The association of cardiovascular mortality with a first-degree family member history of different cardiovascular diseases
2021-11-15CharbelGhariosMireilleLeblebjianSamiaMoraRogerBlumenthalMiranJaffaMarwanRefaat
Charbel Gharios, Mireille Leblebjian, Samia Mora, Roger S. Blumenthal, Miran A. Jaffa,✉,Marwan M. Refaat✉
1. Department of Internal Medicine, Division of Cardiology, American University of Beirut Medical Center, Beirut,Lebanon; 2. Cardiovascular Imaging Research Center, Massachusetts General Hospital and Harvard Medical School,Boston, MA, USA; 3. Epidemiology and Population Health Department, Faculty of Health Sciences, American University of Beirut, Beirut, Lebanon; 4. Center for Lipid Metabolomics, Divisions of Preventive and Cardiovascular Medicine,Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, USA; 5. The Johns Hopkins Ciccarone Center for the Prevention of Cardiovascular Disease, Baltimore, MD, USA; 6. Department of Biochemistry and Molecular Genetics,American University of Beirut Faculty of Medicine, Beirut, Lebanon
ABSTRACT
Cardiovascular mortality is defined as death due to diseases of the heart or blood vessels, most commonly coronary heart disease (CHD), sudden cardiac death, or stroke. CHD and stroke remain the leading causes of death worldwide.[1]Care for cardiovascular disease (CVD)and loss of productivity due to disability or death costs the United States around $219 billion yearly.[2]A variety of modifiable risk factors have been associated with increased risk of CVD and mortality,namely hypertension, hyperlipidemia, diabetes mellitus (DM), obesity and smoking. The identification of such risk factors and their overall assessment along with other non-modifiable risk factors,mainly age and family history of CVD, is crucial to help estimate an individual’s risk and ultimately guide therapy.[3]
The important role of family history in the development of CVD has been shown in many prospective cohorts.[4-8]However, several controversies exist about the prognostic value of a family history of CVD. This is reflected in the variation of the definitions used across different studies and risk calculators.[9]Firstly, there is scarcity of data on what constitutes a clinically meaningful familial history of CVD. For instance, whether different cardiovascular conditions (e.g., CHD, stroke, or peripheral arterial disease) in different first-degree family members(parents, siblings, or children) carry distinct prognostic values remains unclear.[4,5]Secondly, whether a family history of CVD onset at any age (as opposed to a family history of premature CVD) is an important risk factor is unsure.[7]Thirdly, whether there are sex-based differences in the role of a familial history of CVD in individual risk assessment is also unclear.[7,8,10]
Given all these considerations, additional research is needed to further characterize the relationship between familial history and cardiovascular risk.Using patient data from the Lipid Research Clinics Prevalence Study[11]with up to twenty-three years of follow-up, we aimed to determine which specific first-degree familial histories of CVD (CHD, stroke,or peripheral arterial disease) predict cardiovascular mortality.
METHODS
Study Design and Participants
Participants took part in the Lipid Research Clinics Prevalence Study[11], a prospective cohort of North American individuals across ten geographic locations and diverse socioeconomic and occupational groups, as previously described.[12-14]The populations fell into three broad categories: occupational groups, household or residential groups, and parents of school children. At their first visit, individuals were screened from ten primary care centers in North America. A sample consisting of all visit 1 participants with elevated lipid levels, as well as an additional random sample of 15% of visit 1 participants were invited back for visit 2. The random sample constituted 58% of the total study participants, and the rest were individuals with elevated lipid levels. Overall, 52% of participants invited to visit 2 had hyperlipidemia.
During visit 2 (between 1972 and 1976), participants underwent a medical interview, physical examination, fasting blood studies, and a treadmill exercise test. Six participants were lost to follow-up and were excluded from the current analysis, resulting in 8,646 participants. Follow-up was until death or December 31, 1995. All participants gave written informed consent at study enrollment. The Institutional Review Boards of the Brigham and Women’s Hospital and the American University of Beirut approved the present study. This research was considered exempt.
