Homocysteine, hypertension, and risks of cardiovascular events and all-cause death in the Chinese elderly population:a prospective study
2021-11-15ZhongYingZHANGXiangGUZheTANGShaoChenGUANHongJunLIUXiaoGuangWUYanZHAOXiangHuaFANG
Zhong-Ying ZHANG, Xiang GU, Zhe TANG, Shao-Chen GUAN, Hong-Jun LIU,Xiao-Guang WU, Yan ZHAO, Xiang-Hua FANG,✉
1. Geriatric Department, Xuanwu Hospital, Capital Medical University, Beijing, China; 2. Evidence-based Medical Center,Xuanwu Hospital, Capital Medical University, Beijing, China; 3. Medical Affair Department, Beijing Friendship Hospital,Capital Medical University, Beijing, China; 4. Beijing Geriatric Healthcare Center, Xuanwu Hospital, Capital Medical University, Beijing, China; 5. Education Department, Xuanwu Hospital, Capital Medical University, Beijing, China
ABSTRACT
Cardiovascular disease (CVD) is the leading cause of morbidity and mortality globally in the elderly population,[1]leading to functional impairment and high medical treatment costs. Effective prevention and control of risk factors for CVD remain the best approaches for reducing the disease burden of CVD.[2]Hypertension is a well-known modifiable cardiovascular risk factor that contributes to CVD. The rates of treatment and control of hypertension in China were 45.8%and 16.8%, respectively, in 2015, and were significantly higher than in 1991.[3]However, the incidence of ischemic stroke is still increasing by 8.7% per year,[4]and the incidence of coronary heart disease has increased significantly in China.[5]Thus, it remains urgent to control multiple factors and provide comprehensive management for hypertensive patients.
Epidemiological studies have shown that homocysteine is associated with the risk of CVD and death.[6,7]However, most homocysteine-lowering intervention trials designed for secondary prevention did not decrease the incident CVD events.[8-11]Thus, it is controversial whether homocysteine is a marker or a treatable causative risk factor of increased CVD.Recent research has suggested that hyperhomocysteinemia is independently associated with peripheral microvascular endothelial dysfunction[12]and the carotid-femoral pulse wave velocity[13]in hypertensive patients but not in non-hypertensive individuals. A homocysteine-lowering trial with folic acid demonstrated a significant reduction in the relative risk of first stroke by 21% in Chinese hypertensive patients without a history of stroke or myocardial infarction (MI).[14]Thus, hyperhomocysteinemia is considered a modifiable contributor to CVD in hypertensive patients without a history of CVD. Patients with H-type hypertension, a term used to describe individuals with concomitant hypertension and hyperhomocysteinemia,[15]may be at a particular high risk of CVD. However, except for a nested case-control study,[16]most of the evidence for the combined effect of homocysteine and hypertension came from cross-sectional studies.[17-19]
Therefore, we hypothesize that hyperhomocysteinemia would be associated with the risk of incident CVD events and all-cause death independently in a community-based population without a history of CVD and that hyperhomocysteinemia combined with hypertension would amplify the risk of incident CVD events and all-cause death. We tested these hypotheses in the Beijing Longitudinal Study of Aging (BLSA), a prospective cohort study in Beijing,China. The findings of this study could have important clinical and public health implications for the primary prevention of CVD and comprehensive management of hypertension in Chinese elderly patients.
METHODS
Study Design and Population
This study’s data was collected from the BLSA cohort, a prospective cohort of community-dwelling Chinese people. The longitudinal cohort of the BLSA was initiated in 1992, and there are seven waves of data dating from then. In brief, Beijing consists of eighteen administrative districts divided into three categories according to their degree of urbanization and economic status: eight main cities,five suburbs, and five extended suburbs. Firstly,one administrative district was selected randomly from each category. The districts selected were the Xuanwu District (urban), Daxing County (suburb),and Huairou County (extended suburb). Secondly,specific communities/villages were randomly selected from these districts based on demographic characteristics and the educational level of the population. Thirdly, lists of residents aged 55 years and older in the selected communities/villages were obtained from the local government. All residents aged 55 years and older from specific communities/villages in the selected districts were invited to participate in the baseline interview, questionnaire survey, physical examinations, and laboratory tests.The cohort design, implementation, maintenance,and some of the results of the BLSA have also been reported previously.[20-22]The current study employed the last two waves.
