Effects of promoters on carburized fused iron catalysts in Fischer-Tropsch synthesis
2021-11-20LIUXiaolingMACailianZHAOWentaoZHANGJuanCHENJiangang
LIU Xiao-ling ,MA Cai-lian ,ZHAO Wen-tao ,ZHANG Juan ,CHEN Jian-gang
(1. State Key Laboratory of Coal Conversion, Institute of Coal Chemistry, Chinese Academy of Sciences, Taiyuan 030001, China;2. University of Chinese Academy of Sciences, Beijing 100049, China;3. Shanxi Institute of Energy, Taiyuan 030001, China;4. Beijing Sanju Environmental Protection & New Materials Co., Ltd. Beijing 100049, China)
Abstract: The effects of K, Ru or La promoters on the structure, surface area, crystal phase, and catalytic behavior during FT synthesis of carburized and uncarburized fused Fe catalysts were studied by XRD, XPS, TPD, N2-physisorption and catalytic reaction evaluation techniques. Addition of K improved selectivity of C5+ products for both the carburized and uncarburized catalysts. Addition of Ru suppressed catalytic activity of the carburized catalyst, but had little influence on the uncarburized one. Addition of La led to the encapsulation of the iron carbide, which consequently severely inhibited the carburization and decreased the activity. While Ru and La promote the formation of light components due to their ability to promote hydrogen adsorption. The performance of the reaction in the experiment indicated that the U-K catalyst had the best product distribution, in which the methane selectivity was 4.04%, and the C5+ selectivity was 75.84%.
Key words: Fischer-Tropsch synthesis;carburization;promoter;activity;C5+ selectivity
Fischer-Tropsch synthesis refers to the process in which synthesis gas (H2and CO) made from coal,natural gas, and biomass is converted into various hydrocarbon compounds over catalysts under the certain reaction conditions[1−3]. The metallic element iron, cobalt, nickel or ruthenium is commonly regarded as the active species in CO hydrogenation, which can facilitate CO dissociation. Among them, iron-based catalysts were the first ones that have been used in commercialized synthetic oil industry due to cheaper price and higher activity in FTS.
For decades, fused iron catalyst has proven to be an effective catalyst in FTS. The fused iron catalyst is less affected by the preparation conditions. Similar to the ammonia synthesis catalyst, the fused iron catalyst for FTS must be modified with promoters to maintain the texture and tune the chemisorptions of CO or H2.The promoters might play different roles depending on their interaction ways with active species. They can alter the reduction performance, dispersion degree of the active sites, or improve the activity, stability and selectivity. Anderson[4]investigated the effect of promoter and operating conditions on the reactivity of the fused Fe catalyst. The effect of the K promoter on the fused iron catalyst for FTS was ascribed to basicity.The activity of the catalyst enhanced initially and then decreased with the increase of K content. Moreover,methane production and chain growth probability were influenced by K as well. Pour et al[5]studied the effects of Mg, La and Ga promoters on the structure, surface area, reduction, carburization and catalytic behavior of Fe/Cu/SiO2FTS precipitation catalysts. The results showed that Mg, La and Ca promoters could improve the conversion of CO and water gas shift (WGS)reaction activity, inhibit the formation of methane, and increase the selectivity to olefins and C5+products. This was ascribed to the increase in the catalyst surface basicity and decrease in the reducibility. Wang et al[6]investigated the effects of Ru and Cu promoters on the texture properties, phase structure, reduction and carburized behaviors of Fe-based catalysts in FTS. The reaction results showed that addition of Ru or Cu improved the activity of the FT synthesis, but exhibited different influences on the product selectivity. The addition of Ru increased the selectivity of CH4and inhibited the formation of C5+, while the effect of Cu was opposite. In summary, the addition of promoters could improve the FTS activity, the selectivity of the target hydrocarbons and chemical or physical stability of the catalysts.
