Stem cell therapy and diabetic erectile dysfunction: A critical review
2021-11-02CennikonPakpahanRadityaIbrahimWilliamWilliamZakiyatulFaizahJuniastutiJuniastutiMariaLusidaDelvacOceandy
Cennikon Pakpahan, Raditya Ibrahim, William William, Zakiyatul Faizah, Juniastuti Juniastuti, Maria I Lusida,Delvac Oceandy
Cennikon Pakpahan, Zakiyatul Faizah, Department of Biomedical Sciences, Universitas Airlangga, Surabaya 60132, Indonesia
Cennikon Pakpahan, Raditya Ibrahim, William William, Andrology Program, Universitas Airlangga, Surabaya 60132, Indonesia
William William, Department of Medical Biology, School of Medicine and Health Sciences Atma Jaya Catholic University of Indonesia, Jakarta 14440, Indonesia
J uniastuti Juniastuti, Institute of Tropical Disease, Universitas Airlangga, Surabaya 60132,Indonesia
Maria I Lusida, Institute for Tropical Disease, Universitas Airlangga, Surabaya 60132, Indonesia
Delvac Oceandy, Division of Cardiovascular Sciences, The University of Manchester,Manchester Academic Health Science Centre, Manchester M13 9PT, United Kingdom
Abstract Erectile dysfunction (ED) has been identified as one of the most frequent chronic complications of diabetes mellitus (DM). The prevalence of ED is estimated to be about 67.4% in all DM cases worldwide. The pathophysiological process leading to ED involves endothelial, neurological, hormonal, and psychological factors. In DM, endothelial and neurological factors play a crucial role. Damages in the blood vessels and erectile tissue due to insulin resistance are the hallmark of ED in DM.The current treatments for ED include phosphodiesterase-5 inhibitors and penile prosthesis surgery. However, these treatments are limited in terms of just relieving the symptoms, but not resolving the cause of the problem. The use of stem cells for treating ED is currently being studied mostly in experimental animals. The stem cells used are derived from adipose tissue, bone, or human urine. Most of the studies observed an improvement in erectile quality in the experimental animals as well as an improvement in erectile tissue. However,research on stem cell therapy for ED in humans remains to be limited.Nevertheless, significant findings from studies using animal models indicate a potential use of stem cells in the treatment of ED.
Key Words: Stem cells; Diabetes mellitus; Erectile dysfunction; Stem cell therapy;Diabetic erectile dysfunction
INTRODUCTION
Erectile dysfunction (ED) is defined as the persistent or recurrent inability to attain or maintain an adequate erection to reach satisfaction in sexual intercourse[1]. Although this condition does not directly coincide with serious health problems, it correlates with the decline in the quality of life[2]. Moreover, ED has been determined to be more common in men with diabetes mellitus (DM). Previous studies have shown that the prevalence of ED in DM is very high,i.e., at 67.4%, among those with DM[3]. Thus,DM can be regarded as a major risk factor for acquiring sexual dysfunction in men,increasing the risk of ED in men with DM three times higher than that in those without diabetes[4].
MECHANISM OF DIABETIC ED
Erection is a condition when the penile organ becomes rigid and elevated as a result of the erectile tissue being filled with blood. It is modulated by several mechanisms involving endothelial cells and the autonomic nervous function. To better understand the pathogenesis of ED, we need to comprehend the physiological process of erection,as discussed briefly below.
Three key processes occur during erection: (1) The neurologically mediated arterial inflow; (2) The relaxation of smooth muscle within the corpora spongiosa to allow the blood to fill the penile vasculature; and (3) The obstruction of the veins to retain the blood within the penile vasculature[5].
In response to sexual stimuli, the brain transmits parasympathetic signals through the spinal cord. The signal then reaches the non-adrenergic non-cholinergic neuronal terminals, which will then induce the production of nitric oxide (NO) by neuronal NO synthase (nNOS). Likewise, the endothelium also produces NOviathe activation of endothelial NO synthase (eNOS). eNOS and nNOS act as the endothelial and neuronal regulators, respectively. Then, the NO will be transported to the corpora spongiosa where it converts GTP to cyclic guanosine monophosphate (cGMP) with the help of the guanylate cyclase enzyme. The cGMP induces smooth muscle relaxation, allowing arterial flow to fill the cavernosal space. cGMP also potentiates protein kinase G activity that leads to free intracellular calcium being taken up by the endoplasmic reticulum, causing a decrease in calcium flux and an increase in potassium flux from the cells and in turn causing depolarization followed by the relaxation of vascular smooth muscle. When the cavernosal space congests with blood, the veins become compressed and occluded which traps the blood in the cavernosal space during tumescence[1,5,6].
Based on its mechanism, the pathological causes of diabetic-induced ED can be classified into several different categories,i.e., endothelial, neuronal, and endocrinal causes and others, such as psychological and diabetic-related infections and multiple drug prescriptions. However, all of these dysfunctions can intertwine to form ED(Figure 1).