Family History of CVD
Participants were asked during visit 2 about the presence of familial cardiovascular risk factors or diseases among first-degree family members (parents, siblings, or children). Familial factors assessed included history of hypertension, DM, hyperlipidemia, CHD (defined as history of heart attack or angina), premature CHD (before 60 years), stroke,and peripheral arterial disease. A sample question asked was: “Did your father have a heart attack or angina? If so, did this occur before he was 60 years?”. We were not able to properly assess the association between cardiovascular mortality and history of disease in children, as the frequency of disease in offspring was low, given the younger age of the study population.
Participants’ glucose and lipid levels were obtained after an overnight fast. Patients were considered to have hyperlipidemia if they had either low-density lipoprotein cholesterol (LDL-C) ≥ 160 mg/dL, total cholesterol ≥ 240 mg/dL, or triglycerides ≥ 200 mg/dL. Patients were considered to have DM if they had fasting glucose ≥ 126 mg/dL or used antidiabetic medications. Blood pressure was recorded as the mean value of two measurements at rest (supine). Hypertension was defined as a resting systolic blood pressure (SBP) ≥ 140 mmHg,resting diastolic blood pressure (DBP) ≥ 90 mmHg,or the use of antihypertensive medications. Participants were considered smokers if they had ever smoked cigarettes.
Cardiovascular Mortality
The primary outcome of this study was cardiovascular mortality. From 1976 to 1988, participant deaths were reported by mail or phone and then confirmed with either death certificates, family member or witness interviews, or via medical records. Reason of death was determined by cardiologists who were blinded to the identity of the departed. From 1988 to 1995, deaths were reported from death certificates by trained nosologists who used the National Death Index from 1998 to 1991 and then the Epidemiology Research Index from 1992 to 1995.[15]
Statistical Analysis
Baseline demographic and clinical characteristics were compared between groups with and without family history of premature CHD, using the Student’st-test for continuous variables and the Pearson’s chi-squared test or Fisher’s exact probability test for categorical variables (Table 1).
For survival analysis using Cox survival models,paternal, maternal and sibling history of diseases were first individually assessed for their association with time to cardiovascular mortality, after adjusting for risk factors (age, sex, SBP, DBP, body mass index, smoking, fasting glucose, LDL-C and triglycerides) (Table 2).
Only familial histories of disease that were significantly (P< 0.05) predictive of cardiovascular mortality after adjusting for risk factors were considered eligible to enter the final Cox survival model(Table 3). The final model thus aimed to identify which familial factors remain significant predictors of cardiovascular mortality after adjusting for risk factors and familial histories of disease that showed,at an individual level, significant associations with cardiovascular mortality. The proportional hazards assumption was satisfied using Schoenfeld residuals. Subgroup analyses were performed by sex,baseline age (< 60 years and ≥ 60 years), lipid and hypertensive statuses.
Harrell’s C-index, the net reclassification index and the integrated discrimination index were used to evaluate the improvement in predicting cardiovascular mortality gained by adding familial history of CVD to standard clinical variables.
A two-sidedP-value less than 0.05 was considered statistically significant, and statistical analyses were performed using STATA software(version 13.0).