The inclusion criteria were as follows: (1) individuals who lived in the selected district based on the randomized sampling procedure; (2) individuals who were able to participate in normal communication; (3) individuals who were able to read and complete the questionnaire; and (4) individuals who were able to understand and sign the informed consent form. Exclusion criteria were as follows:(1) individuals with a history of MI; (2) individuals with serious congestive heart failure (CHF) (New York Heart Association classes III-IV); (3) individuals with a history of stroke; (4) individuals who refused blood sampling; and (5) individuals who refused to sign the informed consent form.
Participants were classified into four groups according to the homocysteine level (normal or hyperhomocysteinemia) and presence of hypertension(yes or no). The baseline information of the current study was based on the 2009 survey results, and a total of 2,468 participants were enrolled. Among them, 164 participants were excluded because of a history of stroke, MI, or serious CHF. Furthermore,1,010 participants withdrew because they refused to have their blood sampled, and 37 participants were excluded because important information was missing.Eventually, 1,257 participants were enrolled, and their data were carefully collected (Figure 1). The cohort was followed up from November 2014 to February 2015.

Figure 1 Flow diagram of the study participants.
The Ethics Committee of Xuanwu Hospital, Capital Medical University, Beijing, China, approved this study (No.2018-038). This study was conducted in accordance with the Declaration of Helsinki. All participants provided written informed consent.
Baseline Data Collection
From June 2009 to August 2009, face-to-face interviews and questionnaire survey were conducted by well-trained investigators to collect baseline information from each participant. We conducted a standardized questionnaire to obtain the following information: (1) demographics: name, age, sex, ethnicity, date of birth, marital status, and geographical location; (2) lifestyle: smoking habit, alcohol consumption, and physical activity (≥ 1 h/day or < 1 h/day); and (3) medical history and medication use:durations of hypertension, diabetes mellitus (DM),coronary heart disease, and stroke and use of antihypertensive, hypoglycemic, and lipid-lowering therapy. The categories of active smoking/drinking were former, current, and never-smokers/drinkers.In the present report, we refer to smokers/drinkers as those who were former or current smokers or drinkers. All participants were required to answer all of the questions to the best of their knowledge.
Physical examinations were performed by investigators at designated hospitals or on-site. Blood pressure (BP) was measured according to a standard protocol. After a 5-min rest, sitting BP was measured with a digital BP monitor (Omron HEM-4021, Omron,Kyoto, Japan). The measurements were duplicated for each participant on the non-dominant arm within a 2-min interval. The mean value was used for the analysis. Pulse pressure was calculated as the systolic BP (SBP) minus the diastolic BP (DBP).
Laboratory Examinations and Definitions
Fasting blood samples were collected in the morning after the completion of the questionnaire survey and physical examination. Blood sampling procedures have been reported elsewhere.[21]In short, all venous blood samples were collected after 12-h fasting and were centrifuged within 1 h at 3,000 r/min for 15 min immediately after collection.The separated serum samples were stored in a refrigerator at 2 ℃ and 8 ℃ until testing. All laboratory measurements were performed by routine methods or as per the user manual of the test kits in a commercial laboratory (IPE Center for Clinical Laboratory,Beijing, China) within 24 h.
The serum total homocysteine concentration was measured using an enzymatic cycling assay with the Abbott ARCHITECT®System (Architect-i 2000,Abbott Laboratories, Chicago, IL, USA). Fasting glucose was determined using the glucose oxidaseperoxidase method. High-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C) were measured using the direct assay.Total cholesterol, triglyceride, and creatinine levels were determined by the standard enzymatic method using the Hitachi Clinical Analyzer (Hitachi 7600,Hitachi, Tokyo, Japan). The estimated glomerular filtration rate (eGFR) was calculated according to the Modification of Diet in Renal Disease formula.[23]High-sensitivity C-reactive protein (hs-CRP) was measured using a high-sensitivity nephelometric assay with the Behring Nephelometer II system(Dade Behring, Marburg, Germany).