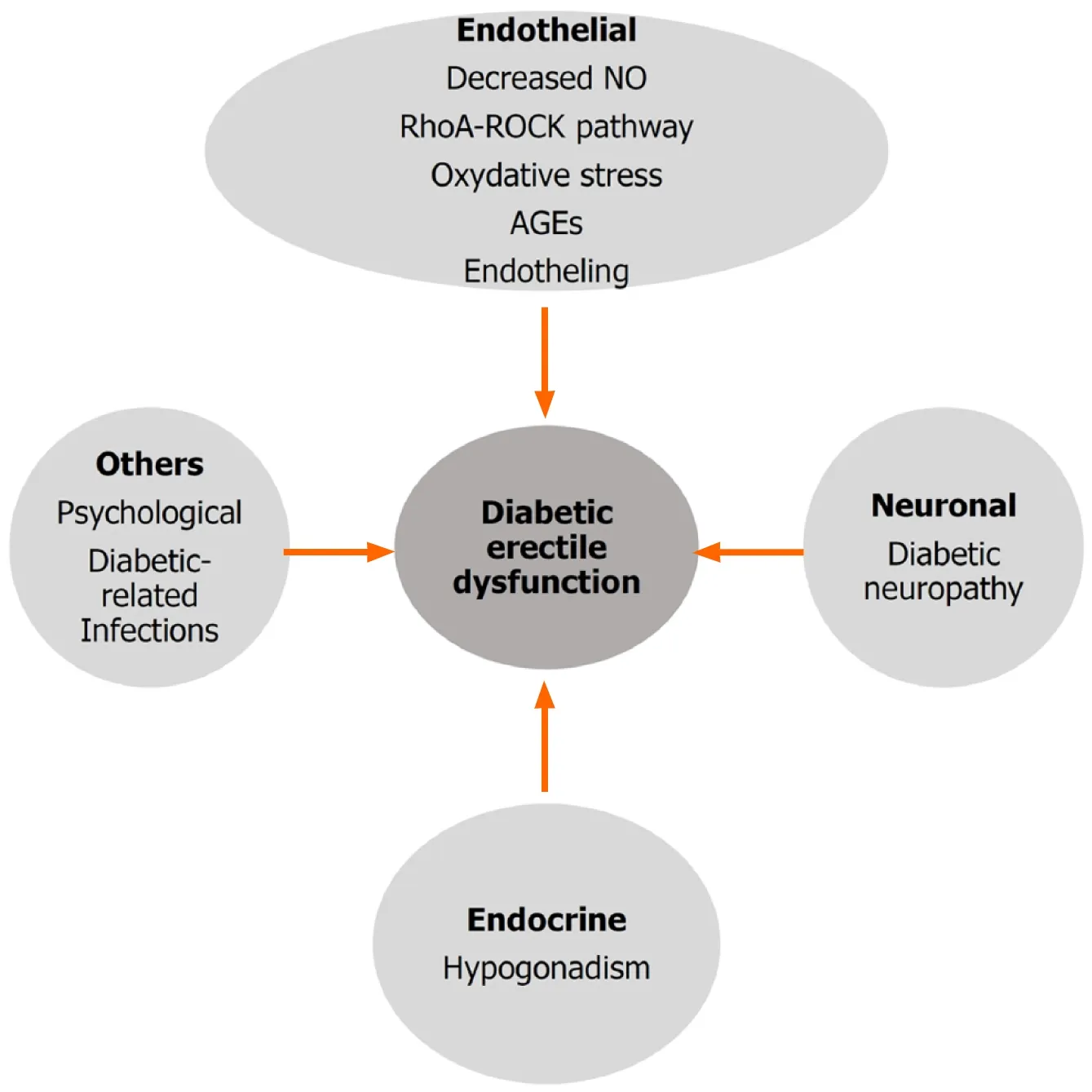
Figure 1 Factors that affect diabetic erectile dysfunction. Major factors that affect the development of diabetic erectile dysfunction include endothelial damage, neuronal dysfunction and endocrine abnormality. Other factors such as psychological factor and diabetic-related infections may also affect the development of diabetic erectile dysfunction. NO: Nitric oxide; AGEs: Advanced glycation end products.
Endothelial factors
One of the most important factors that contribute to the development of diabetic ED is endothelial dysfunction. Endothelial dysfunction is primarily characterized by the loss of NO biological activity and/or biosynthesis at the cellular level, but it may also refer to the reduction in endothelial-dependent vasodilatory response of the smooth muscle cells[7]. The reduction in NO bioavailability is primarily caused by the reduced activity and/or low level of eNOS expression. One possible factor that affects the impairment of eNOS function in diabetic ED is the specific glycosylation process that incapacitates the activation of vascular endothelial growth factor (VEGF) signaling[8].VEGF is proven to be a survival factor for endothelial cells[9] and also contributes to the upregulation of eNOS expression at the molecular level[10]. Despite its important role in modulating eNOS function, it seems that VEGF is not involved in the regulation of nNOS[11,12]. Meanwhile, the role of the RhoA-ROCK (Rho-associated protein kinase) complex in the development of ED has also been comprehensively studied in the past few years. ROCK, which is activated by RhoA, regulates myosin light chain phosphatase (MLCP)viaphosphorylation. This leads to the deactivation of MLCP and subsequently promotes the contraction of the cavernosal body by lowering the levels of calcium in smooth muscles, facilitating the chronic-tonic contraction, thus maintaining the flaccid state of the penis[13]. ROCK has two isoforms: ROCK-1 and ROCK-2. Chiouet al[14] have shown that the induction of type 2 diabetes in rats resulted in the downregulation of eNOS, nNOS, and protein kinase G and the upregulation of the RhoA-ROCK pathway, particularly ROCK-1 isoform. Recently, studies have been conducted to assess the effects of ROCK inhibitors on diabetic ED.Treatment with ROCK inhibitors (SAR407899) has been found to induce penile erection through mechanisms independent of eNOS activity. Thus, this treatment can be targeted to cases of diabetic ED where eNOS activity is impaired[14].
The RhoA-ROCK pathway is also linked with the endothelin-1 (ET1)-induced vasoconstriction[15]. ET1 is a powerful vasoconstrictor, which is released from the penile vascular endothelium. In diabetic patients, the level of plasma ET1 is high[16],which may induce the constriction of the penile blood vessels. ET1 Level may also be elevated in response to the increase in oxidative stress in DM. Chronic hyperglycemia induces the release of free radicals (reactive oxidative species) due to the formation of advanced glycation end products (AGEs). This may contribute to the development of diabetic ED through oxidative cell damage and the suppression of NO action[17,18].
These processes will affect the elasticity of the blood vessels. This may be crucial in the development of ED since elastic blood vessels are important to enable better erections. Moreover, endothelial damage can cause plaque formation and subsequently the blockage of blood vessels. This blockage will result in the inability of the blood vessels to expand properly, thus leading to lower blood capacity and decreased speed of blood flow, resulting in reduced erectile ability.
