The dynamic changes and mechanisms of Rehmanniae radix processing based on Maillard reaction
2021-12-04XiangLongMengBoWangXiaoYanZhangChenZiLyuXiaoJuanSuChenXuNingShuoShengZhang
Xiang-Long Meng,Bo Wang,Xiao-Yan Zhang,Chen-Zi Lyu,Xiao-Juan Su,Chen-Xu Ning,Shuo-Sheng Zhang*
1College of Chinese Materia Medica, Shanxi University of Chinese Medicine, Jinzhong 030619,Shanxi,China; 2Department of Herbology,College of Korean Medicine,Dongguk University,Gyeongju 38066,Korea.
Abstract
Background:Traditional Chinese medicines are usually processed before they are used for clinical treatment.This is done in a way associated with the Maillard reaction.Methods: Based on the Maillard reaction, this paper analyzed the degree of processing of rehmannia root(Rehmanniae radix)relative to the dynamic variation rules of Maillard reaction index parameters, including pH,A420, amino acids, and 5-hydroxymethylfurfural.Furthermore,this study introduced thermal analysis techniques and pyrolysis kinetics to assess the influence of the correlation between processing raw rehmannia root and the Maillard reaction during carbonization.It then went through the whole process of transforming the raw material to end-product in order to explain the scientific connotation of processing it.Results: The results showed that each time rehmannia root was processed, its pH value and amino acid content decreased, while the A420 value and 5-HMF increased.Processing with wine shows a significant difference in these experimental indexes.The position and intensity of the maximum thermal weight loss rate peak of processed rehmannia root at different degrees of processing are different.Comprehensive quantitative 221 ±0.2 °C for processed rehmannia root carbonization was the processing temperature limit.Moreover, the kinetic solution verified that the activation energy corresponding to the carbonization temperature was close to the maximum value of the activation energy of the whole carbonization process, and the optimal mechanism function was g(α) = ((1 −α) −1/3 −1)2.Conclusion: The Maillard reaction occurred during the processing of rehmannia root mixed with carbonization.With each increase of the number of steaming and drying cycles involved in the processing, the level of Maillard reaction increased significantly.The wine-steaming method had a significant effect on the quality of the processed product.
Keywords: Processing of traditional Chinese medicine, Maillard reaction, Radix Rehmanniae Praeparata,Rehmannia root,Thermoanalysis,Pyrolysis kinetics
Background
Chinese herbal medicines are processed using a variety of methods before they are administered to patients.Based on the severity of the illness and a patient's clinical needs, the processing approach taken is meant to give full play to the medicine's preventive and curative effects,while also overcome adverse reactions,and ensuring safety and effectiveness [1].As for rehmannia root (Rehmanniae radix(RR), Dihuang in Chinese), the perennial herb and its processed components have made important tonics used in China for thousands of years [2, 3].As recorded inShennong Bencaojing(Shennong's Classic of Materia Medica),a Chinese medical text book written in the Eastern Han Dynasty (25 C.E.-220 C.E.), RR is derived from the dry roots ofRehmannia glutinosaLibosch, which belongs to the Scrophulariaceae family of flowering plants [4, 5].Because of its medicinal value, various forms of natural and processed RR are often used in clinical practice, such as raw RR, RR, processed RR(RRP, Shu Dihuang in Chinese), and both carbonized RR (CRR, Sheng Dihuangtan in Chinese) and RRP(CRRP, Shu Dihuangtan in Chinese) [6] (Figure 1).Raw RR is notable for its therapeutic properties in traditional Chinese medicine (TCM) [7].However,because of its cold nature, raw RR is not suitable for patients with indigestion.When the RR is processed using steam from water, the resulting RRP is adept at clearing “Heat” (a concept based on TCM theory) [8]and generating body fluid.Alternatively, when it is processed using steam from yellow wine, the RRP is believed to open up blood vessels due to the volatility of the wine.
Figure 1 Rehmannia glutinosa Libosch whole plant,Rehmanniae radix slices and its processed components
Since RR was first recorded, more processing methods have been developed, including some controversial steps, such as the addition of excipients,steam cycles, and steaming times [9, 10].The most widely accepted way to assess the quality of RRP is to follow the maxim: “Black as paint, sweet as sugar”,which is based on a method of processing by steaming and drying the herb typically over nine cycles.But the traditional instructions warn:“If the processing is done for too long,the taste will be sour.”[11].
