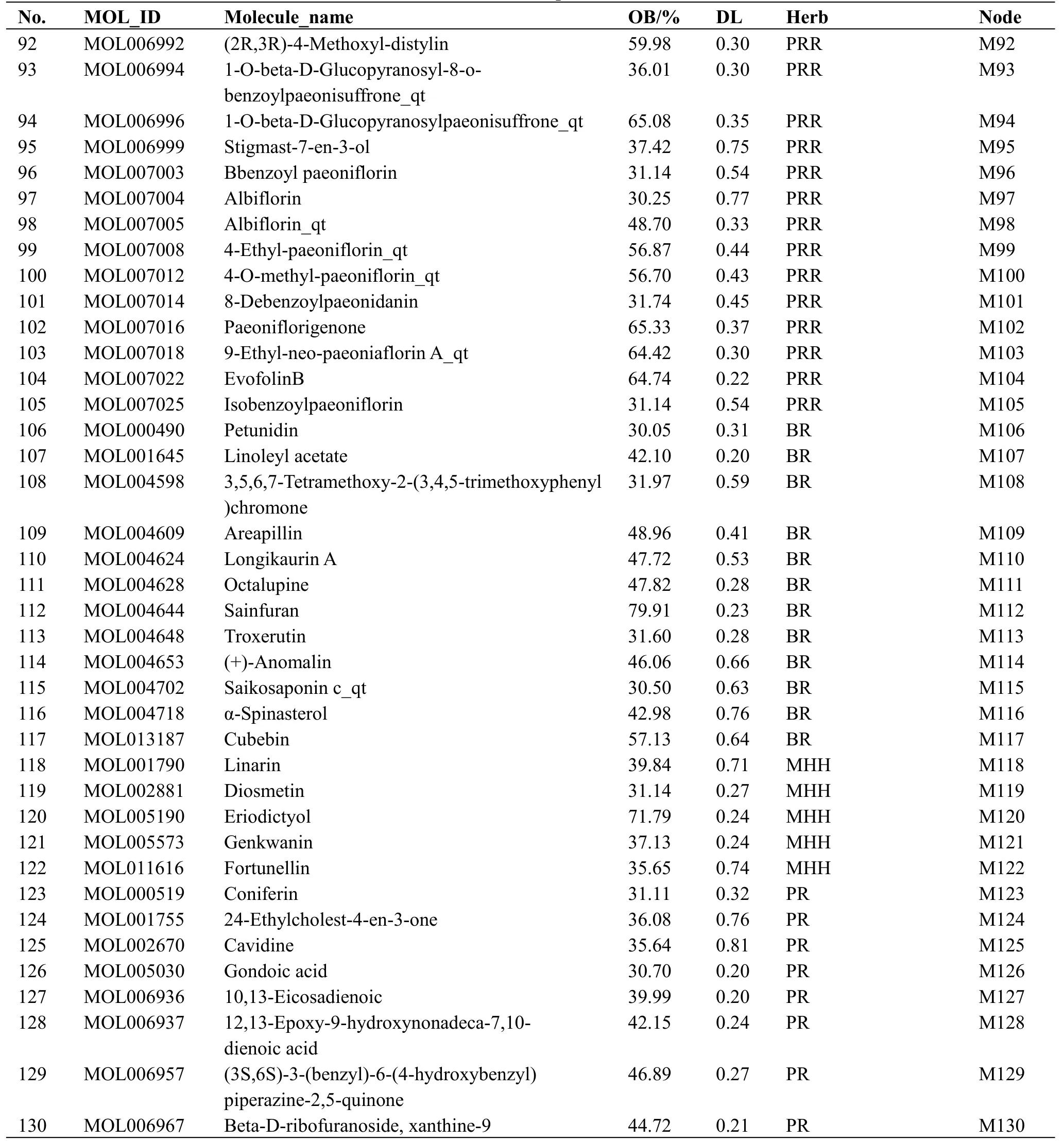
Table 1 List of active candidate components in HZKY(Continued)
Potential targets of active compounds in HZKY.A total of 1,672 targets were obtained from 130 active compounds in HZKY based on the TCMSP, TCMID,Bioinformatics Analysis Tool for Molecular mechANism of Traditional Chinese Medicine, and PUBCHEM databases.Repeated targets were removed,and 269 targets were identified with alignment from the Uniprot database.
