Effect of glycyrrhizic acid and 18β-glycyrrhetinic acid on the differentiation of human umbilical cord-mesenchymal stem cells into hepatocytes
2021-11-02AbihaFatimaTubaShakilMalickIrfanKhanAishaIshaqueAsmatSalim
Abiha Fatima, Tuba Shakil Malick, Irfan Khan, Aisha Ishaque, Asmat Salim
Abiha Fatima, Tuba Shakil Malick, Irfan Khan, Aisha Ishaque, Asmat Salim, Stem Cell Laboratory,Dr. Panjwani Center for Molecular Medicine and Drug Research, ICCBS, University of Karachi, Karachi 75270, Sindh, Pakistan
Abstract BACKGROUND End-stage liver disease is a global health complication with high prevalence and limited treatment options. Cell-based therapies using mesenchymal stem cells(MSCs) emerged as an alternative approach to support hepatic regeneration. In vitro preconditioning strategies have been employed to strengthen the regenerative and differentiation potential of MSCs towards hepatic lineage.Chemical compounds of the triterpene class; glycyrrhizic acid (GA) and 18βglycyrrhetinic acid (GT) possess diverse therapeutic properties including hepatoprotection and anti-fibrosis characteristics. They are capable of modulating several signaling pathways that are crucial in hepatic regeneration. Preconditioning with hepato-protective triterpenes may stimulate MSC fate transition towards hepatocytes.AIM To explore the effect of GA and GT on hepatic differentiation of human umbilical cord-MSCs (hUC-MSCs).METHODS hUC-MSCs were isolated and characterized phenotypically by flow cytometry and immunocytochemistry for the expression of MSC-associated surface molecules. Isolated cells were treated with GA, GT, and their combination for 24 h and then analyzed at three time points; day 7, 14, and 21. qRT-PCR was performed for the expression of hepatic genes. Expression of hepatic proteins was analyzed by immunocytochemistry at day 21. Periodic acid Schiff staining was performed to determine the functional ability of treated cells.RESULTS The fusiform-shaped morphology of MSCs in the treatment groups in comparison with the untreated control, eventually progressed towards the polygonal morphology of hepatocytes with the passage of time. The temporal transcriptional profile of preconditioned MSCs displayed significant expression of hepatic genes with increasing time of differentiation. Preconditioned cells showed positive expression of hepatocyte-specific proteins. The results were further corroborated by positive periodic acid Schiff staining, indicating the presence of glycogen in their cytoplasm. Moreover, bi-nucleated cells, which is the typical feature of hepatocytes, were also seen in the preconditioned cells.CONCLUSION Preconditioning with glycyrrhizic acid, 18β-glycyrrhetinic acid and their combination, successfully differentiates hUC-MSCs into hepatic-like cells. These MSCs may serve as a better therapeutic option for degenerative liver diseases in future.
Key Words: Glycyrrhizic acid; 18β-glycyrrhetinic acid; Hepatocyte differentiation; Human umbilical cord-MSCs; Mesenchymal stem cells
INTRODUCTION
Liver is a complex organ, designed to fulfil variety of tissue-dependent tasks such as metabolic, excretory, circulatory and secretory functions[1]. Parenchymal hepatocytes are polarized polygonal epithelial cells with remarkable proliferation ability, which efficiently repopulates liver cells in response to the injurious stimuli. Tissue mass is restored by their mitotic division and increase in the size of cells termed as compensatory hyperplasia and hypertrophy[2]. Even though liver is a pivotal organ for homeostasis with considerable inherent regenerative ability, massive injury to the liver may prevent regeneration due to the accumulation of extracellular matrix proteins and scar tissue formation (fibrosis) which progress to cirrhosis, a common pathological condition contributing to end-stage liver disease (ESLD)[3]. To prevent ESLD progression, stem cell therapy has also shown promising clinical outcomes and considered as a potential alternative to conventional therapies. Mesenchymal stem cells (MSCs) have gained considerable attention because of their multi-lineage potential, immune-privileged and paracrine properties, making them valuable candidates for translational approach. These cells can be obtained from different sources, such as bone marrow, adipose tissue, peripheral blood, cartilage, skeletal muscle, umbilical cord blood and tissue[4]. Although MSC-mediated therapeutic approach has been effective in ESLD, the major setback is the low cell viability and resistance in impaired tissues post-transplantation[5]. To overcome this consequence,MSCs can be differentiated into functional hepatocytesin vitro,to enhance their therapeutic potential prior to transplantation. Various extrinsic factors including mechanical induction, growth factors, and chemical compounds promote differentiation of stem cells. They are capable of triggering several signaling pathways that control fate of stem cells, directing them towards a desired cell lineage[6]. In recent years, researchers explored the role of phytochemical compounds such as triterpenes in inducing stem cell differentiation. In a clinical finding, it is reported that pentacyclic triterpene, oleanolic acid has a potential to enhance osteogenic differentiation of MSCs by inhibiting Notch signaling pathway[7]. A study conducted on triterpene compound, ginsenosides has highlighted their role in enhancing hepatic gene expression during the induction of hepatic differentiation of MSCs[8].