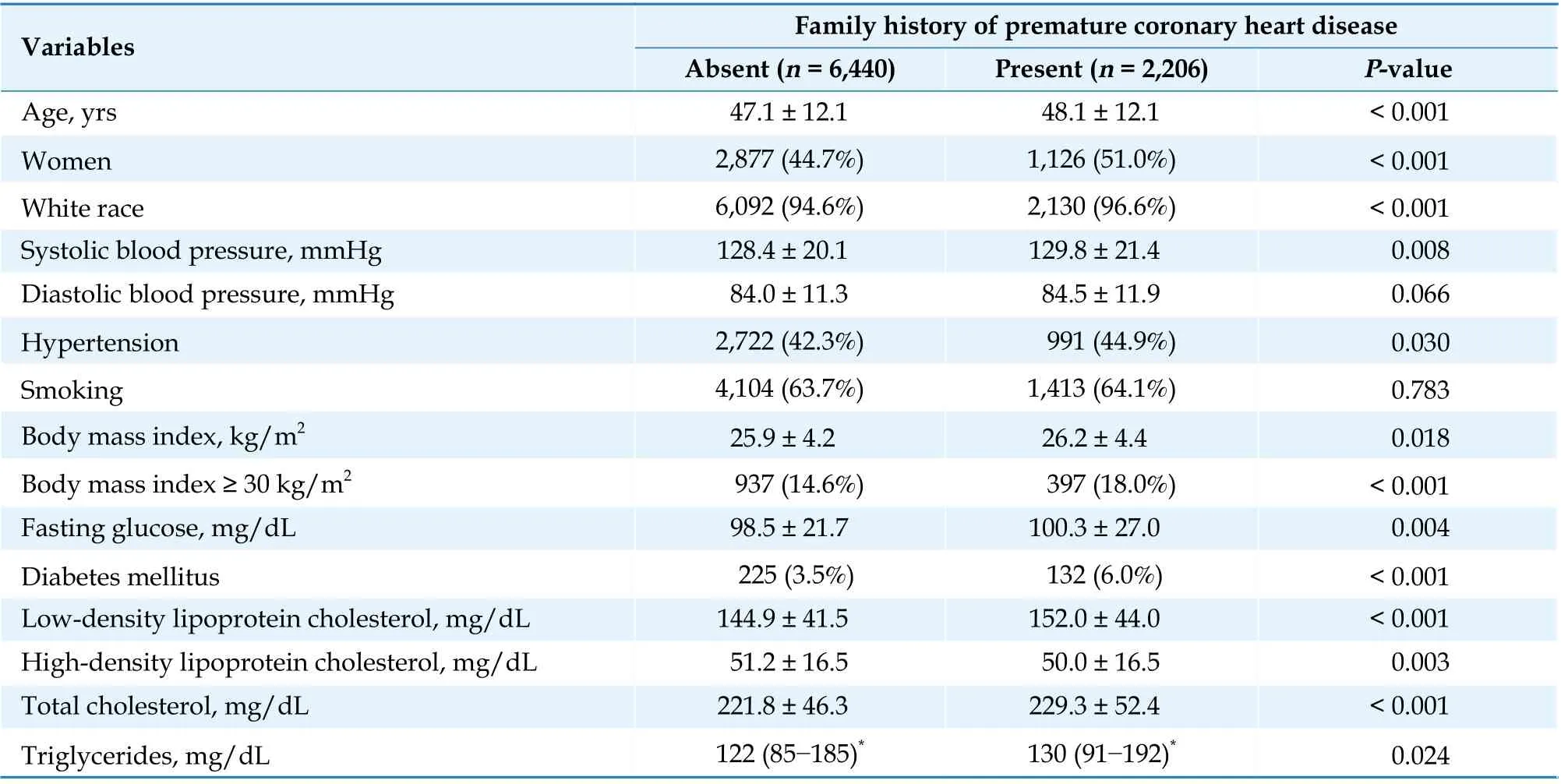
Table 1 Baseline demographic and clinical characteristics grouped by presence of a family (paternal, maternal or sibling) history of premature coronary heart disease (before age 60 years).

Table 2 Adjusted hazard ratio of cardiovascular mortality for first-degree familial histories of different cardiovascular diseases.Estimates were adjusted for age, sex, systolic blood pressure, diastolic blood pressure, body mass index, smoking, fasting glucose, low-density lipoprotein cholesterol and triglycerides.
RESULTS
There were 8,646 individuals (mean age: 47.4 ±12.1 years, 46% women, 52% of participants with hyperlipidemia) who were followed up for a mean duration of 19.4 ± 4.9 years. There were 1,851 deaths(21%), including 852 cardiovascular deaths (46% of deaths). 2,206 of participants (26%) had either a father, mother, or sibling with a history of premature CHD, 2,302 of participants (27%) had either a father, mother or sibling with a history of stroke,and 2,049 of participants (24%) had either a father,mother or sibling with a history of peripheral arterial disease.
Participants with a family (paternal, maternal or sibling) history of premature CHD had higher rates of cardiovascular mortality during follow-up (12.8%vs.8.8%,P< 0.001). They were also more likely to have significantly higher baseline levels of fasting glucose, LDL-C, total cholesterol, triglycerides, and lower levels of high-density lipoprotein cholesterol.They also had higher rates of hypertension and obesity (Table 1).