The Maillard reaction, also known as the carbonyl ammonia reaction, is a chemical process between amino-containing compounds and compounds from the carbonyl group, which polymerize to generate a new compound [12].It was named after the French physicist and chemist Louis Camille Maillard(1878-1936), who initially discovered the process,which is also described as a nonenzymatic browning reaction.When foods are processed or cooked at high temperature, the Maillard reaction takes place between amino acids and reducing sugars, to generate different flavors and brownish colors.This reaction mainly happens through condensation, cyclization, and the rearrangement of Amadori molecules to produce fructosyl amines.Under acidic conditions,intermediate products, such as hydroxymethylfurfural,are generated through 1, 2-enolization reaction,dehydration, and deamination.These intermediate products then undergo further condensation and polymerization to form complex polymer pigments[13].This also takes place during the processing of RR[14].For example, during the processing of concocted RRP, its color becomes darker and 5-hydroxymethylfurfural (5-HMF) is generated,indicating the occurrence of the Maillard reaction.Thermal analysis [15] is a technique used to measure the physical properties of materials under different temperatures and time, and can be called on to assess processing of TCM.
In recent years,many new processes have been used in the study of TCM.For example,the main method of acquiring RRP is through stirring and calcination [16].While other processing techniques have also been developed to it, there is a lack of a common standard for processing charred RRP,since the ingredient CRRP is inconsistent.Moreover, information about RRP processing technology, chemical components, medical effects, optimization of processing technology, and improving CRRP quality is mentioned in the related references.By using the“steaming and drying for nine cycles” method to obtain RRP,Lu Pengwei[17]aimed to change the content of iridoids during processing.Guo Yanxia [18]tried to study the Maillard reaction in the processing of RRP, but the related books and studies recorded neither the dynamic changes nor the chemical components during the RRP processing[19-21].This paper is the first to analyze RR processing time and the dynamics of the Maillard reaction's parameters, including pH value,A420 value,amino acids, polysaccharides, and 5-HMF.We used thermal analysis and pyrolysis kinetics to analyze the influence of RRP processing and Maillard reaction in the carbonization of RRP.We then went through the process from RRP to processed CRRP, in order to explain the scientific connection of processing RRP.
Materials and reagents
Instrument
We purchased an STA-409C multi-atmosphere thermo-differential thermal analyzer (Netzch,Germany), an Ultra-3660 UV-visible spectrophotometer (Beijing Puyuan Precision Technology Co., Ltd., China), a GZX-9140MBE electric blast drying oven (Shanghai Boxun Industrial Co., Ltd., China), an HHS thermostatic water bath(Shanghai Boson Industrial Co., Ltd., China), a DV215CD one-ten-thousandth precision electronic balance (Ohaus Instrument Co., Ltd., China), an ultrasonic cleaning machine (Ningbo Xinzhi Biotechnology Co., Ltd., China), a DE-100g universal high-speed crusher (Zhejiang Hongjingtian Industry &Trade Co., Ltd., China), a PHS-3C+ acidity meter(Chengdu Century Ark Technology Co., Ltd., China),and a U3000 HPLC(Thermo Fisher Scientific,USA).
Medicinal herbs
The raw RR used in this study was collected from Xiangfen in Shanxi Province, China.These were confirmed as the roots ofRehmannia glutinosaLibosch.by Prof.Zhang Shuosheng, of Shanxi University of Chinese Medicine of China.
To make the RRP [22], RR was put into a steamer and heated for 6 h, and then dried in an oven for 24 h at 60 °C.This formed one cycle of steaming and drying, which is traditionally repeated nine times,during which a sample was taken after each cycle.Next, the samples were cut into pieces, dried at 60 °C and labeled as S1 to S9.
In the case of RRP processed with yellow wine[23],the ratio between the wine and the herb was set at 10:4,and all other steps were as above.These samples were labeled as SW1 to SW9.
Standard substances
5-HMF was purchased from Chengdu Pufeide Biotech Co., Ltd (Sichuan, China, standard substances mass fraction ≥98%, batch number MMST-14099).Glycine(Shanghai Standard Technology Co., Ltd., mass fraction ≥98%, batch number 1863) was the other substance used in this study.
Experimental method
Determination of A420 and pH value during RRP processing
Samples of 2.5 g were placed in a 25 mL conical flask,10mL distilled water was added, followed by 40 min of ultrasound.The supernatant was decanted into a 50 mL tube and centrifuged (3,000 r/min), with the supernatant separated.5mL distilled water was then added to the precipitate and the process was repeated.The supernatant was combined three times and placed in a 25 mL volumetric flask.The pH and the absorbance A at 420 nm were measured(divided by 25 to obtain the absorbance of the RRP extract).The sample was frozen at −20°C.
Determination of free amino acid content during RRP processing
Solution preparation.Ninhydrin solution: 1 g ninhydrin was dissolved in ethanol to 50 mL to obtain 2%ninhydrin solution.
Potassium dihydrogen phosphate solution (0.2 mol/L):1.36 g of potassium dihydrogen phosphate was dissolved in distilled water to 50 mL and mixed well.