A normal homocysteine concentration was considered to be < 15 μmol/L. Hyperhomocysteinemia is conventionally described as ≥ 15 μmol/L.[24]Hypertension was diagnosed as a SBP ≥ 140 mmHg and/or a DBP ≥ 90 mmHg and a medical history or current use of antihypertensive medications, according to the Joint National Committee guideline.[25]H-type hypertension was defined as concomitant hypertension and hyperhomocysteinemia. The DM was diagnosed according to the following American Diabetes Association criteria[26]: fasting glucose level ≥7.0 mmol/L, medical history of DM, or currently receiving hypoglycemic therapy.
Prospective Follow-up and Endpoints
Participants who were enrolled in the baseline survey were followed for an average of 4.84 years.We administered a second standardized questionnaire to each participant. The medical history and health insurance records of each participant were reviewed by well-trained staff. New cases of CVD events, deaths, and whether they regularly took folic acid during the entire follow-up period were collected. The participants answered the following question: “Have you taken folic acid since 2009?” The categories were: “never, occasionally, and frequently”.Regular supplementation of folic acid was defined as those who frequently took folic acid until the investigation. The BP measurement was the same as the baseline standard protocol.
Death records were obtained from the participants’ families, medical records, and the local Center for Disease Control and Prevention. Causes of death were codified according to the principles of the 10thversion of the International Classification of Diseases.
The CVD events were defined as a composite of coronary events (i.e., fatal MI, non-fatal MI, percutaneous coronary intervention, coronary artery bypass surgery, or sudden cardiac death), stroke events(i.e., fatal stroke or non-fatal stroke), and peripheral vascular events (i.e., peripheral vascular surgery).During the follow-up process, incident CVD events were assessed by the following questions in a standardized questionnaire: “Have you been told by a doctor that you have been diagnosed with MI or stroke since 2009?” or “Have you had a percutaneous coronary intervention, coronary artery bypass surgery, or peripheral vascular surgery since 2009?”Investigators were responsible for collecting data,including diagnosis certificates, hospital discharge records, imaging data and whether it was diagnosed by a neurologist or cardiologist. The diagnosis time,diagnosis hospital, electrocardiogram and myocardial enzyme records, and other detailed medical history were also collected. Other available information, such as personal health files and inpatient medical records, were also obtained from local community hospitals in the three districts, Xuanwu and Friendship Hospitals. The medical diagnostic team of two attending physicians from the Department of Geriatrics of Xuanwu Hospital and Friendship Hospital judged CVD events.
Statistical Analysis
For continuous variables, normally distributed data are expressed as mean ± SD. The differences between the groups were assessed using analysis of variance, followed by the post-hoc test with the least significant difference correction. Non-normally distributed data are expressed as the median value(interquartile range, IQR), and the median differences between the groups were assessed using the Kruskal-Wallis test. The Pearson’s chi-squared test was used to determine the differences in the categorical variables, which are reported as number(percentage).
Cumulative hazards of CVD events, stroke events, coronary events, and all-cause death according to hyperhomocysteinemia and the presence of hypertension were estimated using the Kaplan-Meier method. Additionally, multivariate Cox regression analysis was used to evaluate the contribution of combined associations of hyperhomocysteinemia and hypertension to the risk of the endpoints. Using the subgroup of normal homocysteine level and normotensive as the reference, the hazard ratios (HRs) and 95% confidence intervals (CIs) of CVD events, stroke events, coronary events, and allcause death were calculated. The Cox regression model comprised the following scenarios: crude(unadjusted), model 1 (adjusted for sex and age),and model 2 (adjusted for variables in model 1 plus smoking status, alcohol consumption, DM, physical activity, LDL-C, hs-CRP, eGFR, antihypertensive treatment and regular supplementation of folic acid).When we evaluated the individual association between hyperhomocysteinemia and the endpoints, we additionally adjusted hypertension in the Cox regression model (adjusted for twelve factors). Possible multiplicative interactions between homocysteine and hypertension were examined by adding a product term (homocysteine multiplied hypertension) to the regression model via the likelihood ratio test under model 2.