It is well-known that the Fe phase is converted into carbides during FTS. Classically, the promoter was introduced during fusion process. However, it might lead to different outcomes if the promoter was added before and after carburized. After systematic research,it was found that K, Ru or La promoters play a certain role in FTS performance. In this paper, the effect of promoters type and introduction of promoter before and after carburized on the performance of the FTS reaction were investigated. In order to avoid the influence of other factors, such as the preparation method of catalyst and the dispersion of iron, a batchprepared fused iron catalyst was used as precursor. The precursor was impregnated with promoters K, Ru or La, before and after carburized, respectively. A variety of characterization techniques, such as N2physical adsorption, X-ray diffraction, H2-TPD and CO-TPD were used to elucidate and correlate relation between structure and the activity, selectivity and stability of the catalysts.
1 Experimental section
1.1 Catalyst preparation
Balanced magnetite (Sinopharm Chemical Reagent Co, Ltd, 98%) powder was mixed with promoters (2% Al2O3, 1.3% KNO3, 2% SiO2and 0.7%MgO, Sinopharm Chemical Reagent Co, Ltd, 99%).Then the feedstock was melted and held at 1400 °C in an induction furnace in an Ar atmosphere for 1 h to ensure the homogeneity of the melt. The molten material was then quenched in water. Subsequently, the catalyst was dried, ground and sieved to 60–80 mesh[7].After that, the promoter was introduced in to the fused iron catalyst at different stages. The one was added after reduction, and the conditions was performed in a hydrogen atmosphere at 600 °C for 6 h, followed by passivation treatment. Afterwards, the promoters were introduced by a wet impregnation method. The content of promoter was 0.3% K2CO3, 2.5% N4O10Ru and 2.5%La(NO3)3, respectively, named as U-promoter. The other was carburized after reduction, and the reduction treatment was same as described above, the catalysts were carburized in a CO atmosphere at 350 °C for 6 h,then subjected to passivation treatment. The promoters were added using the method same as above, named as C-promoter. The blank samples without promoters were named as C and U. After impregnation, the catalysts were dried at 90 °C in the flowing air for 12 h.These dried catalysts were referred to fresh catalysts.
1.2 Catalyst treatments and characterizations
The BET surface area and pore size distribution were measured using N2adsorption-desorption at−196 °C. Prior to the experiments, the samples were degassed at 300 °C for 3 h. The isotherms were measured using a Micromeritics ASAP 2010 system.The total pore volume (TPV) was calculated from the amount of vapor adsorbed at a relative pressure close to unity assuming that the pores are filled with the condensate in the liquid state. The pore size distribution curves were calculated from the desorption branches of the isotherms using the Barrett-Joyner-Halenda (BJH) formula. The catalysts external surface area and micro-pore volumes were calculated using the deBoert-plot method.
X-ray diffraction (XRD) patterns were obtained on a D/max-RA diffractometer with CuKα radiation running at 30 mA and 40 kV. The XRD analysis was used to identify crystalline phase and calculate microstrain.
X-ray photoelectron spectroscopy (XPS)measurements were carried out on the Thermo Scientific ESCALAB 250Xi equipment, which was employed to determine the surface composition and valence state of the surface elements.
CO-TPD experiments were performed on an atmospheric quartz tube reactor equipped with a quadruple mass spectrometer (HIDEN, Hpr-20; Pfeiffer Vacuum Technology AG), using He (CO-TPD) as the carrier gas. The sample loading was 150 mg. The gas flow rate was 20 mL/min and the heating rate was 10 °C/min. The sample was cooled to 80 °C for the TPD tests. CO adsorption on the catalyst was performed at 80 °C for 1 h, and then the sample was purged with He for 1 h to remove weakly adsorbed species, respectively. After this step, CO-TPD was carried out while the temperature was increased to 800 °C at a rate of 10 °C/min under a flow of He.