Identification of potential targets and pathways of HZKY in NAFLD and network analysis of compounds, targets, and pathways.A total of 1,334 targets for NAFLD were identified from the online Mendelian Inheritance in Man and GeneCards databases.After intersecting with 269 targets of HZKY,62 targeted were identified as potential targets of HZKY in NAFLD.
The interactive relationship of 62 potential targets was analyzed and visualized using String 11.0.The candidates with the highest values of network connectivity were BAX, CASP3, CASP9, IL6, EGFR,MAPK8, CYCS, ESR1, VEGFA, LDLR, PPARG,PLAU, VCAM1, CYP3A4, GSK3B, PON1, GSTP1,NQO1, CTSD, and ACACA.BAX, CASP3, and CASP9 were the targets with the highest values of network connectivity.Additionally, as determined using KEGG analysis, apoptosis, fatty acid synthesis,and estrogen signaling pathways were the terms with the highest network connectivity.
The compounds, targets, and pathways of HZKY in NAFLD were imported into Cytoscape 3.7.2 to establish the compound-target-pathway network(Figure 1).
According to the compound-target-pathway network,compounds targeted to BAX included M56, M95, and M116; compounds targeted to CASP9 included M1,M2, M3, M5, M8, M9, M10, M13, M14, M21, M25,M29, M31, M33, M34, M35, M39, M49, M50, M57,M59, M62, and M125; and compounds targeted to CASP3 included M1, M2, M3, M4, M5, M6, M8, M9,M10, M14, M13, M19, M21, M23, M25, M33, M34,M35, M38, M39, M49, M50, M56, M57, M58, M59,M62,M64,M65,M84,M95,M116,M125,and M130.Moreover, BAX, CASP9, and CASP3 are closely associated with the apoptosis pathway.Therefore, the apoptosis pathway was predicted to be the potential mechanism of action of HZKY in NAFLD.
Results of Experimental Validation
HZKY ameliorated HFD-induced NAFLD in rats.Using the NAFLD model rat, we observed that 12 weeks of HFD led to a significant increase in body weight in the model group compared with that in the control group (467.07 ± 15.31 vs.414.29 ± 28.93 g,P< 0.01), whereas HZKY treatment significantly decreased the body weight in the HZKY group compared with that in the model group (420.57 ±15.52 vs.467.07 ± 15.31 g,P<0.01, Table 2).In addition,the serum levels of TG,TC,and LDL-C were significantly elevated and the serum level of HDL-C was decreased in the model group compared with that in the control group (TG: 1.70 ± 0.13 vs.0.28 ± 0.04,TC: 2.58 ± 0.34 vs.1.40 ± 0.26, LDL-C: 1.50 ± 0.23 vs.0.89 ± 0.09, and HDL-C: 1.29 ± 0.16 vs.1.86 ±0.18,P<0.01 for all, Table 2).HZKY treatment decreased the serum levels of TG,TC, and LDL-C and increased the serum level of HDL in the HZKY group compared with those in the model group (TC: 2.16 ±0.22 vs.2.58±0.34,P<0.01;TG:1.28±0.16 vs.1.70± 0.13,P<0.05; LDL-C: 1.14 ± 0.22 vs.1.50 ± 0.23,P<0.05;and HDL-C:1.49±0.14 vs.1.29±0.16,P<0.05,Table 2).
H&E staining demonstrated that the hepatic lobule and nucleus were clearly visible, the sizes of the hepatocytes were similar, and the hepatic cord was arranged neatly in the control group.Compared with that in the control group, in the model group, hepatic steatosis was obvious, indicating that there were vacuolar adipose droplets of various sizes and more balloon-like changes in hepatocytes, and the structure of the hepatic tissue could not be clearly observed.HZKY treatment markedly alleviated the hepatic steatosis and balloon-like changes in the HZKY group compared with that in the model group(Figure 2a).
Similarly, oil red O staining revealed that slight lipid deposition was observed in the control group.The lipid droplets in the cytoplasm of hepatocytes were stained red and were of various sizes and widely distributed in the cytoplasm, indicating the presence of lipid deposition in a large number of hepatocytes.However,HZKY significantly ameliorated lipid deposition and reduced the number of intracellular lipid droplets(Figure 2b).