Neuronal factors
The neuronal aspects of diabetic ED are less well understood due to lacking diagnostic tools available to support this etiology. Several studies have been conducted to assess nocturnal penile erection and whole sexual cycleviamagnetic resonance imaging and positron emission tomography scan to characterize the disorder in the central aspects of erection[19]. Diabetic neuropathy is proven to be an important factor in the development of diabetic ED.
In DM, microangiopathy and nerve damage are caused by increased oxidative stress, accumulation of AGEs, impaired axonal transport, increased flux through the polyol pathway, altered protein kinase C activity, and poly(ADP-ribose) polymerase activity[20]. In a study comparing the significant microangiopathy and macroangiopathy factors in DM, the results indicated that microangiopathy factors, especially diabetic neuropathy, have a more significant impact than macroangiopathy factors in the development of diabetic ED[6].
Endocrine factors
Hypogonadotropic hypogonadism occurs in approximately 30%-40% of men with type 2 diabetes[21]. This condition reduces the production of testosterone, which. has been identified to stimulate the synthesis, storage, and release of pro-erectogenic neurotransmitters; modulate neuronal activity, receptor sensitivity, neurotransmitter liberation, and socio-sexual behavior (increasing libido); and positively influence the levels of dopamine, NO, oxytocin,etc.[1]. In a population-based cohort, the prevalence of hypogonadism in men with ED was 35% compared with 22.7% in men without ED[22]. A study in Japan conducted by Imaiet al[23] showed that decreased testosterone has been associated with severe or moderate ED as opposed to men with mild or no ED and is a risk factor for the development of ED.
EVALUATION OF ED
Decreased erectile capacity is the most common symptom of patients with ED. The standard way to evaluate ED is by using the International Index of Erectile Function-5(IIEF-5) or Erectile Hardness Score (EHS) criteria. IIEF-5 has five questions that can be used to evaluate a person’s sexual life. This questionnaire has a sensitivity of 98% and a specificity of 88%[24].
Moreover, the EHS scoring system can also be used to evaluate the progression of ED following treatment as this scale is convenient to use. The EHS scale categorizes ED into five levels of erectile ability: (1) If the penis does not change; (2) If the penis is slightly enlarged without hardening; (3) If the penis is enlarged and hardens but cannot be used for sexual intercourse; (4) When the penis is enlarged, hardens, and can be used for sexual intercourse, but not maximally; and (5) If the penis is enlarged and hardens to the maximum[25].
In various studies using experimental animals, the evaluation of ED can be performed at the molecular and tissue level, starting from intracavernosal pressure measurement, assessment of angiogenesis process using certain markers, and analysis of erectile tissue profile including the blood vessel content and smooth muscle structure. Other molecular processes can also be used as parameters in the evaluation of ED.
TREATMENT APPROACHES OF DIABETIC ED
Lifestyle modifications
In an effort to regain the capability of erection in diabetic ED, many approaches in terms of lifestyle change have to be applied. Long-term control of glycemic levels is important. Using HbA1c as an index of hyperglycemia, Choet al[26] showed a significant connection between the severity of ED and the level of HbA1c. Physical activity has beneficial effects on the prevention and/or improvement of ED in several prospective studies[27]. With regard to consistent physical activity, weight loss in obese or overweight diabetic patients is strongly correlated with ED, as observed in a prospective study with a study duration of 5 years to 25 years showing that overweight and obese individuals displayed an increased probability in developing ED compared to individuals with normal weight[28]. Importantly, smoking[5] and alcohol appear to be risk factors for ED. In a recent study focusing on alcohol intake and erectile function, it was shown that moderate alcohol consumption conferred the highest protection of erectile function[29].
Pharmacological approach
Phosphodiesterase-5 (PDE5) inhibitors are the first-line therapy for treating ED. The mechanism of the PDE5 inhibitor drug is to inhibit the PDE5 enzyme in vascular smooth muscle cells, thus preventing cGMP degradation to GMP resulting in a sustained erection as the penile blood vessels will be kept dilated. Currently, there are four different types of PDE5 inhibitors available for ED treatment: sildenafil,vardenafil, tadalafil, and avanafil[5].
The most common adverse effects related to the treatment with PDE5 inhibitors are headache and flushing due to the vasodilatory effects of PDE5 inhibitors on the blood vessels. Abnormal vision is experienced by about 6% of individuals taking sildenafil,which could be attributed to the inhibitory action of the drug against PDE6, which is abundant in the retina[5]. On the other hand, back pain and muscle cramps are common adverse effects of tadalafil[5].
In the early 1980s, the first report of intracavernosal injection with papaverine was published and opened a new line of treatment of ED[30]. This second line of treatment is normally used for patients who demonstrated low response or low effectivity or those displaying severe side effects following PDE5 inhibitor treatment. Drugs that are available for ICI are alprostadil (10 mcg, 20 mcg, 40 mcg) and papaverine. ICI treatment induces vasodilation in the arterial smooth muscles, inducing blood flow and blood entrapment within the lacunar spaces of the penis. This treatment has shown better efficacy than oral pharmacological treatment. However, the dropout rate for ICI therapy remains relatively high, and it may be associated with priapism,ecchymoses, hematoma formation, and penile fibrosis[31]. The intraurethral application of alprostadil is also used for second line treatments of ED[30]. The most common side effects of this therapy are penile pain and urethral burning sensation.
Vacuum and surgical approach
Vacuum therapy uses negative pressure to distend the corporal sinusoids and to increase blood inflow to the penis[32]. However, studies that evaluate the use of vacuum constriction device (VCD) reported up to 30% patient dropout due to inadequate rigidity, penile pain, difficulty to ejaculate, and aesthetic causes[33].