Cumulative incidence curves of cardiovascular mortality grouped by presence of paternal, maternal, or sibling history of premature CHD (Figure 1A)or stroke (Figure 1B) diverge very early (log rankP<0.001). The cumulative incidence curves of cardiovascular mortality grouped by presence of paternal, maternal, or sibling history of peripheral arterial disease exhibit however substantial overlap(Figure 1C).

Table 3 Hazard ratio of cardiovascular mortality for standard clinical and familial factors included in the final model.
Association Between Individual Familial Factors and Cardiovascular Mortality
After adjustment for participant risk factors (age,sex, SBP, DBP, body mass index, smoking, fasting glucose, LDL-C and triglycerides), only a history of CHD in parents and siblings, a history of DM in the mother, and a history of hypertension and hyperlipidemia in a sibling were significant predictors of cardiovascular mortality. Importantly, a familial history of stroke or peripheral arterial disease did not predict cardiovascular mortality after adjusting for risk factors (Table 2).
Familial Factors that Independently Predicted Cardiovascular Mortality
After adjusting for risk factors and for paternal,maternal and sibling histories of CHD before 60 years, maternal history of DM, and sibling history of hypertension or hyperlipidemia, only parental histories of premature CHD remained independent predictors of cardiovascular mortality, with somewhat higher associations for a maternal history of premature CHD [adjusted hazard ratio (aHR) =1.99, 95% CI: 1.36-2.92,P< 0.001 for maternal history of premature CHD; aHR = 1.52, 95% CI: 1.10-2.10,P= 0.011 for paternal history of premature CHD] (Table 3). Of note, replacing in the final model history of parental premature CHD with history of parental CHD at any age rendered similar results,albeit with more conservative estimates (aHR =1.35, 95% CI: 1.02-1.78,P= 0.035 for maternal history of CHD; aHR = 1.28, 95% CI: 1.01-1.63,P=0.043 for paternal history of CHD).
Adding parental history of premature CHD to a model that included age, sex, SBP, DBP, body mass index, smoking, fasting glucose, LDL-C and triglycerides resulted in statistically significant albeit small improvement in risk discrimination as indicated by Harrell’s C-index (which improved from 0.840 0 to 0.844 6) and an integrated discrimination improvement index of 0.004 9 (95% CI: 0.0027-0.0072).The improvement in the category-free net reclassification index was not significant (Table 4).
Subgroups Analyses

Figure 1 Cumulative incidence curves of cardiovascular mortality grouped by presence (red) or absence (blue) of a family (paternal, maternal or sibling) history of premature coronary heart disease (before age 60 years) (A), stroke (B), and peripheral arterial disease (C).
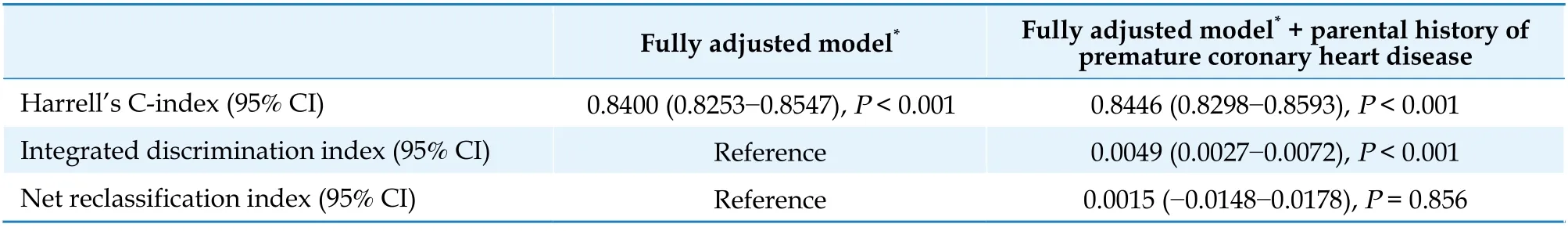
Table 4 Discrimination and reclassification statistics for a parental history of premature coronary heart disease with 23-year risk of incident cardiovascular mortality.