H2-TPD experiments were performed on an atmospheric quartz tube reactor equipped with a quadruple mass spectrometer (HIDEN, Hpr-20; Pfeiffer Vacuum Technology AG). The samples were saturated with pure H2for 1 h and then flushed with Ar to remove the physically adsorbed molecules. The TPD experiments were performed at the heating rate of 10 °C/min at 50−800 °C under an Ar atmosphere.
1.3 Catalytic performance tests for FTS
The evaluation of catalyst was conducted in a fixed bed reactor with a tube diameter of 10 mm. The electrically heated vertical stainless steel tubular reactor is cladded with a 50 cm copper cylinder. The reactor can be supplied with H2, synthesis gas (molar ratio H2/CO = 2). The gas flow was controlled by Books 5850E mass flow controllers (MFC). The pressure was controlled via a back pressure regulator.The reaction products passed a 130 °C hot trap and a 5 °C cooling trap at working pressure and can be measured online by a GC. The FTS performances of the catalysts were tested in a fixed bed reactor, 1 mL of catalyst mixed with 2 mL of quartz was charged in the isothermal region of the reactor. Synthesis gas with a 2∶1 H2/CO ratio was introduced into the reactor. The FTS reaction conditions were set as H2/CO = 2,p=2.0 MPa,t= 240 °C and GHSV = 3000 h−1. The gaseous products were analyzed by GC (Agilent Technologies 7890A, 60/80 Carboxen 1000 column).The liquid and wax products were collected by a hot trap (130 °C, 2 MPa) and cold trap (10 °C, 2 MPa),respectively.
2 Results and discussion
2.1 Textural properties of the catalysts
BET specific surface area, pore size distribution and total pore volume were measured to determine the texture properties of the catalyst. The results were shown in Table 1. The N2adsorption/desorption isotherms were plotted in Figure 1(a) and 1(b). All samples displayed type IV Langmuir isotherms with H3 type hysteresis loop, indicating that both carburized and uncarburized catalysts were typical mesoporous materials. It was observed that the incorporation of Ru or La promoter into the fused iron catalyst during the impregnation step significantly changed the texture properties. By means of adding Ru or La to the fused iron catalyst, the surface area and pore volume were significantly increased. According to the added element, the order of the observed specific surface area is Ru > La > K. The specific surface area of the catalyst was increased after loading Ru and La might be due to the accumulation of Ru and La on the surface of the catalysts, forming new pores and increasing the surface area. Zhao et al.[8]reported that the presence of lanthanum significantly increased the BET surface area of iron-based catalysts, and this result was also confirmed by our results. Some studies[9]have shown that the addition of an appropriate amount of K can reduce the specific surface area of the iron-based catalyst. The specific surface area of the fused iron catalyst was small. After introduction of K, the specific surface area was reduced.
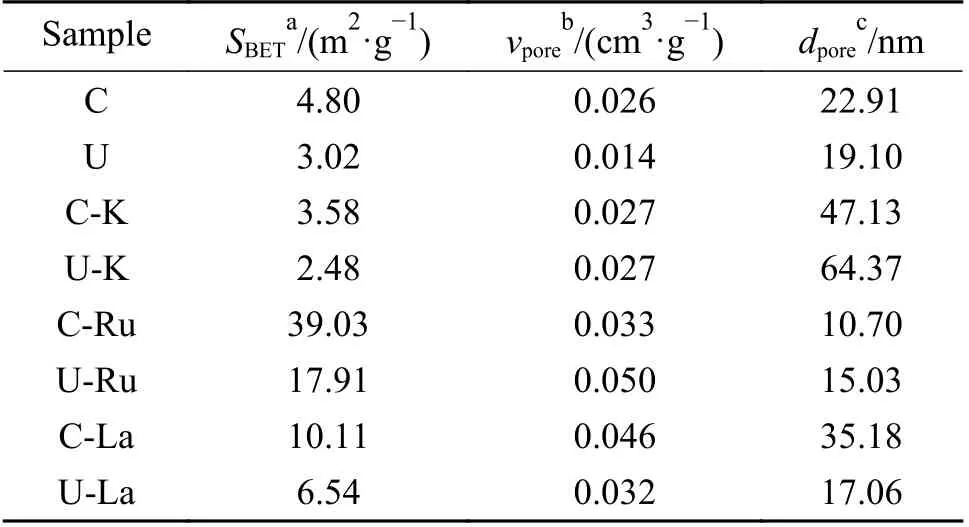
Table 1 Textural properties of the catalysts

Figure 1 N2 physisorption results of the catalysts(a): Isotherms of the nitrogen adsorption-desorption curves;(b): BJH pore size distribution curves calculated from the nitrogen desorption isotherms
As shown in the Figure 1(b), we can see that the C, U, C-K and U-K fused iron catalyst samples exhibited a wide pore distribution from 10 to 200 nm.The samples impregnated with Ru and La showed a pore distribution from 0 to 10 nm. The order of the average pore size calculated from the desorption curve of the isotherm is K > La > Ru. This had a certain impact on the Fischer-Tropsch reaction performance.