Sodium hydroxide solution (0.2 mol/L): 0.2 g of sodium hydroxide was dissolved in distilled water to 25 mL and mixed well.
pH phosphate buffer preparation: 25 mL of 0.2 mol/L potassium dihydrogen phosphate solution plus 11.8 mL of 0.2 mol/L sodium hydroxide solution was mixed in distilled water to 100 mL and mixed well.
Glycine reference solution: 0.05 g of dry glycine reference substance was dissolved in distilled water to 50 mL, obtaining 1 mg/mL of glycine reference solution.
Test solution:2 mL of RRP water extract was mixed with 8 mL of 95% ethanol, placed at 4 °C for 6 h and centrifuged(3,000 r/min,15 min).The supernatant was separated.Distilled water was added to dissolve the precipitate,and then mixed with 4 mL of 95%ethanol,placed at 4°C for 6 h,and centrifuged(3,000 r/min,15 min).The supernatant was separated, and the combined supernatant was dried at 45°C and dissolved in distilled water to volume 5 mL.
Standard curve.0.15, 0.2, 0.25, 0.3, 0.35, 0.4, and 0.45 mL of 1 mg/mL glycine reference solutions were weighed,adding up to 0.45 mL with the distilled water,respectively.Then 1 mL of 2% ninhydrin solution and 1 mL of pH 6.8 phosphate buffer were added and mixed well.After heating in a 100°C water bath for 15 min, the solutions were cooled down and distilled water was added to 100 mL.After 15 min, it had been diluted 1.67 times.The absorbance was measured at 567 nm.Then a standard curve was drawn with glycine concentration C(mg/mL)as abscissa and absorbance A as ordinate.Linear regression was used to calculate the regression equation:A=285.3C −0.208,R2=0.9994.
Sample content determination.Sample determination: 0.5 mL of sample solution was mixed with 0.5 mL of 2% ninhydrin solution and 0.5 mL of pH 6.8 phosphate buffer, followed by heating in a 100°C water bath for 15 min.After the solution cooled,distilled water was added to volume 5 mL after 15 min.The absorbance was measured at 567 nm.
Sample blank determination: 0.5 mL of sample solution was mixed with 0.5 mL of anhydrous ethanol and 0.5 mL of pH 6.8 phosphate buffer, followed by 100 °C water bath for 15 min.After cooling down,distilled water was added to volume 5 mL after 15 min.The absorbance was measured at 567 nm.Actual sample light absorption A=A(sample)−A(blanksample)
Methodological study.According to methodological investigation requirements, the precision, stability,repeatability, and sample recovery rate were investigated.SW1 was measured successively at 1, 2,4, 8, 16, and 24 h, and repeated 6 times for stability and repeatability.The relative standard deviation(RSD)of stability and repeatability were 3.29% and 1.16%,respectively.The control sample was continuously injected 6 times to obtain the precision RSD 2.01%.The average recovery was 100.01%, and the RSD was 0.72%.
Determination of 5-HMF content during RRP processing[24]
Preparation of the test solution.Each sample(S1-S9,SW1-SW9) of 0.5 g was put into a 50 mL volumetric flask; methanol was added to volume 50 mL, followed by soaking for 1.5 h at room temperature and ultrasonic extraction at room temperature overnight.Then the sample was replenished, filtered, and 25 mL of continuous filtrate was collected.The residue evaporated into dried material and then dissolved in 5 mL of 15% methanol.The solution was filtered with a 0.45 μm microporous membrane to obtain the test solution.All samples were prepared in triplicate.
Preparation of reference solution.Reference 5-HMF at 22.76 g was dried to a constant weight and weighed.Added to was 10 mL methanol to obtain 2269 μg/mL 5-HMF as a control solution.
Chromatographic conditions.A Hypersil GOLD aQ C18(Thermo Fisher Scientific,USA;250 nm×46 nm)column was used with a flow rate of 1 mL/min and column temperature of 25 °C.Mobile phase of acetonitrile: 0.1% phosphoric acid water (11:89).Figure 2 shows a detection wavelength of 284 nm.