Triterpenes, structurally similar to steroids, are diversified class of chemical compounds containing isoprene units. They can occur as glycosides or aglycones and categorized as pentacyclic and tetracyclic[9]. They are abundantly found in vegetables,fruit peels, medicinal plants, and stem bark[10,11]. Glycyrrhizic acid (GA) and 18βglycyrrhetinic acid (GT) are potent triterpenes possessing variety of therapeutic properties for liver diseases. This includes anti-hepatotoxic, anti-fibrotic, anti-tumor,anti-inflammatory, anti-arthiritic, anti-allergic and anti-viral properties[12,13]. Their beneficial effects in cell culture and animal models of liver toxicity have been investigated. Interestingly, these triterpenes have been used in the treatment of hepatitis,liver cirrhosis, and cancer and have shown to improve liver function[14]. Considering the characteristics of these compounds in hepatic anomalies, we hypothesized that these triterpenes may have the ability to directly differentiate hUC-MSCs into hepatocytes or aid in the process of differentiation.
MATERIALS AND METHODS
Ethics committee approval and human umbilical cord collection
Present study was approved by the Independent Ethics Committee, International Center for Chemical and Biological Sciences (IEC, ICCBS) under protocol #:ICCBS/IEC-037-HT-2018/Protocol/1.0. The umbilical cord samples (n= 10) were collected from Zainab Panjwani Memorial Hospital (ZPMH) with an informed consent from healthy donors following full-term cesarean delivery. Using aseptic techniques,cord samples (approximately 8-10 cm in length) were collected in a phosphate buffered saline (PBS) transfer medium containing anticoagulant (5 g/L EDTA) in a sterile bottle and kept at 4 °C until transferred to the experimental research facility in ice box and processed within 2-4 h of collection.
Processing and culturing of human umbilical cord tissue using explant culture
Sample processing was carried out in a biosafety cabinet class II, type A2 (ESCO,United States). Cord tissue sample was placed in a culture dish and rinsed multiple times with sterile PBS to remove blood clots and debris. Cord tissue was chopped into small pieces of about 1-3 mm in size and plated in T-75 flasks having 10-13 mL of DMEM (GIBCO, United States) supplied with 100 mL/L FBS, 0.1 g/L streptomycin,100000 Units/L penicillin, and 0.001 mol/L sodium pyruvate. Explants were placed at 37 °C in humidified CO2incubator (Hera Cell, Germany). Tissue culture was observed for cell growth and medium was replaced after every 72 h. hUC-MSCs migrated out from the small pieces of umbilical cord Wharton’s jelly to the surface of the culture flask during 10-14 d of initial culture. After sufficient attachment of hUC-MSCs,explants were removed and fresh medium was added for further expansion. At this stage, cells were termed as P0 passage. Once the adherent cells reached 80%confluence, they were sub-cultured for next passages using 2.5 g/L trypsin. hUCMSCs of P4 passage were used in all subsequent experiments.
Characterization of hUC-MSCs by immunocytochemical analysis and flow cytometry
Isolated hUC-MSCs were characterized by immunocytochemistry for the expression of MSC associated surface molecules, as reported previously[15]. Approximately, 8000 cells were seeded on cover slips placed in 24-well plate with 200 µL DMEM per well.The cells were then kept at 37 °C in incubator containing 50 mL/L CO2for 24-48 h until the monolayer was formed. After confirming cell expansion, media was removed, and cells were washed with 1 × PBS. Cells were fixed with 200-300 µL 40 g/L PFA for 10 min at room temperature followed by washing with PBS. 300 µL of 0.1% Triton X-100 was added in each well for cell permeabilization for 10 min at room temperature and washed 3 times with PBS. Cells were then kept at 37 °C for 1 h in the blocking solution containing PBS supplemented with 20 g/L BSA and 1 mL/L Tween 20 to prevent nonspecific binding. Blocking solution was removed and cells were incubated with monoclonal primary antibodies against CD73, CD105, vimentin, and CD45. Plate was kept overnight at 4 °C. Subsequently, primary antibodies were removed and wells were washed with 1 × PBS. Alexa Fluor 546 goat anti mouse secondary antibody was added in each well and incubated at 37°C for 1 h followed by washing with PBS. Cells were counterstained with 0.0005 g/L solution of DAPI-PBS for 15 min at room temperature and again washed with PBS. Lastly, 5 µL mounting medium was added on the glass slide and coverslip (cell-side) was placed on it. Slides were analyzed using fluorescence microscope (TE2000-S, Nikon, Japan).