2.2 Crystalline phases of the catalysts
Figure 2(a) shows the XRD patterns of the fresh catalysts impregnated with different promoters after carburization. The catalysts showed a well-crystallized Fe5C2phase (JCPDS No. 19-0629) with characteristic diffraction peaks at 2θangles of 39.8°, 40.7°, 43.7°,44.7°, 45.1°, 45.9°, 48.7°, 49.3°, 51.9°, 58.2°, 78.5°and 82.5°. The promoters impregnation after carburization had no effect on the iron carbide crystal phase detected by XRD. The peak shape and intensity of C-K was little different from that of the blank catalyst. The intensity of the peaks C-Ru and C-La catalysts was relatively weak, indicating that addition of Ru and La could reduce the crystallinity of Fe5C2.
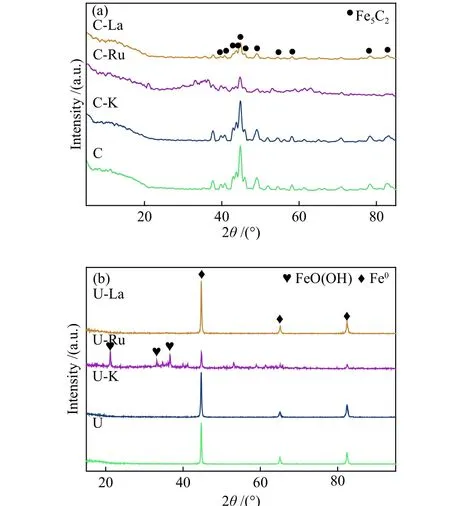
Figure 2 XRD patterns of fused iron catalysts(a) carburized by CO; (b): reduced by H2
Figure 2(b) shows the XRD patterns of uncarburized catalysts. At a reduction temperature of 600 °C, Fe3O4completely reduced to α-Fe. The result showed that once the iron phase formed, it remained stable during the subsequent impregnation and thermal treatment of the uncarburized catalysts.
Figure 3 shows the XRD patterns of the catalysts after 240 h of FTS. It can be seen that the diffraction peaks of the iron carbide active phase still appeared in profiles of the carburized catalysts. There were no obvious diffraction peaks of the iron carbide in the uncarburized catalysts. In particular, α-Fe phase was detected in U-La after the reaction, indicating that inhibition of carburized in FTS is an important reason for the decline in activity.