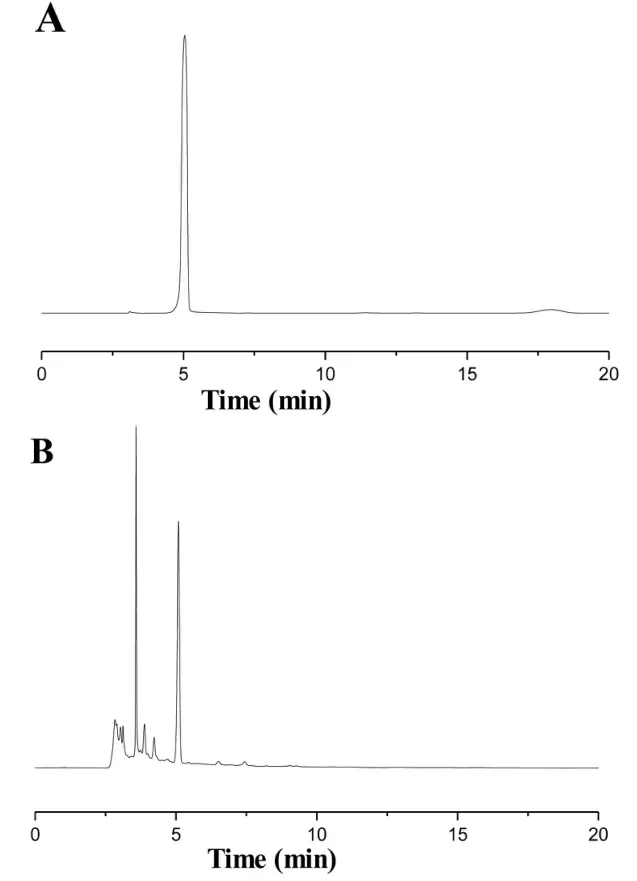
Figure 2 HPLC analysis of 5-HMF in the RRP.(A)5-HMF as a standard compound; (B) 5-HMF in the sample.RRP, processed Rehmanniae radix; 5-HMF,5-hydroxymethylfurfural; HPLC, high-performance liquid chromatography.
Linear relationship investigation.The control substances were prepared as solutions at concentrations from 0.455 to 227.6 μg/mL.The injection volume was 10 μL.The peak area was determined for each concentration, and the regression equation was calculated:for 5-HMF it was Y=1.073X+0.458,R²=0.999.
Methodological study.According to the methodological investigation requirements, the precision, stability, repeatability, and sample recovery rate were investigated.SW1 was measured successively at 1, 2, 4, 8, 16, and 24 h, and this was repeated 6 times for stability and repeatability.The RSD of stability and repeatability were 2.13% and 0.77%.The control sample was continuously injected 6 times to obtain the precision RSD 1.78%.The average recovery of 5-HMF was 98.15%, and the RSD was 0.73%.
Thermogravimetric test
In the thermogravimetric simulation experiment, N2was used as the carrier gas and the flow rate was 100 mL/min.The samples were placed in a crucible and the temperature was increased from room temperature to 600°C at a rate of 10°C/min.The finished sample was used for qualitative analysis.
Kinetic test
In this experiment, the activation energy of a certain conversion rate was solved based on the Kissinger-Akahira-Sunose method, the Ozawa-Flynn-Wall method, and the Friedman method under the isoconversional model.These methods neglect the effects of heating rate and independent reaction mechanism on pyrolysis and combustion,while emphasizing the first-order process from feedstock to pyrolysis products.The specific formula is as follows:
ln(β/T2)=ln(AE/Rg(x))−E/RT
log (β) = log (AE/Rg(α)) −2.315 −0.457E/RT(Kissinger-Akahira-Sunose method)
ln (dα/dt) = ln f(α) + ln A − E/RT (Friedman method)
At a later stage of the experiment, based on the commonly used solid-state reaction mechanism function,the reaction mechanism was explored using a single scan rate of uncertainty.In the process, the common mechanism function g(α) of solid-state thermal decomposition (Table 1) was brought into the Coats-Redfern equation ln (g(α)/T2) = ln (AR/βE) −E/RT for linear fitting,to solve the dynamic parameters,where α is the weight loss rate,T is the temperature, R is the gas constant, β is the heating rate, and dα/dt is the decomposition reaction rate.After comparison and verification, the mapping analysis was carried out to select the mechanism function that best described the pyrolysis reaction of RR charcoal.