Subgroup analyses were performed according to categories of sex and baseline age (< 60 years and≥ 60 years), hypertension, and hyperlipidemia. There was no significant difference in the ability of a parental history of premature CHD to predict cardiovascular mortality among those with and without baseline hypertension or hyperlipidemia or among those in the younger (< 60 years) versus older (≥ 60 years) age subgroups, after adjusting for the full set of cardiovascular risk factors and as evidenced byPinteraction> 0.05 (Figure 2). Subgroup analysis by sex showed that the ability of a parental history of premature CHD to predict cardiovascular mortality was less significant in women (aHR = 1.10, 95% CI:0.74-1.65) than in men (aHR = 1.79, 95% CI: 1.38-2.31),Pinteraction= 0.021 (Figure 2).
DISCUSSION
In this study, we examined the risks of cardiovascular mortality that different first-degree familial histories of CVD carry. After adjusting for cardiovascular risk factors, only a parental history of CHD, especially before the age of 60 years, was a significant predictor of cardiovascular mortality.Importantly, the presence of stroke or peripheral arterial disease in first-degree relatives were not associated with increased risk of cardiovascular mortality. In this study, the importance of our findings lies in the ability to predict cardiovascular mortality using a simple, targeted question “Did your father or mother have a heart attack or angina? If so, did this occur before he or she was 60 years?”. This is important given the evidence that a more detailed or complex familial history of CVD did not prove to be more effective at predicting cardiovascular events than more simple histories.[16]
While we did not find that a history of premature CHD in a sibling predicted cardiovascular mortality after adjusting for parental history in the final survival model, a previous study from the Framingham offspring cohort found that history of premature CVD in a sibling conferred increased risk of cardiovascular events after adjusting for risk factors and parental history of premature CVD.[4]Our findings might have been different due to differences in endpoints assessed (incidence of cardiovascular events versus cardiovascular mortality), definitions used for a significant family history of premature CVD(see next section), statistical methodology (logistic regression versus survival analysis), and potentially due to the younger mean baseline age of our cohort.
Another important point is that the additional prognostic value of a familial history of CVD beyond standard clinical risk factors seems to be limited in women. Our findings are in accordance with previous work showing that a positive family history of myocardial infarction (MI) in a parent, sibling, or child independently predicted cardiovascular mortality in men, but not in women.[10]

Figure 2 Plot of hazard ratio with 95% CI of cardiovascular mortality for a parental history of premature coronary heart disease,stratified by subgroups of sex, baseline age, lipid, and hypertensive statuses. Estimates were adjusted for age, sex, systolic blood pressure, diastolic blood pressure, smoking, body mass index and fasting glucose and lipids.
Familial History of Premature CVD
What precisely constitutes a familial history of premature CVD remains a debate. Studies from the Framingham offspring cohort defined it as any cardiovascular event (CHD, stroke, peripheral arterial disease, CHD mortality, CVD mortality) before 55 years in a father or brother, and before 65 years in a mother or sister.[4,5]Bachmann,et al.[6]used a more conservative cutoff of CHD before 50 years in their analysis of the Cooper Center Longitudinal Study in men. Risk score calculators that incorporate premature family history as a predictor of cardiovascular risk also exhibit variation in their definitions.The Joint British Societies 3 (JBS3) calculator defines it broadly as a close relative under 60 years suffering from CVD.[17]The QRISK2 calculator defines it as angina or heart attack in a first-degree relative younger than 60 years.[18]The Reynolds Risk Score for cardiovascular risk in women defines it as a parent with MI before 60 years.[19]The latter definition is in closest accordance with our findings.
Clinical Implications
Many widely used risk score calculators, such as the Framingham Risk Score,[3]the Pooled Cohort Equation,[20]and the European SCORE[21]do not incorporate family history of premature CVD as a risk predictor. This is not only due to the lack of agreement on a specific definition; studies have shown that it only carries a minimal incremental prognostic value once other standard cardiovascular risk factors are adjusted for.[16,22]This finding was also replicated in our cohort. That is no surprise given that DM, hypertension, obesity and hyperlipidemia have strong genetic determinants. However, familial history captures important additional prognostic information that genetic history or precise genomic polygenic scores do not, that is, familial environmental determinants such as diet type, smoking habits and exposure, and living conditions.