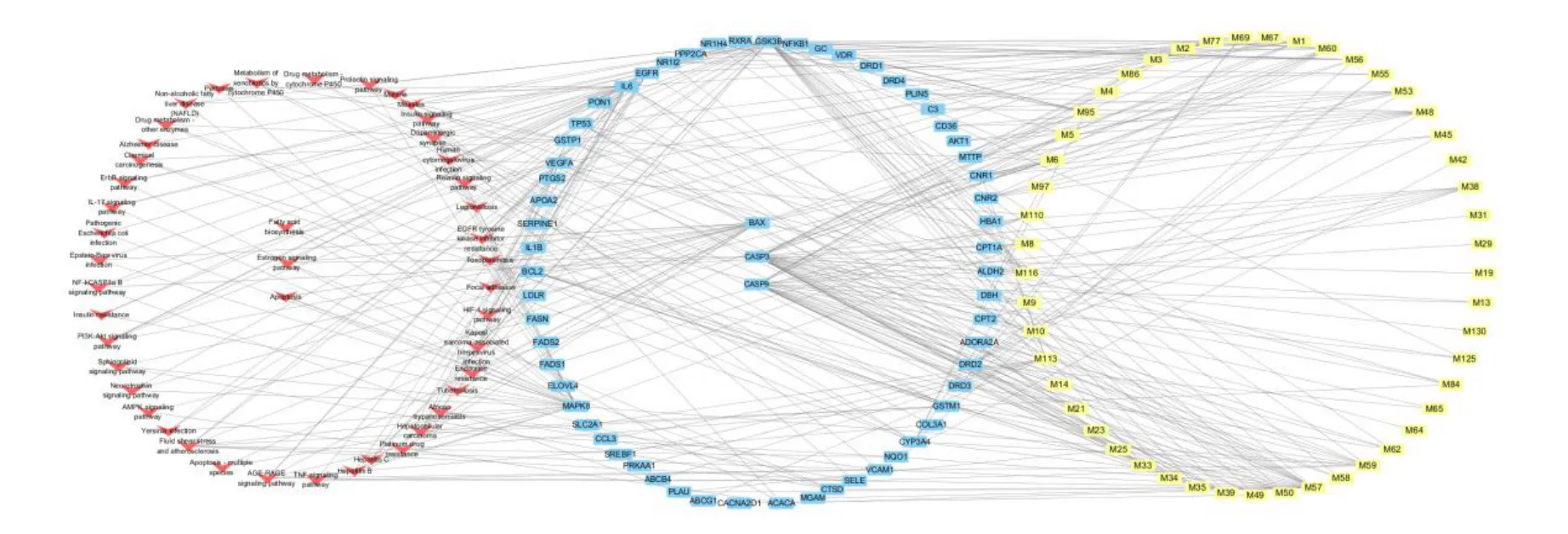
Figure 1 Compound-target-pathway network.The blue nodes represent the targets, the yellow nodes represent the active components of HZKY,and the red nodes represent pathways.Every active ingredient can act on multiple targets.
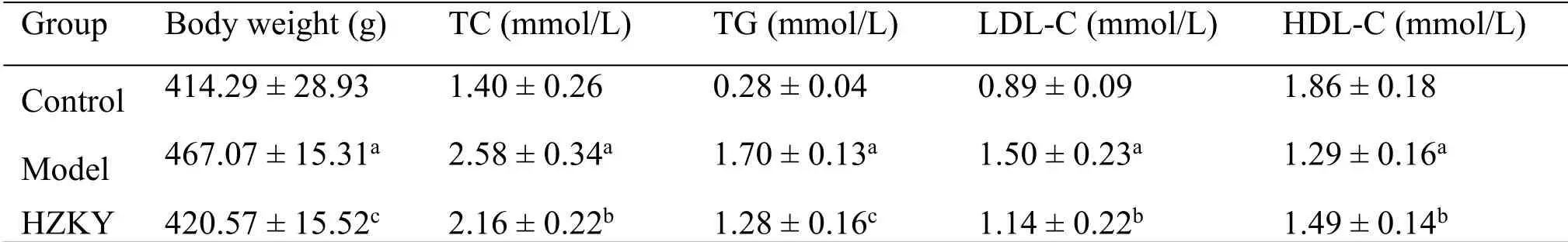
Table 2 HZKY treatment decreased body weight and changed the blood lipid composition in NAFLD model rats.