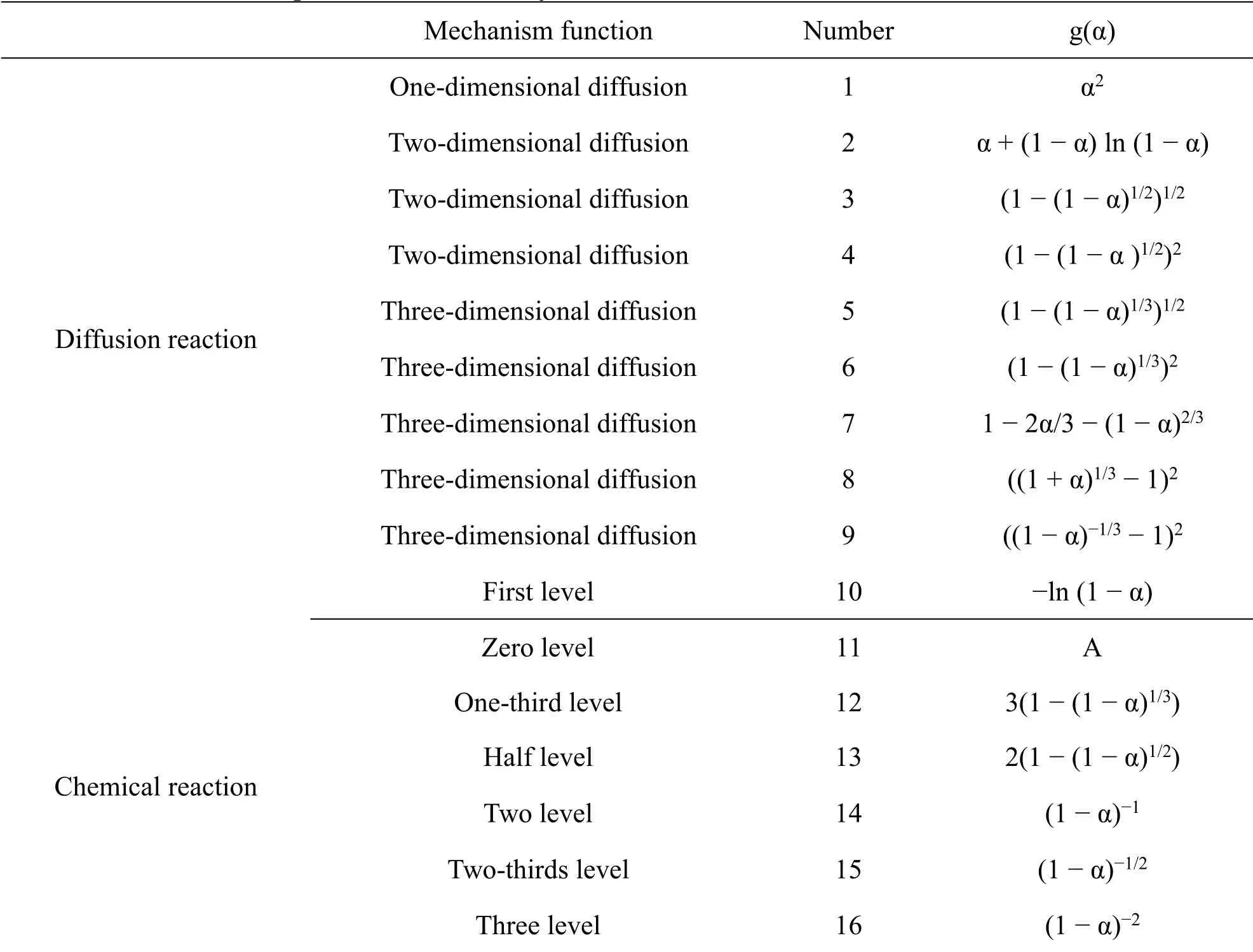
Table 1 Expression of commonly used solid-state reaction mechanism function
Results
Determination of pH value and color change during different RRP processing cycles
The pH values and the absorbances A at 420 nm are shown in Figure 3A and Figure 3B.The pH values significantly decreased with each step of the processing cycle.For the water-steaming group,although there were fluctuations,though the overall pH values decreased from the first cycle (5.23 ± 0.02) to the ninth cycle (4.82 ± 0.03).For the wine-steaming group, the overall pH values decreased from the first cycle (5.16 ± 0.02) to the ninth cycle (4.45 ± 0.02).Moreover, the pH values in the water-steaming group were significantly higher than in the wine-steaming group,especially after the third cycle.
The absorbances A at 420 nm significantly increased with each step of the processing cycle.For the water-steaming group, although there are fluctuations,the overall absorbances A at 420 nm increased from the first cycle (0.10 ± 0.01) to the ninth cycle (0.32 ±0.01).For the wine-steaming group, the overall absorbances A at 420 nm decreased from the first cycle(0.19 ± 0.01) to the ninth cycle (0.55 ± 0.01).Meanwhile, the absorbances A at 420 nm of the wine-steaming group were significantly higher than the water-steaming group for every processing cycle,especially after the third.
Determination of free amino acid content during the different RRP processing cycles
The amino acid content of all samples is shown in Figure 3C.In both processing methods, the content decreased sharply from the first to the third cycle(from 0.655 ± 0.006% to 0.327 ± 0.001% in the wine-steaming group, and from 0.3440 ± 0.001% to 0.242±0.019%in the water-steaming group),and then remained at a relatively low level from the third to ninth cycle (S9, SW9: 0.450 ± 0.030%, 0.320 ±0.030%, respectively), in spite of small fluctuations.Moreover,the amino acid content in the wine-steaming group was significantly higher than the water-steaming group in every processing cycle.
Determination of 5-HMF during different RRP processing cycles[25,26]
As shown in Figure 3D, the 5-HMF content increased with each processing cycle.Moreover, it also showed significant differences between the two groups over each processing cycle,especially after the sixth cycle.