Flow cytometric analysis was performed according to the previously reported method with some modifications[16]. Cells were cultured in T-75 flasks. Upon reaching 80% confluence, cells were washed twice with 1 × PBS and treated with 5 mL cell dissociation buffer. Cells were incubated in 50 mL/L CO2at 37 °C for 30-40 min.Cell suspension was collected in 15 mL conical tube and centrifuged for 10 min at 400× g. Supernatant was removed and pellet was mixed with 600 µL cold FACS solution(10 g/L BSA-1 × PBS, 0.001 mol/L EDTA and 1 g/L sodium azide) and transferred equally into 6 conical tubes. Each cell suspension was centrifuged at 400 × g for 5 min.Supernatant was discarded and blocking solution was added in each tube. 200 µL monoclonal primary antibodies against vimentin, CD73, CD105, and CD45 were added in separate tubes, and kept for 1 h at room temperature. Cell suspension was centrifuged at 400 × g at 5 min. Supernatant containing unbound primary antibodies were removed and pellet was washed twice with 500 µL ice cold FACS solution by centrifugation at 400 × g for 8 min each. After discarding the supernatant, 200 µL Alexa Fluor 546 goat anti mouse secondary antibody was added in each tube and incubated for 2 h at room temperature in dark. Cells were again washed with 500 µL FACS solution. Pellet was suspended in 300 µL FACS solution and transferred in 5 mL FACS tubes. Cells labeled only with secondary antibody were used as control. Single cell suspension was analyzed by flow cytometer (FACScaliber, Becton Dickinson) and data was interpreted using BD Cell Quest Pro software.
MTT assay for cell viability
MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay was conducted as previously reported[17], to find out the non-toxic concentration of GA,GT and their combination. The assay is based on the ability of converting watersoluble tetrazolium salt into water-insoluble formazan particles by metabolically active cells. Approximately, 5000 cells were seeded with 200 µL DMEM per well in a flatbottom 96-well plate. Plate was kept in CO2incubator overnight at 37 °C. Next day,medium was removed and cells were incubated for 24 h with different concentrations of GA and GT in FBS-free medium. Following day, medium was removed and cells were incubated with 200 µL of 0.5 g/L MTT solution for 4 h in the CO2incubator. MTT solution was removed and 200 µL DMSO was added to dissolve formazan granules formed by the metabolically active cells. Absorbance was monitored at 570 nm using spectrophotometer (SpectraMax, United States).
Treatment of hUC-MSCs
To induce hepatic differentiation, 10 µM concentration of both compounds and their combination was selected based on MTT cell viability assay. hUC-MSCs were divided into the following experimental groups; untreated control, GA-treated, GT-treated,and combination group of (GT + GA)-treated hUC-MSCs. Working solutions were prepared from their 0.01 mol/L stock solutions. Cells grown in T-25 flasks were treated in FBS-free medium for 24 h. Subsequently, medium was removed, fresh medium was added and cells were kept in CO2incubator for different time-points; day 7, 14 and 21 for morphological examination and RNA isolation. Exhausted medium was replaced after every 72 h.
Evaluation of hepatic markers by gene and protein expression analysis
For gene expression analysis, total cellular RNA was isolated from untreated and treated cells at day 7, 14 and 21 by Trizol method according to the manufacturer’s guidelines. Micro-volume UV-Vis spectrophotometer (Nanodrop 2000, Thermo Fisher,United States) was used to quantify and assess the purity of extracted RNA.Absorbance was measured at 260 nm in µg/µL and an absorbance ratio of 260/280 was calculated for sample purity. cDNA was synthesized equivalent to 1 µg of extracted RNA using RevertAid First Strand cDNA synthesis kit (Thermo Fisher,United States). Gene expression levels of hepatic markers were determined by qRTPCR (Mastercycler ep realplex, Eppendorf, Germany) using primer sequences listed in Table 1. Total of 40 PCR cycles were run according to the program layout shown in Table 2. All reactions were carried out in triplicates to validate them statistically.Human specificβ-actingene was used as an internal control to normalize gene expression in all the experimental groups. Results obtained in the form of Ct values were used to find relative gene expression.