Two-sidedP-value < 0.05 were considered statistically significant. Statistical analysis was performed using SPSS 19.0 (SPSS Inc., IBM, Armonk, NY, USA).
RESULTS
Participants’ Baseline Information
A sampling diagram of the study population is shown in Figure 1. One hundred fifty-six participants were lost to follow-up due to relocation and noncompliance throughout the follow-up. The baseline characteristics of the follow-up participants according to hyperhomocysteinemia and hypertension are presented in Table 1. Among the 1,101 follow-up participants, the mean age was 69.13 ± 8.04 years, and the median homocysteine level was 16.57 μmol/L (IQR: 12.98-21.88). The prevalence of hyperhomocysteinemia was 61.85%(681/1,101) and 62.03% (415/669) in all follow-up participants and hypertensive participants, respectively. Compared with the reference group (participants without hypertension and hyperhomocysteinemia), the participants with H-type hypertension were older and had a significantly higher proportion of males. In addition, the SBP, DBP,pulse pressure, triglyceride level, and hs-CRP level were significantly higher in the H-type hypertension group than in the reference group, and the HDL-C level and eGFR were significantly lower in the H-type hypertension group than in the reference group.
Individual Association of Hyperhomocystein emia with CVD Events and All-cause Death
During a mean follow-up of 4.84 years (6,081.8 person-years), 155 deaths occurred, and 156 participants (12.41%) were lost to follow-up, 19 participants took folic acid regularly during the follow-up.During the follow-up, a total of 168 participants had 173 CVD events, and five participants had coronary events and stroke events simultaneously. Of the total 173 CVD events, there were 58 coronary events(33.5%), 113 stroke events (65.3%), and 2 peripheral vascular events (1.2%).
Table 2 details the crude and adjusted HRs and 95% CIs of hyperhomocysteinemia for the risks ofCVD events and all-cause death. After adjusting for sex, age, smoking status, alcohol consumption, DM,hypertension, physical activity, LDL-C, hs-CRP,eGFR, antihypertensive treatment and regular supplementation of folic acid, hyperhomocysteinemia independently increased the risk of CVD events and all-cause death by 45% (HR = 1.45, 95% CI:1.01-2.08) and 55% (HR = 1.55, 95% CI: 1.04-2.30),respectively.

Table 1 Baseline characteristics of the follow-up participants according to hyperhomocysteinemia and hypertension.
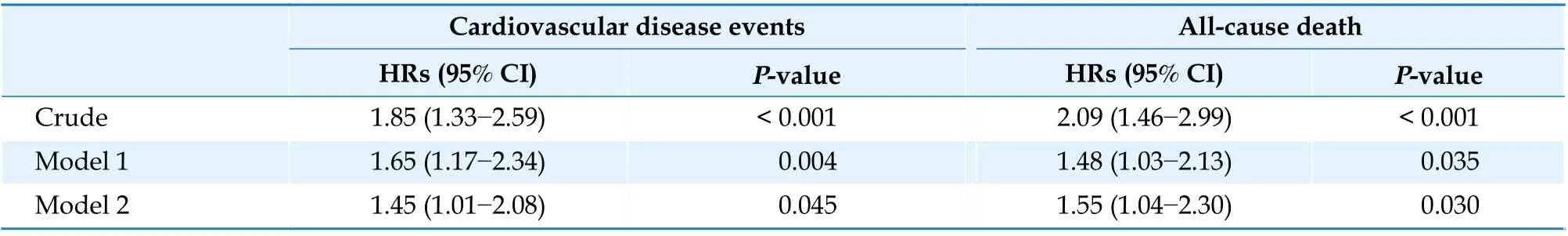
Table 2 Cox regression analyses of hyperhomocysteinemia for cardiovascular disease events and all-cause death.