Pyrolysis characteristics of the different RRP processing cycles[27,28]
The pyrolysis combustion characteristics of RRP in simulated air (N2:O2= 4:1) has been investigated in previous studies [29].In TCM theory, CRRP is produced by the carbonization of RRP within an enclosed container in an anoxic state.Therefore, we studied the pyrolysis characteristics of RRP in an inert atmosphere (N2) (Figure 4), in order to understand the correlation between different processing degrees, or Maillard reaction degrees,and the processing results of CRRP.
We focused on the pyrolysis stage, and our analysis follows the reference [30].At the pyrolysis stage, two obvious thermal weight loss phases and two thermal weight loss rate peaks were found for RR, with different processing cycles and adjuvants.For RRP processed with steam from yellow wine, the initial and final temperatures over two stages were 155.9 ±7.76 °C to 279.48 ± 2.95 °C and 279.48 ± 2.95 °C to 326.7±2.21°C,respectively.Moreover,the intensities of thermal weight loss rate peaks were 5.76 ±1.21 %/min and 2.52 ± 0.08%/min, which appeared at 211.83 ± 4.11 °C and 326.7 ± 2.21 °C, respectively.Similarly, for RRP processed with no adjuvant, the initial and final temperatures over two stages were 162.4 ± 8.39 °C to 278.44 ± 7.15 °C and 278.44 ±7.15 °C to 341.4 ± 7.15 °C, respectively.The intensities of thermal weight loss rate peaks were 6.50± 1.23 %/min and 2.43 ± 0.08 %/min, which appeared at 211.52±3.3°C and 315.41±14.31°C,respectively.
With each increase in processing cycle, both the mean intensity of the two thermal weight loss rate peaks and temperature intervals (initial and final temperature) decreased at the pyrolysis and combustion stage.
Altogether, both the processing cycles and adjuvant are important conditions for the carbonization process of CRRP.The intensity of thermal weight loss rate peak near 223.45±4.32°C,or 221.33±0.2°C,was an important indicator for the degree of RRP processing.Moreover, the corresponding temperature of the thermal weight loss rate peak in the second stage of pyrolysis combustion near 279.48 ± 2.95 °C or 278.44± 7.15 °C was the upper temperature limit for RRP carbonization.
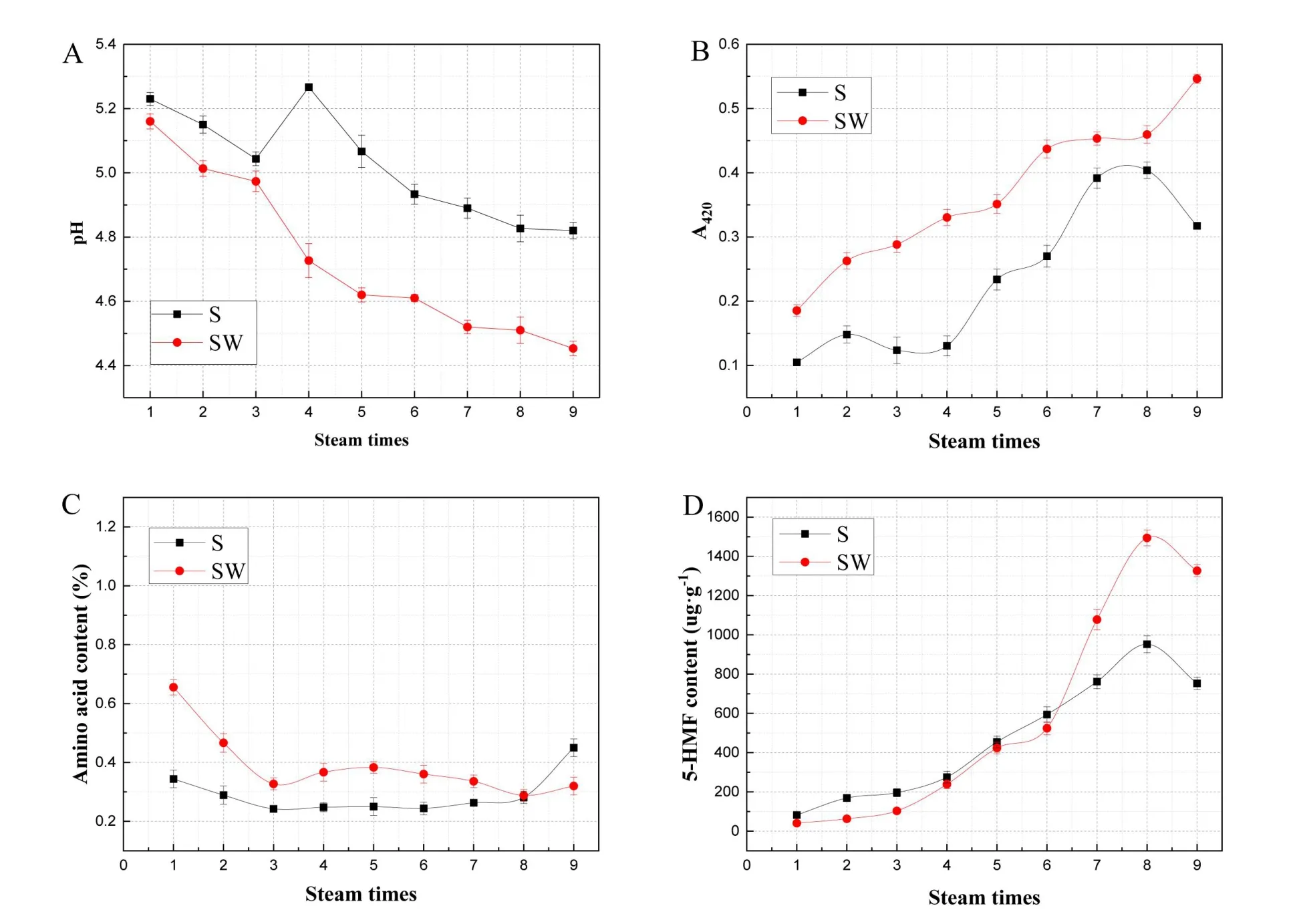
Figure 3 Dynamic changes of pH value, A420, amino acid content, and 5-HMF content in processed RRP,with steaming and drying times.(A) Dynamic changes of pH value; (B) absorbance at 420 nm; (C) amino acid content; (D) content of 5-HMF in processed RRP with steaming and drying times.RRP, processed Rehmanniae radix; 5-HMF, 5-hydroxymethylfurfural; S, water steaming method; SW, yellow wine-steaming method; N, time.Data represent mean±SD(n=3 per group).
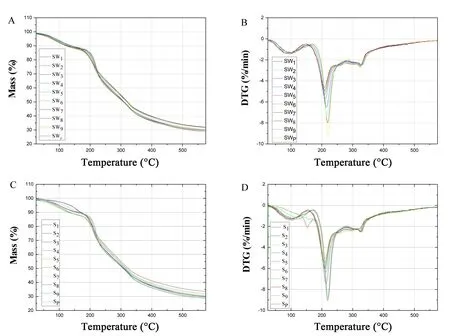