The implantation of penile prosthesis as a surgical therapeutic approach for ED is used when the pharmacological (oral, injection, or trans-urethral) and mechanical(VCD) approaches do not achieve satisfactory effects or induce side effects that bothered the patient. The implantation of prosthesis provides a reliable and predictable erection and the highest satisfaction rate to both partners when compared with all of the treatments in ED[34].
All of the treatments available for diabetic ED are developed to achieve sexual satisfaction by improving the erection, but none of them have the capacity to repair the endothelial blood vessels in patients with diabetic ED. New therapeutic strategies to address the main problem in ED, for example, regenerative therapy, have not been widely explored, despite their potential to improve endothelial function. Regenerative therapy, such as stem cell-based therapy, has been identified to have the potential to address the root of the problem in diabetic ED. In the next part of this review, we discuss this therapeutic approach for the treatment for diabetic ED.
STEM CELLS AS A FUTURE THERAPY FOR DIABETIC ED
Ernst Haeckel first introduced stem cells (stammzellein German) in 1868 to delineate unicellular organism from which all multicellular organisms originated[35]. Since then, many studies were conducted to identify different types of stem cells and their potency to treat various human diseases. In 2006, Yamanaka successfully developed induced pluripotent stem cells (iPSCs), which were derived from adult somatic cell reprogramming, increasing the hope of using this type of stem cells as a therapeutic approach for many chronic diseases[35]. Stem cells are unique because they have several features such as the capability to self-renew, extensive proliferation capacity,the potential to differentiate to different cell types, the ability to minimize DNA damage through several DNA repair system, and the ability to maintain a very low metabolism to reduce reactive oxygen species level (quiescent stem cells)[35-37].
Classification of stem cells
Stem cells are undifferentiated cells which are able to differentiate to specialized cell types because of their ability to self-renew and to differentiate to one or more cell lineages. Stem cells can be classified based on their origin and potency[36,38]. To date,adipose-derived mesenchymal stem cell (ADSC) is considered as one of the most frequent cells used in the field of uro-andrology because it is abundant and easy to obtain[39-41]. Moreover, ADSCs have anti-inflammatory properties and an ability to repair vascular and nerve damage, which are the hallmarks in the development of diabetic ED[42].
Three fundamental aspects in stem cell therapy, including that for diabetic ED, need to be considered. These include (1) the origin of the stem cells, tissue biopsy, and cell harvesting, (2)ex vivostem cell culture and clonal expansion through numerous distinct growth signals to produce specified cell type that we need, and (3) the delivery of these stem cells to the organ of interest[38,40].
Different routes of delivery are still being investigated to determine which one gives the best results. So far, several preclinical and clinical trials using various stem cell types have been conducted.
After delivery, two different mechanisms may occur in the tissues following stem cell implantation. First, the stem cells may differentiate to specific cells and hence,regenerate the damaged tissue. Second, stem cells may secrete various growth factors through the paracrine pathway[40,43], which can induce propagation and the differentiation of resident progenitor cells resulting in the repair of damaged tissue[43].
Current progress in stem cell therapy for diabetic ED
We have described that there are currently three main approaches to ED therapy which have been adopted worldwide, these are oral medication, intra-cavernosal injection and vacuum devices and penile prosthesis. However, all of these available options can only ameliorate the symptoms without restoring the damage of the penile tissue[39]. Stem cell therapy may address this problem.
The use of stem cells for the treatment of ED has been developed in several cases of ED post-prostatectomy. Several studies have reported many cases of cavernous nerve damage following prostatectomy[44], and the use of stem cells is now being investigated for the treatment of diabetic ED. It is estimated that ED occurs in approximately 67.4% of all cases of DM[3], illustrating that ED requires effective treatment to improve the quality of life of many patients with diabetes affected with ED.
Erection occurs as a result of the release of NO from sinusoidal endothelial cell walls. The vasodilation that occurs in these blood vessels in response to NO causes the compression of venules located between the trabecula and the tunica albuginea so that the blood becomes trapped within the blood vessels[43]. In simpler terms, it can be described that endothelial cells, cavernous smooth muscle cells, NO and nerve cells are key factors in erection, and DM damages these components. Stem cells, which have the ability to differentiate to specialised cells, may have the ability to correct these defects and restore proper erectile function[43]. This is the rationale for using stem cells as a therapy for ED in the future. However, the underlying mechanism still cannot be fully explained and needs further exploration.
Stem cell research in cases of ED has been pursued since the 2000s. However, most studies were limited to experimental animals. Of the 25 studies reviewed in this article,we found that 21 were conducted on animals (Table 1) and four on humans (Table 2).Of those experiments on animals, the stem cells used were isolated from adipose tissue, bone and human urine. Further, the type of stem cells used was autologous and allogenic, and the average number of stem cells injected per animal was 1 × 106cells,which were delivered intra-cavernosally. However, the number of cells injected among these studies ranged from 2 × 106to 5 × 105cells per animal[46,47]. Moreover, in addition to stem cell therapy, several studies added growth factors, hormones and drugs, including insulin[48], magnetic iron oxide nanoparticles[49], microtissue[50],inducible NOS[47], myocardin[51], icariin[52], corin[53], exosomes from corpus cavernosum smooth muscle cells[54], fibroblast growth factor 2 (FGF2)[55], stromal cell-derived cell factor-1 (SDF-1)[56], VEGF[57] and hepatocyte growth factor[45].