Combined Association of Hyperhomocysteinemia and Hypertension with CVD Events and Allcause Death
After adjusting for all confounding variables and as compared with the reference group (subgroup of normal homocysteine level and normotensive), the HRs (95% CIs) of CVD events, stroke events, and coronary events were 1.76 (0.91-3.44), 1.62 (0.77-3.43), and 5.59 (0.71-43.80) for hyperhomocysteinemia alone and 1.82 (0.92-3.63), 1.38 (0.63-3.05),and 8.07 (1.03-63.07) for hypertension alone, respectively. Of note, the participants with hyperhomocysteinemia in combination with hypertension carried the highest risk of CVD events, stroke events,and coronary events compared to the participants without either condition (HR = 2.44, 95% CI: 1.28-4.65; HR = 2.07, 95% CI: 1.01-4.29; and HR = 8.33,95% CI: 1.10-63.11, respectively) (Table 3). Figure 2 presents the crude and adjusted HRs with 95% CIs of all-cause death. Similar findings were obtained for all-cause death. The risk of all-cause death was significantly higher in the group of participants with a combination of hyperhomocysteinemia and hypertension than in the reference group. The participants with H-type hypertension manifested the highest risk of all-cause death. The HRs (95% CIs) of all-cause death in the unadjusted model, model 1(adjusted for age and sex), and model 2 (adjusted for variables in model 1 plus smoking status, alcohol consumption, DM, physical activity, LDL-C, hs-CRP, eGFR, antihypertensive treatment and regular supplementation of folic acid) were 4.05 (2.10-7.82), 2.28 (1.17-4.42), and 2.31 (1.15-4.62), respectively. The HRs (95% CIs) were 2.70 (1.34-5.45), 1.84(0.91-3.72), and 1.70 (0.83-3.48) for hyperhomocysteinemia alone and 2.17 (1.06-4.43), 1.67 (0.81-3.41), and 1.55 (0.73-3.29) for hypertension alone,respectively.
Although the highest risks of CVD events and allcause death were observed in the subgroup withH-type hypertension, multiplicative interaction terms between hyperhomocysteinemia and hypertension for CVD events, stroke events, coronary events, and all-cause death were not statistically significant in model 2 (P= 0.299,P= 0.290,P=0.293, andP= 0.325, respectively).

Table 3 Hazard ratios with 95% CI of cardiovascular disease events, stroke events, and coronary events according to the presence of hyperhomocysteinemia and hypertension.

Figure 2 HRs with 95% CIs for all-cause death according to hyperhomocysteinemia and hypertension. (A): Unadjusted; (B): model 1; and (C): model 2. Model 1: adjusted for sex and age. Model 2: adjusted for variables in model 1 plus smoking status, alcohol consumption, diabetes mellitus, physical activity, low-density lipoprotein cholesterol, high-sensitivity C-reactive protein, estimated glomerular filtration rate, antihypertensive treatment and regular supplementation of folic acid. CI: confidence interval; HHcy: hyperhomocysteinemia; HRs: hazard ratios; HT: hypertension.
DISCUSSION
In this community-based prospective cohort study, hyperhomocysteinemia was independently associated with the risk of incident CVD events and all-cause death in the Chinese elderly population without a history of CVD. We did not find a synergistic effect on the endpoints between homocysteine and hypertension, but we found an additive effect.Participants with H-type hypertension had the highest risk of incident stroke events, coronary events, and all-cause death after adjusting for multiple risk factors, including antihypertensive treatment and regular folic acid supplementation. This finding may provide evidence of hyperhomocysteinemia for incident CVD events among the elderly population and shed light on a new target for the primary prevention of CVD in hypertensive individuals.