Figure 3 XRD patterns of the fused iron catalysts after FTS for 240 h
2.3 Surface chemical states of the catalysts
XPS was used to identify the surface properties of catalysts. XPS investigation of the catalysts showed that C, O and Fe are the main surface species. The XPS spectra of Fe 2p, Ru 2pand La 3dare compiled in Figure 4. Figure 4(a) and 4(b) shows the Fe 2pspectra of all catalysts, in which peaks at binding energies (EB)of 710.21, 724.69 eV and 712.10, 725.98 eV are assigned to 2p3/2, 2p1/2split orbitals of FeⅡion and 2p3/2,2p1/2split orbitals of FeIIIion, respectively.Additionally, the peaks at 707.39 and 720.0 eV are tentatively attributed to metallic Fe or FexC[10,11]. While a small amount of Fe3+and Fe2+exist in all catalysts,which might be formed by the oxidation of Fe0in the sample preparation and transfer process. Figure 4(b)shows that there is no peak at 707.39 eV, which is due to passivation of the reduced catalysts before discharge to avoid ignition.

Figure 4 XPS spectra of the catalysts: (a) Fe 2p (carburized); (b) Fe 2p (uncarburized); (c) Ru 3p; (d) La 3d
Binding energy of Fe in the K-doped sample shifted to lower values. The changes in the binding energy of Fe with the C-K and U-K catalysts were slight, which have a certain impact on the FTS reaction performance. The XPS spectra of Ru and La are depicted in Figure 4(c) and 4(d) , respectively. The peaks at 465.3 and 488.4 eV were ascribable to Ru0.Nevertheless, two 835.2 and 853.0 eV peaks assigned to La3+can be found in the XPS spectrum of La 3d,which indicates that La cannot be reduced after reaction processes, but only existed in the form of La2O3[12]. Figure 4(a) shows that the peaks corresponding to iron carbide were not observable in C-Ru and C-La catalysts, indicating that Ru or La covered the surface, which is confirmed by the XRD results.
2.4 Adsorption behaviors of the catalysts
H2-TPD was used to investigate the effect of promoters on the H2adsorption behavior of the fused catalysts (Figure 5). As displayed in Figure 5(a) and 5(b), H2desorption on the fused iron catalysts mainly appeared in two zones: below 500 °C and above 500 °C.Different surface structures might result in different H2-TPD profiles. Therefore, the peaks below 500 °C should be attributed to the desorption of H2from the surface of metallic iron[7]. It can be seen from Figure 5(b) that an obvious peak below 500 °C appeared in the TPD profile of the U-Ru catalysts, which was due to the decomposition of Ru species and the desorption of H2. When the adsorbed H2species is desorbed at a lower temperature, it means that the Fe−H bond strength is weak. For the carburized catalysts, it is different that the adsorption peak area was increased by the incorporation of Ru and La. Contrarily, the lower temperature range obviously decreased over catalysts U-Ru and U-La. The carburized catalysts exhibited two peaks above 350 °C. One (350−450 °C) might be due to the adsorption of H2on the metallic iron that strongly interacts with Fe3O4. The other increase in adsorption peak area (above 580 °C) could be ascribed to the hydrogenation function of La or Ru species function. Furthermore, with the addition of Ru and La promoters, no matter which addition method, H2will be activated in a large amount. This means that Ru and La help to enhance the adsorption of hydrogen and promote the production of methane[13,14].
The CO-TPD profiles of the catalysts are exhibited in Figure 5(c) and 5(d) . The main CO desorption peaks of all catalysts were located in the temperature range 100−650 °C. The first peak (150−350 °C) of the uncarburized catalysts could be ascribed to the weak adsorption of CO on catalyst, while the second in the range of 500–650 °C gradually shifts to higher temperature corresponding to the desorption peaks of strong CO adsorption[15]. The carburized catalysts have only a strong adsorption peak, with the addition of promoters, and the peak temperature and peak area both increase. It can be found that the appropriate addition of promoters is beneficial to enhance the strong adsorption of CO, causing a higher CO concentration on the surface of the catalyst. It is evident that promoter K can transfer electrons to CO through iron oxide and strengthen the Fe–C bond[16].The adsorption of CO in U-La and U-Ru might be attributed to the adsorption of La or Ru species on the surface. In particular, the coverage of Ru species serves as adsorption sites.