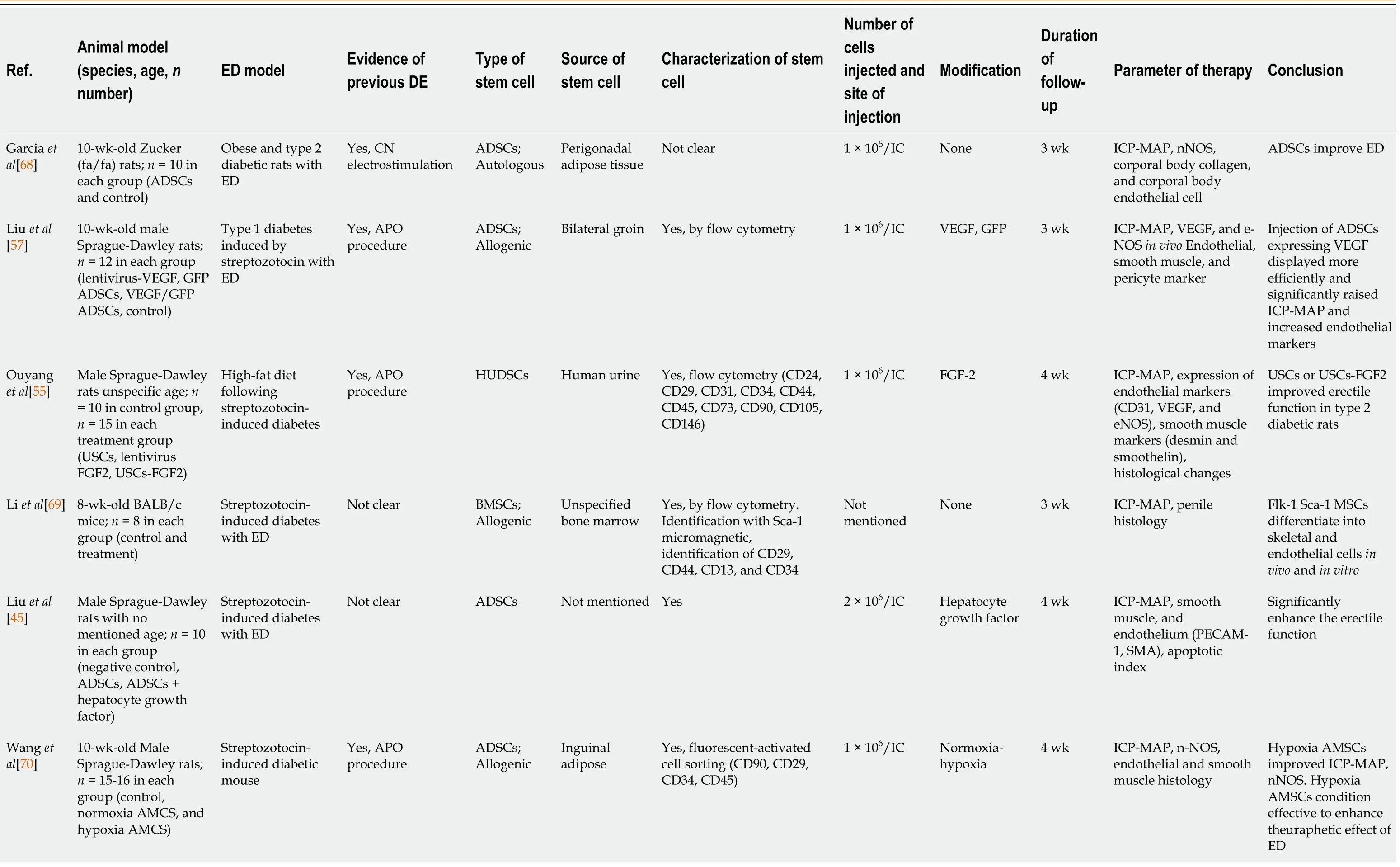
Table 1 List of articles on stem cell therapy in animal models of diabetic erectile dysfunction

Kovanecz et al[71]7-mo-old Zucker(fa/fa) rats; n = 8 in each group(untreated, early diabetic treated with SC, early diabetic with high glucose treated with SC, late diabetic treated with SC, nondiabetic untreated)Not mentioned specifically Not clear MSDCs;Allogenic Hindlimb muscles Not clear 1 × 106/IC Early and late diabetes 8 wk ICP-MAP, nNOS-eNOS,collagen ratio, calponin,inflammation marker Stem cell decreased collagen and fat infiltration,upregulated nNOSeNOS, and improved erectile function Ryu et al[46]12-wk-old C57BL/6J mice; n = 6 in each group (control,diabetic, diabetic with PBS, and diabetic with BMSCs)Streptozotocininduced diabetic mouse Not clear BMSCs;Allogenic Tibiae and Femur Yes, flow cytometry (CD3,CD44, CD45, CD103, CD105,CD117, MHC-1, and Sca-1)3 × 105/IC None 2 wk e-NOS-nNOS,endothelial and smooth muscle content histology BMSCs improved significant recovery of erectile tissue Zhou et al[48]8-wk-old male Sprague-Dawley; n =5 in each group(negative control,ASCs + ad-lucmyocardin, ASCs +ad-luc, ED without treatment)Streptozotocininduced diabetic mouse Not clear ADSCs;Autologous Paratesticular fat tissue Not clear 1 × 106 ADSC/IC Insulin and neutral protamine hagedorn 4 wk ICP-MAP, AGEs, and RAGE; growth factors and cytokine in penis ADSCs combined with insulin improved erectile function and pathological changes Wang et al[52]10-wk-old male Sprague-Dawley rats;n = 14-15 in each group (negative control, icariin,ADMSCs, ADMSCs +icariin)Streptozotocininduced diabetic mouse Yes, APO procedure ADSCs;Allogenic Inguinal adipose Yes, fluorescent-activated cell sorting (CD90, CD29,CD34, CD45)1 × 106/IC Icariin 4 wk ICP-MAP, histology and immunohistology of penis tissue,intracellular ROS levels Icariin-enhanced ADSCs in erectile function Icariin could protect ADSCs against oxidative stress Zhou et al[50]8-wk-old male Sprague-Dawley rats;(n = 8 in control, n =20 in treated groups)Streptozotocininduced diabetic mouse Yes, APO procedure ADSCs;Allogenic Paratesticular fat tissue Not clear 1 × 106 ADSC and 1 × 104 ADSCs per MT/IC Microtissues(MTs)4 wk ICP-MAP, nNOS,smooth and endothelial content histology MTs improved histopathology and erectile function rather than traditional ADSC Zhu et al[49]10-wk-old male Sprague-Dawley rats;n = 8-10 in each group(non-diabetic controls,diabetic with PBS,ADSCs, ADSCs +Magnetic application Streptozotocininduced diabetic mouse Not