HZKY inhibited apoptosis in NAFLD model rats
According to pathways identified from the network pharmacology, apoptosis was selected as the mechanism for HZKY in NAFLD.Expressions of BAX, CASP9, and CASP3, which were predicted as the top targets of HZKY in NAFLD, were investigated to determine the anti-apoptotic activities of HZKY in NAFLD.The expressions of BAX, CASP9, and CASP3 were elevated in the model group compared with those in the control group(for BAX,P<0.05;for CASP9 and CASP3,P<0.01; Figure 3), whereas HZKY treatment decreased the expressions of BAX,CASP9, and CASP3 in the HZKY group compared with those the model group(for BAX and CASP9,P<0.05;for CASP3,P<0.01;Figure 3).
Discussions
In this study,the potential targets of HZKY in NAFLD were predicted using network pharmacology.Many compounds in HZKY, such as baicalein,o-nornuciferine, and quercetin, have been demonstrated to have treatment effects in NAFLD.Baicalein, derived from Banxia (Pinelliae Rhizoma),Huangqin (Scutellariae Radix), and Shaoyao(Paeoniae Radix Alba), is shown to reduce lipid accumulation in NAFLD model rats by inhibiting oxidative stress [25].O-Nornuciferine, derived from Heye (Nelumbinis Folium), decreases the serum TG and TC levels in NAFLD mouse models [26].Quercetin, derived from Chaihu (Bupleuri Radix) and Heye (Nelumbinis Folium), improves NAFLD via downregulation of FAS and SREBP-1C expression and upregulation of IRS1 expression in the liver [27].In addition, BAX, CASP9, and CASP3 were the HZKY targets with the highest values of network connectivity,and apoptosis was the main signaling pathway of HZKY in NAFLD.The effects of HZKY in NAFLD model rats were also investigated in the present study.The results showed that HZKY treatment significantly improved the serum levels of TG, TC, LDL-C, and HDL-C in NAFLD model rats.Moreover, lipid accumulation in the liver was reduced following HZKY treatment, indicating the significant curative effect of HZKY in NAFLD.
NAFLD is accompanied by the apoptosis of hepatocytes [28]; electron microscopy previously showed that the ultrastructures of mitochondria were damaged in the hepatocytes of patients with NAFLD[29].Steatosis in hepatocytes can impair the function and decrease the membrane potential of mitochondria.A decrease in the mitochondrial membrane potential can trigger the release of cytochrome C and activate caspase family proteins, ultimately inducing apoptosis of hepatocytes [30].Furthermore, dysfunction in fatty acid synthesis can cause obesity and NAFLD.The uptake and synthesis of fatty acids in the liver is significantly increased in the liver [31].Excessive amounts of fatty acid can trigger IR by inducing oxidative stress and decreasing sensitivity in hepatocytes.IR can cause dysfunction of lipase activity,thereby triggering lipolysis in the peripheral organs and increasing the levels of fatty acids in the serum[32].Moreover,IR induces hyperinsulinemia, and high levels of insulin inhibit the β-oxidation of fatty acids.The excess fatty acids can be transported to the liver and cause steatosis in hepatocytes and NAFLD [33].Estrogen has also been demonstrated to be associated with NAFLD.Compared with premenopausal women,postmenopausal women exhibit an increase in body weight, lipid accumulation, hepatic inflammatory responses, and oxidative stress [34].The activation of estrogen receptors can increase the production of bile acid in NAFLD model mice.Bile acid can activate the farnesoid X receptor to inhibit lipogenesis in the liver.Estrogen receptors can also directly reduce the lipid contents and liver injury in NAFLD model mice by inhibiting the activation of pregnane X receptor.Estrogen can also improve NAFLD by regulating the TLR-MYD88-IL-6 pathway.Estrogen has been demonstrated to improve hepatocyte injury in NAFLD by inhibiting the activation of NF-κB and C/EBPβ and decreasing the activity of IL-6 promoter [35] .In addition, lack of estrogen can contribute to the progression of NAFLD[36-37].BAX,CASP9, and CASP3 are important factors that modulate apoptosis in NAFLD.Accumulation of free fatty acids is shown to activate and dimerize BAX in the cytoplasm [38].Dimerized BAX is transported to the outer membrane of the mitochondria and activates CASP-9 and CASP-3 to initiate apoptosis[39].