Figure 5 H2 and CO-TPD profiles of the fused Fe catalysts(a): H2-TPD (carburized); (b): H2-TPD (uncarburized); (c): CO-TPD (carburized); (d) CO-TPD (uncarburized)
2.5 Catalytic performance for FT synthesis
The results of CO hydrogenation on the catalysts doped with different promoters before and after carburized, under the conditions of 240 ℃, 2.0 MPa,GHSV = 3000 h−1, H2/CO = 2∶1 are summarized in Figure 6. The change in activity with time on stream can be used as an indicator of catalyst stability. The stability of the catalysts was good enough to obtain reproducible data. The CO conversion rate initially increased with the elapse of reaction time, then decreased slightly with the further operation for most catalysts[7]. It can be seen from Figure 6 that compared with the blank catalyst, the addition of the promoters K, Ru or La all suppressed the CO conversion. As demonstrated in the literature[17], it was proposed that coverage of surface Co atoms by Mn and La oxides results in a decrease of catalytic activity. The results of this research provide proof for our research. The Kdoped one showed the least decrease in activity among the promoters investigated. This might be due to the alkaline effect[18,19]. The decreased in catalysts activity caused by the C-Ru and C-La are more significant,with Ru acting as the most negative promoter in the case. This might be due to the coverage of the Fe5C2surface with Ru, which had little reactivity under such an ambient pressure. This conjecture was confirmed by the characterization of BET, XRD and XPS as well.

Figure 6 CO conversion versus time on stream for fused iron catalysts with different promoters
It can be seen from Figure 7 that the methane selectivity of the reaction increased with the reaction time, and the C5+selectivity showed the opposite trend.After 240 h time-on-stream, the C-K catalyst showed high selectivity to heavy hydrocarbons (C5+, 75.84%),while the methane fraction was less than 6%, which is ideal for the application in the FTS process. In the XPS characterization, we found that the binding energy of iron shifted to a low energy after doping with K. As promoter K is a typical alkali metal reagent, which provides electrons and increases the density of the electron cloud around the iron. It can also be seen from TPD that the K promotes the adsorption of CO, which promotes the production of heavy hydrocarbon products. The promotion effect of K, Ru or La on the catalytic performance agrees well with that reported in literature[2,4, 8,20], in which K facilitates the formation of long-chain hydrocarbon components, while both Ru and La promote the formation of light components. It is believed that the reactants and products diffuse slowly in the narrow pores and rapidly diffuse in the wider pores, thereby inhibiting the re-adsorption of 1-olefins and leading to high selectivity to heavy hydrocarbons[21−23]. It can be seen from the pore size distribution diagram that the order of the average pore size calculated from the desorption curve of the isotherm is K > La > Ru. As mentioned in the previous H2-TPD, promoters Ru and La could improve the hydrogenation capacity, thereon explaining why the CH4selectivity is higher in the C-Ru or C-La catalysts and the CH4selectivity in the C-K catalyst is lower, but the C5+selectivity is the opposite.

Figure 7 CH4 and C5+ selectivity versus time on stream for the catalysts with different promoters
Under the same conditions, the CO hydrogenation on uncarburized samples (addition of promoters after reduction) were studied, the results of which were shown in Figure 8. The most obvious change in the CO conversion rate was that the extent of reactivity suppression was different. The introduction of Ru promoter has a marginal effect on activity, which is also had the smallest degree of activity inhibition.During the evaluation process of U-La catalyst, we found that there was actually no activity under the same reaction conditions. The activity occurred only after increasing the reaction temperature to 260 °C.According to previous studies, rare earth oxides can increase the alkalinity of the catalysts surface, and will inhibit the formation of iron carbide[8], which was shown via carrying out an XRD on the used catalyst.The carbide is believed to be the active phase and the uncarburized phase (α-Fe) might be the cause of the inactivity[24]. The changes in the distribution trends of products CH4and C5+are roughly the same as the results after carburized.