There is recent compelling evidence about a growing list of non-traditional risk factors for CVD,namely socioeconomic status, particulate pollutants,noise exposure, and stressful lifestyle.[23-25]Familial history, in virtue of its encompassing and holistic nature, can be a simple tool to capture such factors before we are able to precisely quantify and routinely incorporate these non-traditional risk factors into cardiovascular prediction models. Interestingly, British prediction models have already incorporated area of residence (UK postcode) for QRISK2[18]and the Townsend deprivation score for JSB3[17]as surrogates for socioeconomic status.
While the decision to incorporate familial history as an integral part of prediction models remains debatable, it remains an important risk-enhancing factor to consider in clinical decision-making, especially in younger patients with no or few borderline traditional risk factors. Importantly, the final decision to initiate preventive measures remains a shared physician-patient decision.[22]
STRENGTHS AND LIMITATIONS
One major advantage provided by the Lipid Research Clinics Prevalence Study cohort is the richness of the information collected about familial history of CVD. This enabled us to study the prognostic value of each familial factor individually and then to understand which factors remain significant after all important familial factors are accounted for. Another unique aspect of this cohort is that it enabled us to study such associations while being relatively unaltered by a mix of cardiovascular medications.Statins, beta-blockers, and antihypertensives targeting the renin-angiotensin axis were not widely used at the time of study start. This provides insight into disease associations with mortality independently of modern interventions. Whether such interventions alter the association between familial factors and cardiovascular mortality remains to be investigated.
One limitation of this study is the subjective nature of how information about familial history was collected. Self-reporting of familial disease by participants is subject to recall bias. One other important consideration in the ascertainment of familial history is that maternal history of CHD might have been a stronger predictor of cardiovascular mortality than paternal history due to higher biological accuracy in determining the maternal relationship. The collection of genetic information could have provided more insight into the significance of this observation.Additionally, although data about offspring CVD was available, frequency of disease was low that reliable estimates could not be drawn.
An important point to mention is that half the participants had hyperlipidemia at baseline, which can potentially limit the generalizability of our findings. However, as shown in our subgroup analysis,a parental history of premature CHD predicted cardiovascular mortality independently of baseline hyperlipidemia. Finally, more precise and consistent definitions of premature CHD would allow for more refined estimates of the actual cardiovascular risk.
CONCLUSIONS
A parental history of CHD (defined as angina or MI), especially before age 60 years, was the best familial predictor of cardiovascular mortality in this study. This finding could help better identify highrisk patients and possibly initiate or intensify strategies to prevent cardiovascular mortality.
ACKNOWLEDGMENTS
This study was supported by the Fogarty International Center and Office of Dietary Supplements of the National Institutes of Health (D43 TW009118)via the Scholars in HeAlth Research Program(SHARP) at the American University of Beirut, and the NHLBI K24HL 136 852 of Dr. Mora. All authors had no conflicts of interest to disclose. The authors would like to acknowledge the SHARP faculty, staff,and director, Dr. Ghada El-Hajj Fuleihan, at the American University of Beirut for providing trainees with the necessary quantitative skills to analyze large clinical datasets. The authors would also like to acknowledge the investigators, staff and participants in the Lipid Research Clinics Prevalence Study. The findings and conclusions in this study are those of the authors and do not necessarily represent the official position of the National Institutes of Health.
杂志排行
Journal of Geriatric Cardiology的其它文章
- The incidence and predictors of high-degree atrioventricular block in patients with bicuspid aortic valve receiving selfexpandable transcatheter aortic valve implantation
- The role of electrocardiographic imaging in patient selection for cardiac resynchronization therapy
- Homocysteine, hypertension, and risks of cardiovascular events and all-cause death in the Chinese elderly population:a prospective study
- Impact of SGLT2 inhibitors on major clinical events and safety outcomes in heart failure patients: a meta-analysis of randomized clinical trials
- Are angiographic culprit lesions true? Disagreement between angiographic and optical coherence tomographic detection
- Inflammatory abdominal aortic aneurysms treated with leflunomide: an eight-year follow-up case report and literature review