Homocysteine is a sulfur amino acid derived from the essential amino acid methionine. Hyperhomocysteinemia can increase oxidative stress, endothelial cell injury, and vascular inflammation.[27]In the current study, the prevalence of hyperhomocysteinemia was 62.03% (415/669) in hypertensive participants.Given the selection bias, the prevalence of H-type hypertension may be underestimated. Most homocysteinelowering intervention trials did not show a significant effect in reducing the risk of incident CVD events in secondary prevention among the general population.[28]However, hyperhomocysteinemia accompanied by hypertension jointly contributes to microvascular endothelial dysfunction,[12]the carotidfemoral pulse wave velocity,[13]early carotid artery atherosclerosis,[21]and further CVD events.[19]A primary prevention trial further identified an additional 21% reduction (HR = 0.79, 95% CI: 0.68-0.93)in the risk of stroke in 20,702 hypertensive patients when folic acid was supplemented with antihypertensive drugs.[14]
Our results align with those of a prospective, nested, case-control study in Chinese adults who had no history of stroke at baseline.[16]Homocysteine and hypertension appear to act additively but not synergistically to increase the risk of stroke and stroke death. After adjustment for the major covariates and using normal BP/normal homocysteine as the reference group, the odds ratios (ORs) with 95%CIs of incident stroke were increased in subjects with hyperhomocysteinemia (≥ 10 μmol/L) alone[3.5 (0.7-16.5)] and in subjects with hypertension alone [9.7 (1.7-56.4)]. The highest odds were found among subjects with H-type hypertension [12.7(2.8-58.0)]. A similar pattern was found for stroke death. The effect of interaction between homocysteine and hypertension on the risk of stroke and stroke death was not significant. These results indicated that homocysteine and hypertension appear to act additively on a multiplicative scale to increase the risk of stroke and stroke death.
Two previous retrospective studies found that the interaction effect between homocysteine and BP on the risk of increased arterial stiffness[29]or stroke was statistically significant. Chen,et al.[29]reported a stronger positive association between homocysteine and increased arterial stiffness in patients with high SBP levels (≥ 145 mmHg, 16,644 participants) than in those without (Pinteraction= 0.048).Another research study found that homocysteine was independently associated with stroke in hypertensive patients (OR = 1.03, 95% CI: 1.02-1.04), and a significant interaction was identified between homocysteine and SBP (OR = 1.18, 95% CI: 1.11-1.26,Pinteraction< 0.001) and DBP (OR = 1.22, 95% CI:1.15-1.30,Pinteraction< 0.001).[30]Additionally, Fan,et al.[19]revealed a significant multiplicative interaction of hypertension and homocysteine for the presence of moderate/severe neurological severity in patients with first-ever ischemic stroke (OR = 13.15, 95% CI:5.29-32.69) after adjustment for confounding factors,and in 69.5% of patients, this was attributed to the biological interaction in all patients with moderate/severe neurological severity (0.70 of the attributable proportion due to interaction, 95% CI: 0.44-0.95). These inconsistencies in the interaction effect between the current study and previous studies may be caused by differences in the research design(prospective or retrospective study), diagnostic criteria for hyperhomocysteinemia, and endpoints (incident CVD events or outcome of neurological severity after stroke).
In the current study, participants with H-type hypertension had a higher BP and dyslipidemia (higher triglyceride level/lower HDL-C level) than the reference group, which is congruent with previous findings from the BLSA. Homocysteine was independently associated with pulse pressure[31]and increased the risk for wide pulse pressure by 33% (OR =1.33, 95% CI: 1.04-1.68). Hyperhomocysteinemia had a mild-to-moderate independent effect on early carotid artery atherosclerosis, considered the prodromal stage of CVD, and the strength of the effect increased when combined with hypertension.[21]Triglyceride/HDL-C represents an atherogenic signature of insulin resistance,[32]CVD, and mortality.[33]Homocysteine is involved in insulin resistance, as it activates the adipocyte nucleotide-binding oligomerization domain-like receptor protein 3.[34]Zhang,et al.[15]found that patients with H-type hypertension had higher HOMA-IR (Homeostatic Model Assessment for Insulin Resistance) values, a surrogate marker of insulin resistance, than those who only had hypertension [3.58 (2.59-4.85)vs.2.96(1.90-3.49),P< 0.01]. Hyperhomocysteinemia (> 10 μmol/L) interacted with hypertension to exacerbate insulin resistance (ß = 0.501,P< 0.01). In addition, homocysteine is associated with increased BP variability[35]and disturbed circadian BP variation[36]in hypertensive individuals. These results indicate that hyperhomocysteinemia may exacerbate target organ damage and cause further CVD events in patients with hypertension due to dyslipidemia, insulin resistance, and abnormal BP rhythm and variation.