Figure 8 CO conversion, CH4 and C5+ selectivity versus time on stream for the fused iron catalysts with different promoters(U-La 260 °C for measured activity)
The effects of promoters on the carburized and uncarburized catalysts were comprehensively analyzed.Compared with the catalysts added with promoters after carburized, the uncarburized catalysts were more sensitive to promotion. The comparison of results was shown in Figure 9.
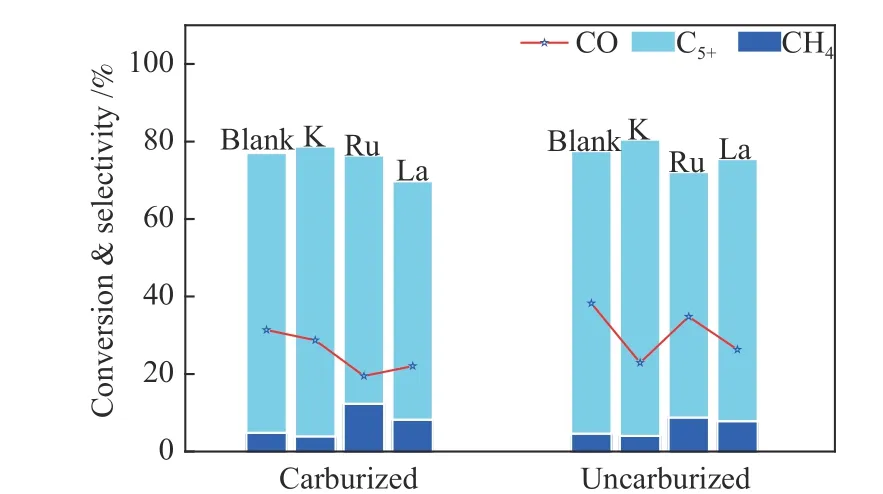
Figure 9 CO conversion, CH4 and C5+ selectivity for the catalysts with different promoters and pretreatment methods(after 144 h time-on-stream)
The material balance formula expressed in the figure issC5+(%)=1−sCH4−sC2−C4. The CO conversion rate for carburized and uncarburized catalysts was reduced to a different degree, and the suppression of activity has a different sequence, C: K < La < Ru; U:Ru < K < La. Combined with the XRD characterization of the catalyst after the evaluation, it can be seen that there were clear iron carbide peaks of the spent catalysts for the carburized one, while there were no iron carbide peaks for the uncarburized one. The change in activity is stronger after being uncarburized,and carbon deposition and sintering are more obvious.For the product distribution, it is also obvious that the C5+selectivity after being uncarburized is higher than the catalysts after being carburized.
3 Conclusions
In summary, the promoters play an important role in affecting the texture properties, phase composition and reaction performance of fused iron catalyst. The addition of K promoters improved the formation of long-chain hydrocarbons, while Ru or La promoter enhanced the formation of light components due to their ability to facilitate hydrogenation while inhibiting iron carbide formation. Comparing the effects of adding promoters before or after being carburized, it was found that the promotion efficiency was dependent on pretreatment methods. The introduction of the uncarburized promoters resulted in an excellent performance.
杂志排行
燃料化学学报的其它文章
- Mo-Sn 催化剂上甲醇低温氧化制甲缩醛
- 酸活化蒙脱土在二甲醚水蒸气重整制氢中的应用
- Ni 对MoS2 基催化剂活性相及加氢脱氮脱硫性能的影响
- Insight into reaction path and mechanism of catalytic cracking of n-hexane in HZSM-5 zeolites
- CO2/CH4/N2 在MER 型沸石中扩散和分离的分子动力学模拟
- Addition of bismuth to Pt and Pd for electric power generation with selective cogeneration of acetate from ethanol in a fuel cell type reactor