clear ADSCs;Autologous Paratesticular fat tissue Yes, by flow cytometry(CD34, CD45, CD44)1 × 106 ADSC Magnetic iron oxide nanoparticle 4 wk ICP-MAP, contents,smooth muscle (α-SMA), endothelium(von Willebrand factor),VEGF ADSCs improved erectile function External magnetic field improved efficiency of labeled ADSC in the corpus cavernosum 8-wk-old male Sprague-Dawley rats;n = 12 in each group(control, DM ED, BMMSC, SDF-1 (stromal Jeon et al[56]Streptozotocininduced diabetic with ED Not clear BMSCs Not mentioned Not mentioned 1 × 106/IC SDF-1 4 wk ICP-MAP, nNOS-eNOS,FGF-VEGF in vivo SDF-1 improved ED recovery and smooth muscle content,increased nNOSeNOS, FGF-VEGF
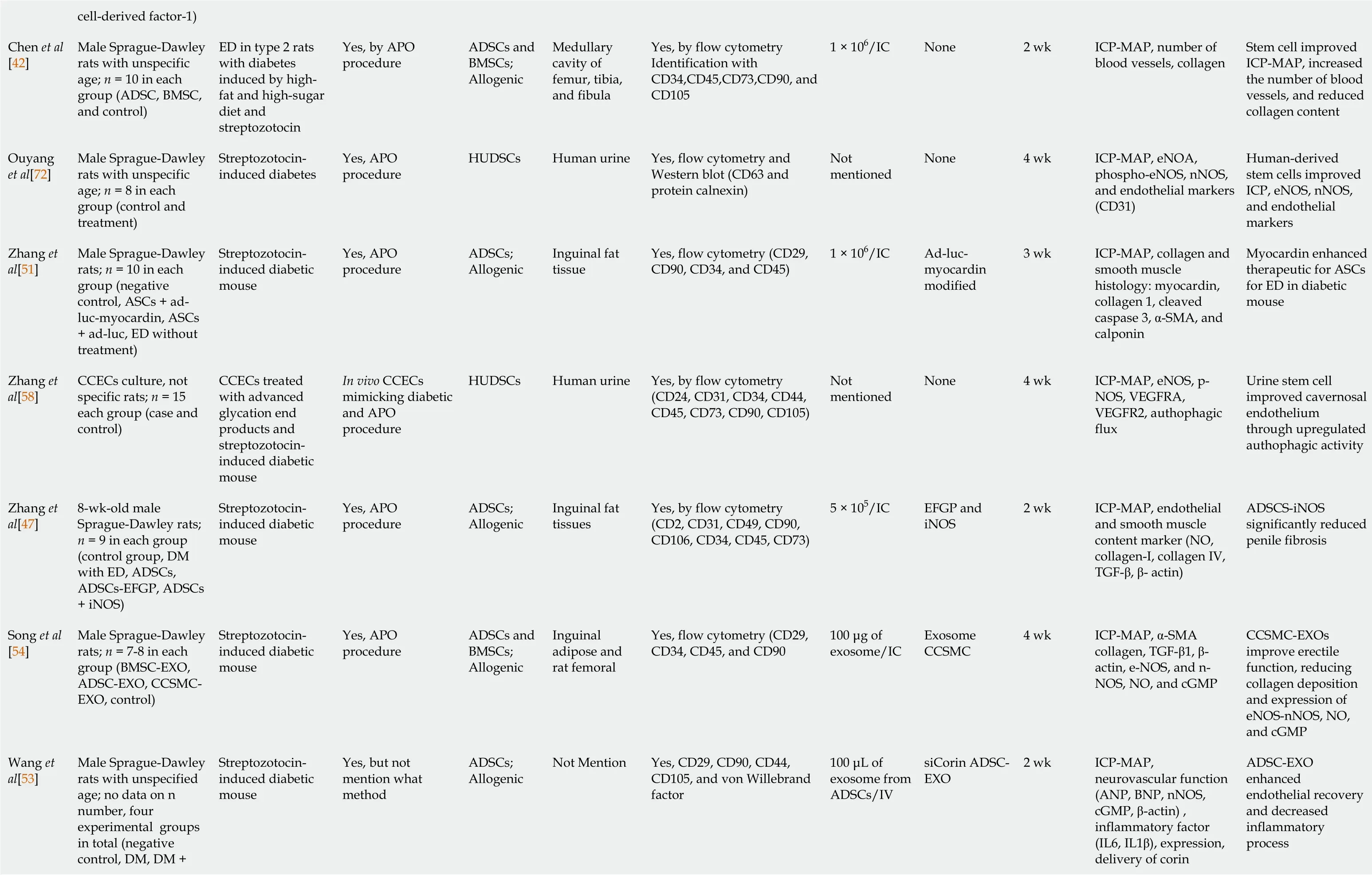
cell-derived factor-1)Chen et al[42]Male Sprague-Dawley rats with unspecific age; n = 10 in each group (ADSC, BMSC,and control)ED in type 2 rats with diabetes induced by highfat and high-sugar diet and streptozotocin Yes, by APO procedure ADSCs and BMSCs;Allogenic Medullary cavity of femur, tibia,and fibula Yes, by flow cytometry Identification with CD34,CD45,CD73,CD90, and CD105 1 × 106/IC None 2 wk ICP-MAP, number of blood vessels, collagen Stem cell improved ICP-MAP, increased the number of blood vessels, and reduced collagen content Ouyang et al[72]Male Sprague-Dawley rats with unspecific age; n = 8 in each group (control and treatment)Streptozotocininduced diabetes Yes, APO procedure HUDSCs Human urine Yes, flow cytometry and Western blot (CD63 and protein calnexin)Not mentioned None 4 wk ICP-MAP, eNOA,phospho-eNOS, nNOS,and endothelial markers(CD31)Human-derived stem cells improved ICP, eNOS, nNOS,and endothelial markers Zhang et al[51]Male Sprague-Dawley rats; n = 10 in each group (negative control, ASCs + adluc-myocardin, ASCs+ ad-luc, ED without treatment)Streptozotocininduced diabetic mouse Yes, APO procedure ADSCs;Allogenic Inguinal fat tissue Yes, flow cytometry (CD29,CD90, CD34, and CD45)1 × 106/IC Ad-lucmyocardin modified 3 wk ICP-MAP, collagen and smooth muscle histology: myocardin,collagen 1, cleaved caspase 3, α-SMA, and calponin Myocardin enhanced therapeutic for ASCs for ED in diabetic mouse Zhang et al[58]CCECs culture, not specific rats; n = 15 each group (case and control)CCECs treated with advanced glycation end products and streptozotocininduced diabetic mouse In vivo CCECs mimicking diabetic and APO procedure HUDSCs Human urine Yes, by flow cytometry(CD24, CD31, CD34, CD44,CD45, CD73, CD90, CD105)Not mentioned None 4 wk ICP-MAP, eNOS, p-NOS, VEGFRA,VEGFR2, authophagic flux Urine