Figure 4 Pyrolysis characteristic TG/DTG curves for RRP.(A) Pyrolysis characteristic curve of TG by yellow wine-steaming method; (B) pyrolysis characteristic curve of DTG by yellow wine-steaming method; (C) pyrolysis characteristic curve of TG by water-steaming method;(D)pyrolysis characteristic curve of DTG by water-steaming method.Different the water-steaming method in processed RRP with steaming and drying times.TG,thermogravimetric; DTG, differential thermogravimetric; S, water steaming method; SW, yellow wine-steaming method; Sp, steaming method according to the Chinese Pharmacopoeia; SWP, yellow wine-steaming method according to the Chinese Pharmacopoeia.
Pyrolysis reaction kinetics results
The corresponding activation energy results are shown in Figure 5.Combined with the combustion pyrolysis characteristics of each sample, the RRP processing showed a heat loss rate peak (intensity −14.00 ±1.00 %/min) at a conversion rate of about 0.25, 221 ±0.2 °C, which was at the extreme point of activation energy, indicating that 221 ± 0.2 °C was the temperature limit for RRP carbonization.
In addition, the corresponding pyrolysis mechanism(Coats-Redfern method) is shown in Figure 6.According to the Coats-Redfern method, the optimum mechanism function for the pyrolysis reaction of RRP carbonation is g(α)=((1 −α)−1/3 −1)2.
As shown in Table 1, the commonly used solid-state reaction mechanism function for the pyrolysis mechanism of RRP is diffusion reaction.With the increase of processing cycles, the carbonation reaction process is always a diffusion reaction.
Discussion
According to TCM theory, herbal medicines processed in different ways or with different adjuvants can exert different therapeutic effects.During the RRP processing, there are significance changes in herbal characteristics, and the composition of the compounds changes at the same time.Water-steaming and wine-steaming are two common processing methods for RRP,and each finished product can have a different pharmaceutical effect.Water-steamed RRP is adept at clearing “Heat” and generating body fluid, while wine-steamed RRP can open up blood vessels because of the volatility of the wine.
Compared to food processing, a more complicated Maillard reaction occurs during TCM processing.This reaction can change the color and taste of the medicine,and also produce new ingredients with pharmacological activity.Thus,the Maillard reaction is directly related to the quality of TCM processing,scientifically explaining the connotation “black essence”in Chinese medicine processing.The Maillard reaction involves free amino, free carboxyl, 5-HMF,pH,temperature,and other parameters.Compared with previous research on RRP, this paper has studied the pyrolysis characteristics of RRP under inert atmosphere (N2), as well as the related parameters of the Maillard reaction.The goal of this study is to understand the correlation between different processing methods and Maillard reaction degrees.