Given the high correlation of the apoptotic pathway predicted by network pharmacology, we determined the anti-apoptotic activities of HZKY in NAFLD model rats.In agreement with the results of network pharmacology analysis, our results show that HZKY treatment significantly decrease the levels of apoptosis-related proteins, including BAX, CASP9,and CASP3,in the liver.
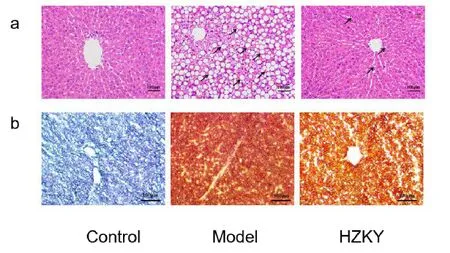
Figure 2 Hepatic steatosis was improved after HZKY treatment.
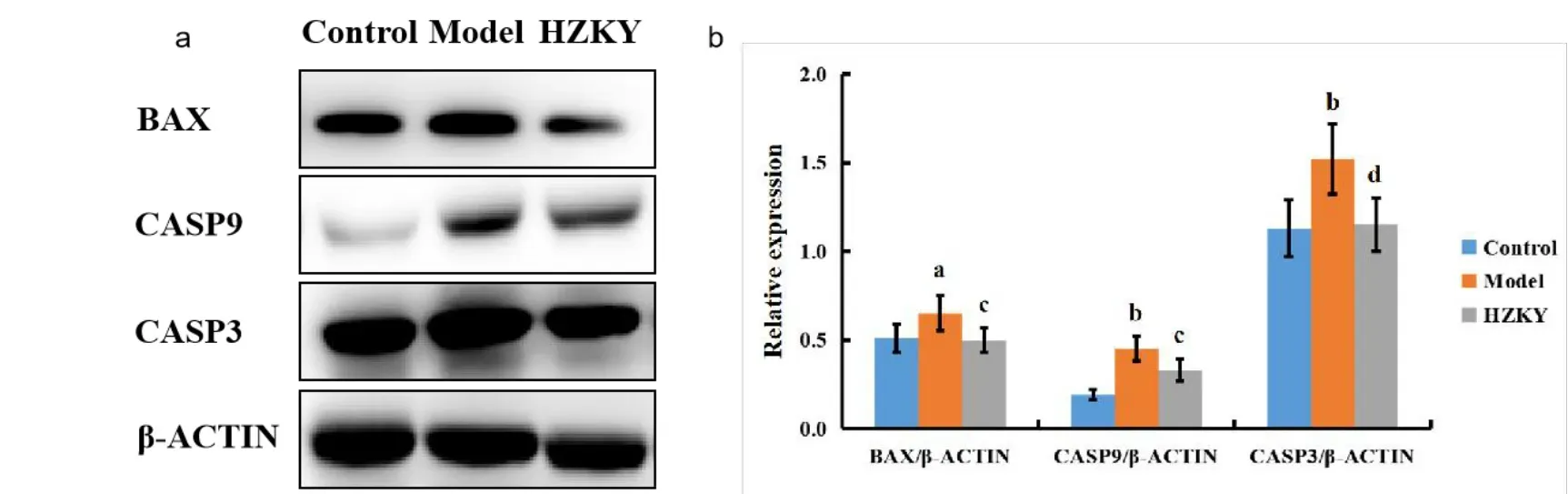
Figure 3 HZKY treatment regulated the expressions of BAX,CASP9 and CASP3 in NAFLD model rats.
Conclusion
The present study demonstrates that HZKY improves NAFLD by inhibiting apoptosis in the liver by decreasing the levels of apoptosis-related proteins,including BAX,CASP9,and CASP3.
杂志排行
Traditional Medicine Research的其它文章
- The dynamic changes and mechanisms of Rehmanniae radix processing based on Maillard reaction
- Investigation of in vitro antioxidant activity of dihydromyricetin and flavonoids rich extract from vine tea(Ampelopsis grossedentata)
- Advances in anti-inflammatory and immunoregulatory mechanisms of sinomenine
- Qiming granule in treating type 2 diabetic kidney disease patients:study protocol for a randomized controlled trial
- Bibliometric analysis of acupuncture research through the Web of Science database from 1990 to 2019
- Jian-Gan-Xiao-Zhi decoction ameliorates high-fat high-carbohydrate diet-induced non-alcoholic fatty liver disease and insulin resistance by regulating the AMPK/JNK pathway