In addition, the association between homocysteine and CVD was more pronounced in elderly individuals in previous studies. In very older people (aged 85 years and older) with no history of CVD, homocysteine concentrations alone could accurately identify cardiovascular mortality, whereas classic risk factors were not used in the Framingham risk score.[37]A subanalysis of a prospective study of pravastatin use in elderly individuals at risk of CVD(PROSPER) suggested that homocysteine levels indicate older people at high risk for fatal and nonfatal coronary heart disease and all-cause mortality.[38]Towfighi,et al.[39]analyzed the effect of age in the Vitamin Intervention for Stroke Prevention trial and revealed that the benefit of homocysteine-lowering therapy exists only among older individuals, not younger individuals.
STRENGTHS AND LIMITATIONS
The strengths of the current study are as follows:(1) we conducted the study in a community-based population in Beijing, a northern city in China, with a high risk of stroke. The initial sampling process was determined by a stratification-random clustering procedure to ensure the representativeness of the Beijing population older than 55 years of age; (2) we designed a prospective cohort study to explore the relationship between hyperhomocysteinemia, hypertension, incident CVD events and all-cause death.This prospective study design enabled us to obtain less biased results; and (3) the current study was performed under strict quality control. Potential confounding risk factors, including antihypertensive treatment and regular supplementation of folic acid, were collected carefully and adjusted for as covariables in the multivariate Cox regression analysis.
However, our study has certain limitations. Firstly,and most importantly, selection bias existed because 1,010 participants (43.84%) were excluded for refusing to have their blood sampled, and 37 participants (1.61%) were excluded for missing important information. We compared the characteristics of participants with or without laboratory assessments/important information from a total of 2,304 participants without a history of CVD (Table 4).Compared with the participants who completed the laboratory assessments/important information, those who did not complete the laboratory assessments/important information were older, consumed less alcohol, the lower prevalence of DM and had a higher proportion of males. Smoking status, physical activity,the prevalence of hypertension, as well as the proportion of antihypertensive therapy were not statistically significantly different between the two groups (allP> 0.05). Representativeness of the population could thus be weaker than expected. Secondly,a single test of serum homocysteine was conducted in the current study. Although a previous study showed no corresponding seasonal variation in plasma homocysteine levels,[40]homocysteine might be affected by the intake and status of relevant B vitamins,age, genetic factors, and hypotensive medications.[41]A single serum homocysteine test cannot provide enough information to establish a causal relationship between homocysteine and incident CVD events or all-cause death. Thirdly, loss to follow-up bias existed in the current study. The patients who were lost to follow-up had fewer drinkers and aData are presented as means ± SD orn(%).higher prevalence of DM than those who were followed up (Table 5). Fourthly, hyperhomocysteinemia is associated with renal insufficiency, although eGFR was measured and adjusted for in the current study, it is only a crude indicator of a reduction in renal function, not an early indicator. Fifthly, laboratory measurements were not performed at the end of follow-up due to limited budget and manpower.We measured the BP of the surviving participants,however, the difference in SBP and DBP among the four groups was not statistically significant after 4.84 ± 1.48 years of follow-up. The BP value of surviving participants at the end of the follow-up has been listed in Table 6. Last but not least, the results were not generalizable since we only investigated a Chinese community-based population without a history of CVD.