When RR's color turns black, it indicates the generation of new colored components during processing.In food processing, the newly generated colored components are mainly melanoid-like, which are usually measured at a detection wavelength of 420 nm [31].In this study, we have shown that as the number of cycles increased, A420 also increased.Consistently, the contents of amino acids, one of the initial reactants of the Maillard reaction, decreased with an increase in processing cycles at the beginning,and then remained at a low level.At the same time,the pH value of the RRP extract gradually decreased.Moreover, with the increase of processing cycles, the absorbance of RRP at 420 nm gradually increased: the water-steaming method showed large fluctuations at 2 to 4 cycles and 6 to 8 cycles, and the wine-steaming method showed a slowed increase at 6 to 8 cycles.In both methods, the contents of 5-HMF in RRP showed a rising trend with the increase in processing cycles.
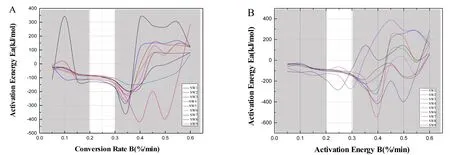
Figure 5 The relationship between conversion rate and activation energy of RRP with 9 different steaming and drying times by two methods.(A)Relationship between conversion rate and activation energy of RRP with 9 different steaming and drying times by the wine-steaming method;(B)the relationship between conversion rate and activation energy of RRP with 9 different water steaming and drying times by steaming method.S,water steaming method;SW,yellow wine-steaming method;RRP,processed Rehmanniae radix.
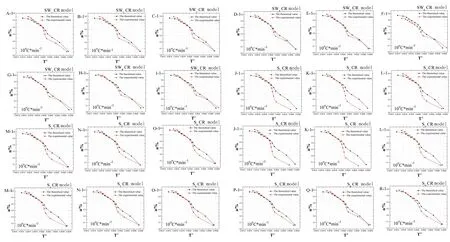
Figure 6 Verification of the mechanism function of pyrolysis reaction in the process of carbonization of RRP.A-1 to I-1 are the verification of the mechanism function of pyrolysis reaction in the process of carbonization of RRP by the wine-steaming method; J-1 to R-1 are the verification of the mechanism function of pyrolysis reaction in the process of carbonization of RRP by water steaming method.S, water steaming method; SW, yellow wine-steaming method; a, weight loss rate; RRP, processed Rehmanniae radix.Due to length limitations, the only retained dynamics results were under 10°C/min heating rate.
But there were also significant differences between the two methods: before the fourth to fifth cycles, the 5-HMF content is slightly higher in the water-steaming group; after the sixth cycle, the 5-HMF content increased clearly in the wine-steaming megroupthod,and became higher than the water-steaming groupe.
Comprehensive analysis shows that with the increase in number of steaming and drying cycles, the two RRP processing methods have a significant difference in Maillard reaction.Adjunct yellow wine can accelerate the Maillard reaction rate by virtue of its alcohol-enhancing properties.Analysis of relevant index components and previous studies [26] suggest that both water-steamed and wine-steamed RRP reached met the necessary processing requirements at the fifth steaming.As for the reason why RR processing traditionally involves steaming and drying over nine cycles, we need to further combine these results with pharmacology to confirm the reasons[26].
Conclusion
The Maillard reaction occurred during the processing of RRP mixed with CRRP.The yellow wine had a significant effect on the quality of processed products.The thermal analysis found that 221 ± 0.2 °C was the temperature limit for RRP carbonization.And the optimum mechanism function during pyrolysis is g(α)= ((1 −α) −1/3 −1)2.The pyrolysis mechanism of RRP charcoal is a diffusion reaction.With the increase of processing cycles, the carbonation reaction is always based on diffusion reaction.Analysis of relevant index components suggests that both water-steamed and wine-steamed RRP reached the necessary processing requirements at the fifth steaming.With each new steaming and drying step, the two processing methods of RRP showed a significant difference in the process of Maillard reaction.
杂志排行
Traditional Medicine Research的其它文章
- Investigation of in vitro antioxidant activity of dihydromyricetin and flavonoids rich extract from vine tea(Ampelopsis grossedentata)
- Advances in anti-inflammatory and immunoregulatory mechanisms of sinomenine
- Hua-Zhuo-Kai-Yu decoction inhibits apoptosis in nonalcoholic fatty liver disease
- Qiming granule in treating type 2 diabetic kidney disease patients:study protocol for a randomized controlled trial
- Bibliometric analysis of acupuncture research through the Web of Science database from 1990 to 2019
- Jian-Gan-Xiao-Zhi decoction ameliorates high-fat high-carbohydrate diet-induced non-alcoholic fatty liver disease and insulin resistance by regulating the AMPK/JNK pathway