stem cell improved cavernosal endothelium through upregulated authophagic activity Zhang et al[47]8-wk-old male Sprague-Dawley rats;n = 9 in each group(control group, DM with ED, ADSCs,ADSCs-EFGP, ADSCs+ iNOS)Streptozotocininduced diabetic mouse Yes, APO procedure ADSCs;Allogenic Inguinal fat tissues Yes, by flow cytometry(CD2, CD31, CD49, CD90,CD106, CD34, CD45, CD73)5 × 105/IC EFGP and iNOS 2 wk ICP-MAP, endothelial and smooth muscle content marker (NO,collagen-I, collagen IV,TGF-β, β- actin)ADSCS-iNOS significantly reduced penile fibrosis Song et al[54]Male Sprague-Dawley rats; n = 7-8 in each group (BMSC-EXO,ADSC-EXO, CCSMCEXO, control)Streptozotocininduced diabetic mouse Yes, APO procedure ADSCs and BMSCs;Allogenic Inguinal adipose and rat femoral Yes, flow cytometry (CD29,CD34, CD45, and CD90 100 μg of exosome/IC Exosome CCSMC 4 wk ICP-MAP, α-SMA collagen, TGF-β1, βactin, e-NOS, and n-NOS, NO, and cGMP CCSMC-EXOs improve erectile function, reducing collagen deposition and expression of eNOS-nNOS, NO,and cGMP Male Sprague-Dawley rats with unspecified age; no data on n number, four experimental groups in total (negative control, DM, DM +Wang et al[53]Streptozotocininduced diabetic mouse Yes, but not mention what method ADSCs;Allogenic Not Mention Yes, CD29, CD90, CD44,CD105, and von Willebrand factor 100 μL of exosome from ADSCs/IV siCorin ADSCEXO 2 wk ICP-MAP,neurovascular function(ANP, BNP, nNOS,cGMP, β-actin) ,inflammatory factor(IL6, IL1β), expression,delivery of corin ADSC-EXO enhanced endothelial recovery and decreased inflammatory process

APO: Apomorphine; ADSCs: Adipose-derived stem cells; BMSCs: Bone marrow stem cells; CCECs: Cavernosal vascular endothelial cells; CCSMC: Corpus cavernosal smooth muscle cell; DM: Diabetes mellitus; ED: Erectile dysfunction;HUDSCs: Human urine-derived stem cells; IC: Intracavernosal; ICP-MAP: Intracavernosal pressure-mean arterial pressure; MSCs: Muscle-derived stem cells; PMSCs: Placental matrix stem cells.
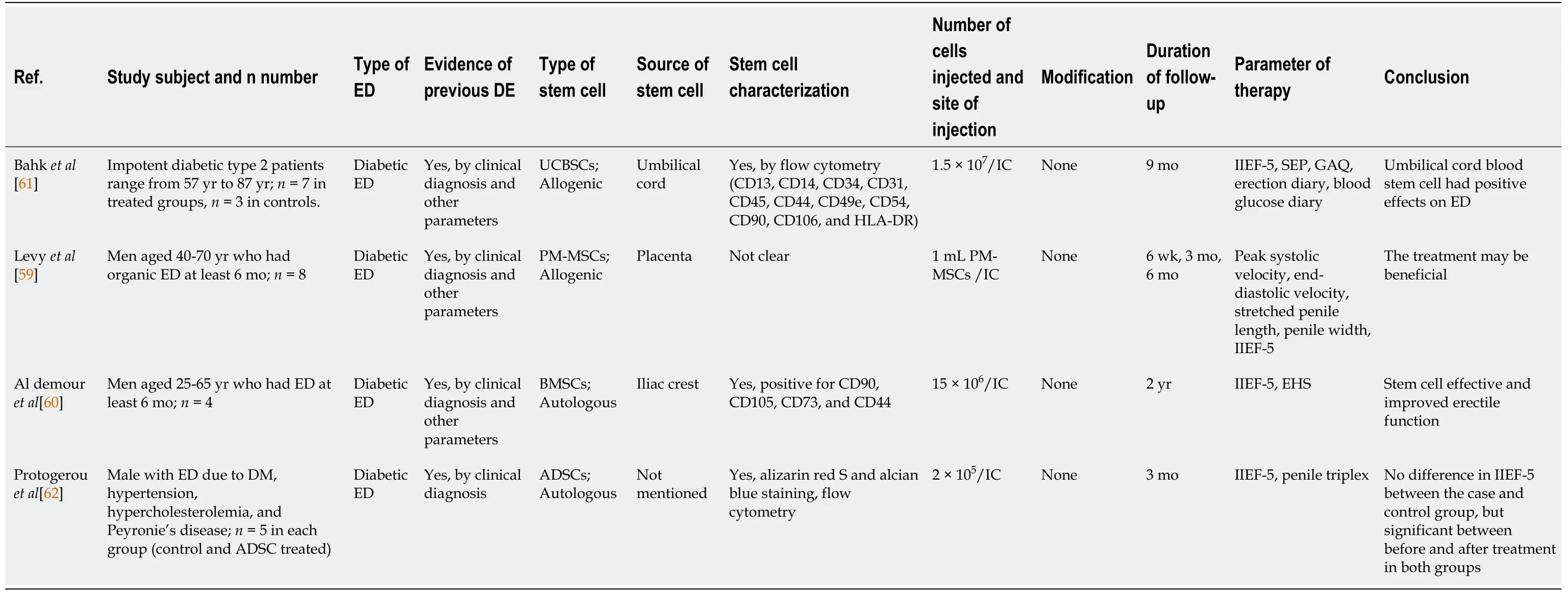
Table 2 List of articles on stem cell therapy in humans with diabetic erectile dysfunction
All of the studies have reported that stem cells provide improvements of the erectile function both functionally and structurally in various animal models. As eNOS and nNOS play a major role in penile erection, these two factors are used as parameters in stem cell studies. Nearly all studies have reported improved levels of these two factors. This indicates that stem cells improve endothelial function at the molecular level.