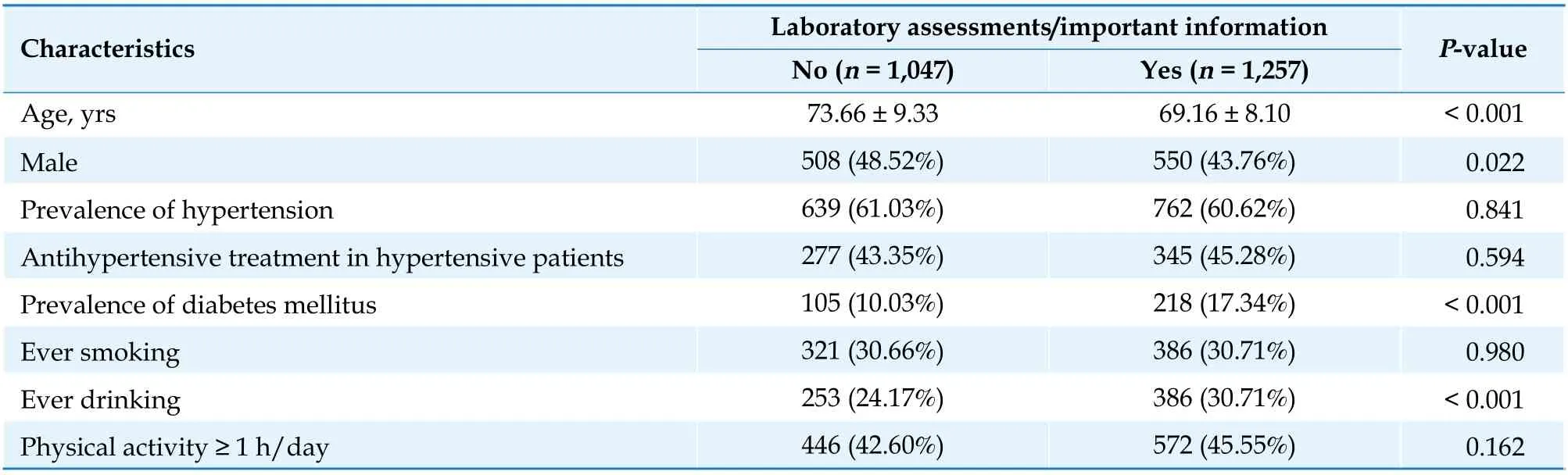
Table 4 Baseline characteristics of participants with or without laboratory assessments/important information from a total of 2,304 participants without a history of cardiovascular disease.
CONCLUSIONS
In conclusion, our findings suggest that hyperhomocysteinemia was an independent risk factor,and when accompanied by hypertension, it contributed to the incident CVD events (stroke and coronary events) and all-cause death in the Chinese communitybased elderly population without a history of MI, CHF,and stroke. The results indicate that hyperhomocysteinemia may be a therapeutic target for the primary prevention of CVD, especially in hypertensive patients. Homocysteine levels were affected by different genetic factors, thus, future research should focus on examining H-type hypertension as a risk factor for incident CVD events and all-cause death in different populations.
ACKNOWLEDGMENTS
This study was supported by the Commission of Science and Technology of Beijing (D121100004912002),the Beijing Natural Science Foundation (No.7152068),and the Project for Collaboration between Basis and Clinic of Capital Medical University (No.17JL69).All authors had no conflicts of interest to disclose.

Table 5 Baseline characteristics of the participants who completed follow-up or were lost to follow-up.

Table 6 Blood pressure value of surviving participants at the end of follow-up.
杂志排行
Journal of Geriatric Cardiology的其它文章
- The incidence and predictors of high-degree atrioventricular block in patients with bicuspid aortic valve receiving selfexpandable transcatheter aortic valve implantation
- The role of electrocardiographic imaging in patient selection for cardiac resynchronization therapy
- Impact of SGLT2 inhibitors on major clinical events and safety outcomes in heart failure patients: a meta-analysis of randomized clinical trials
- Are angiographic culprit lesions true? Disagreement between angiographic and optical coherence tomographic detection
- Inflammatory abdominal aortic aneurysms treated with leflunomide: an eight-year follow-up case report and literature review
- The misfit mitral valve