Moreover, factors, such as VEGF, FGF, and von Willebrand factor, are believed to be vital in the improvement of ED in these studies. The increase of these factors suggests an induction of angiogenesis in the penile tissue. Furthermore, stem cells are also reported to reduce the fibrotic process in penile erectile tissue as well as modulate autophagy process[58]. Autophagy was reported to increase following stem cell treatment, which is believed to be a sign of tissue recovery and regeneration. All of these findings indicate that stem cells play a role in cell regeneration and recovery by increasing the levels of growth factors and suppressing inflammatory factors. Addition of specific factors may further improve tissue repair and erectile function compared to the administration of stem cells alone.
So far, only four studies have focused on stem cell therapy for ED in humans. Levyet al[59] have reported the administration of PM-SCs (placental matrix stem cells) in eight people with ED. After 6 mo following treatment, Levyet al[59] concluded that stem cell therapy improves erectile function. Another study by Al Demouret al[60]reported that the administration of bone marrow stem cells to people with ED resulted in the improvement of both the IIEF-5 and EHS scores; they further claimed that stem cell administration in the context of ED was well tolerated and safe for humans.However, these two studies have some weaknesses, for example, the studies did not include placebo control, were not randomized or blinded, and had a very small number of samples[60].
Bahket al[61] conducted a study on seven diabetic patients aged 57-87 years and compared the effects of stem cell treatment with three patients who receive treatment for ED, such as PDE5 inhibitor alone. The stem cells used were umbilical cord-derived mesenchymal stem cells with a dose of 1.5 × 107cells administered intracorporeally.This study was followed up for 9 mo, and at the end of the study, it was found that the stem cells produced a positive effect on ED as seen in the improvement of IIEF-5, SEP,GAQ, and erectile diary[61].
Protogerouet al[62] reported a different approach by using autologous adipose stem cells to treat ED in five people with DM. This group was compared with five other people who received platelet lysate. The number of stem cells given was 2 × 105cells for 3 mo. At the end of the study, no difference in IIEF-5 was observed between the treatment and the control groups. However, a significant improvement was observed before and after treatment in both groups[62].
Possible mechanisms of stem cell therapy for diabetic ED
There are at least two possible mechanisms underlying the therapeutic effects of stem cell implantation for diabetic ED. First, stem cells may differentiate to specific cells and hence, regenerate the damaged tissue. In diabetic ED, the implanted stem cells may differentiate to myogenic cell precursor that subsequently in the presence of growth factors such as FGF and TGF-Beta can differentiate to myoblasts[40,63]. The myoblastic cells are known to have the capability to differentiate to vascular smooth muscle cells.The new smooth muscle cells may repair and improve damaged vascular tissues and hence improve the erectile function[64,65].
Second possible mechanism may involve the capability of stem cells to secrete beneficial paracrine factors. Stem cells are known to secrete angiogenic factors such as VEGF, basis fibroblast growth factor and SDF-1. These factors are potent inducers of angiogenesis and neovascularization[66,67]. Stem cells can also produce factors such as Wnt4 and Wnt7b, which are able to promote the differentiation of myoblasts to smooth muscle cells[63]. Together, the ability of stem cells to differentiate to smooth muscle cells and to produce beneficial paracrine factors that induce angiogenesis and neo-vascularization may be the underlying mechanisms of the therapeutic effects for diabetic ED.
CONCLUSION
Overall, based on the published data, it seems that stem cell therapy can become an alternative approach for the treatment of diabetic ED in the future. Stem cells can promote recovery and regeneration of the penile tissue following damage due to inflammation and free radicals. Studies reviewed in this paper, which are almost entirely conducted on experimental animals, certainly require further follow-up research. Information on stem cell research for ED treatment remains to be limited, but it can form the basis to develop further research in this area. In particular, larger human studies with appropriate research designs are needed to provide more objective information on the possibility of translational application.
杂志排行
World Journal of Stem Cells的其它文章
- Impact of senescence on the transdifferentiation process of human hepatic progenitor-like cells
- Effect of glycyrrhizic acid and 18β-glycyrrhetinic acid on the differentiation of human umbilical cord-mesenchymal stem cells into hepatocytes
- Current knowledge on the multiform reconstitution of intestinal stem cell niche
- Overview of nutritional approach in hematopoietic stem cell transplantation:COVID-19 update
- Age and genotype dependent erythropoietin protection in COVID-19
- Considerations for the clinical use of stem cells in genitourinary regenerative medicine