Table 1 Primer sequences of the targeted genes
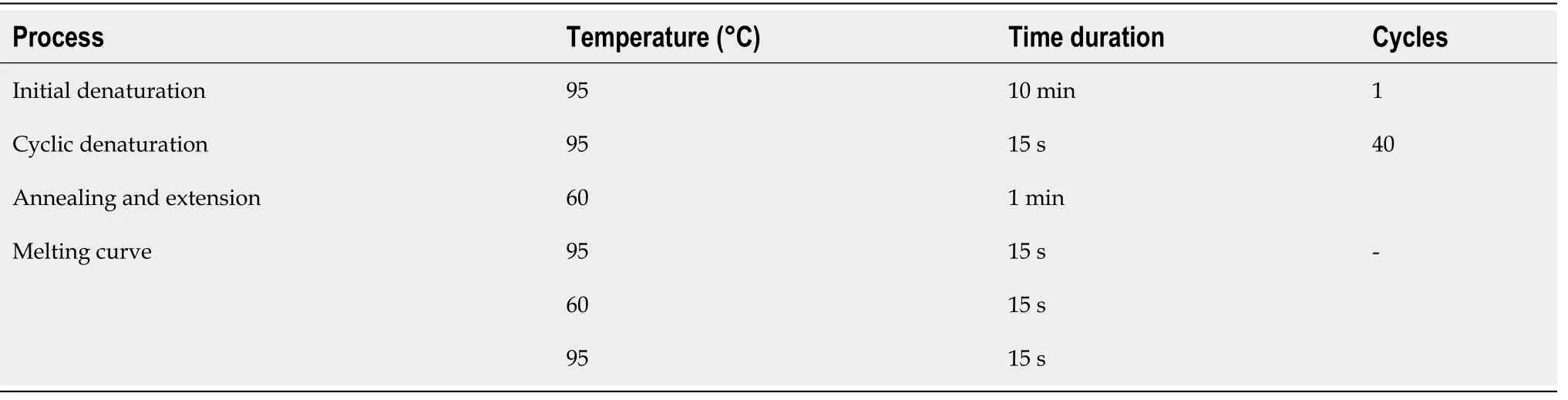
Table 2 qRT-PCR program layout
For protein expression, immunofluorescence staining of untreated and 21 day treated hUC-MSCs was performed using primary antibodies for hepatocyte specific proteinsi.e.,alpha-fetoprotein (AFP), albumin (ALB), hepatocyte nuclear factor-3α(HNF-3α) and tyrosine-aminotransferase (TAT). Protein expression was examined under fluorescence microscope.
Periodic acid Schiff staining for glycogen detection
For functional characterization, presence of intracellular glycogen in 21 d treated hUCMSCs was assessed by periodic acid Schiff staining in accordance with the protocol described previously[18]. Cells were fixed in 40 g/L PFA and permeabilized with 0.1%Triton X-100 for 10 min in 24-well plate. After multiple washings with PBS, cells were oxidized with 1 mL/L periodic acid for 5 min and then washed three times with PBS.Cells were then incubated with Schiff’s reagent for 15 min and again washed with PBS.Subsequently, cells were counterstained with hematoxylin for 1 min and washed with PBS in the same manner. Finally, 10 mL/L mounting medium was added on the slides and cells were examined under light microscope (YS100, Nikon, Japan).
Statistical analysis
Statistical analysis of the data was performed by IBM SPSS software, version 22.Independent samplet-test, and One-way analysis of variance (ANOVA) with Post-HocBonferroni corrections were done to assess statistical significance. All experiments we
RESULTS
Morphological features of cultured hUC-MSCs
hUC-MSCs began to migrate from explants after approximately 8-10 d in culture and adhere to the surface of tissue culture flask. The adherent cells having fibroblast and fusiform appearance, exhibited distinct ability to expand following adherence. After sub-culturing, cells showed greater proliferative ability and maintained their morphology beyond several passages (Figure 1).
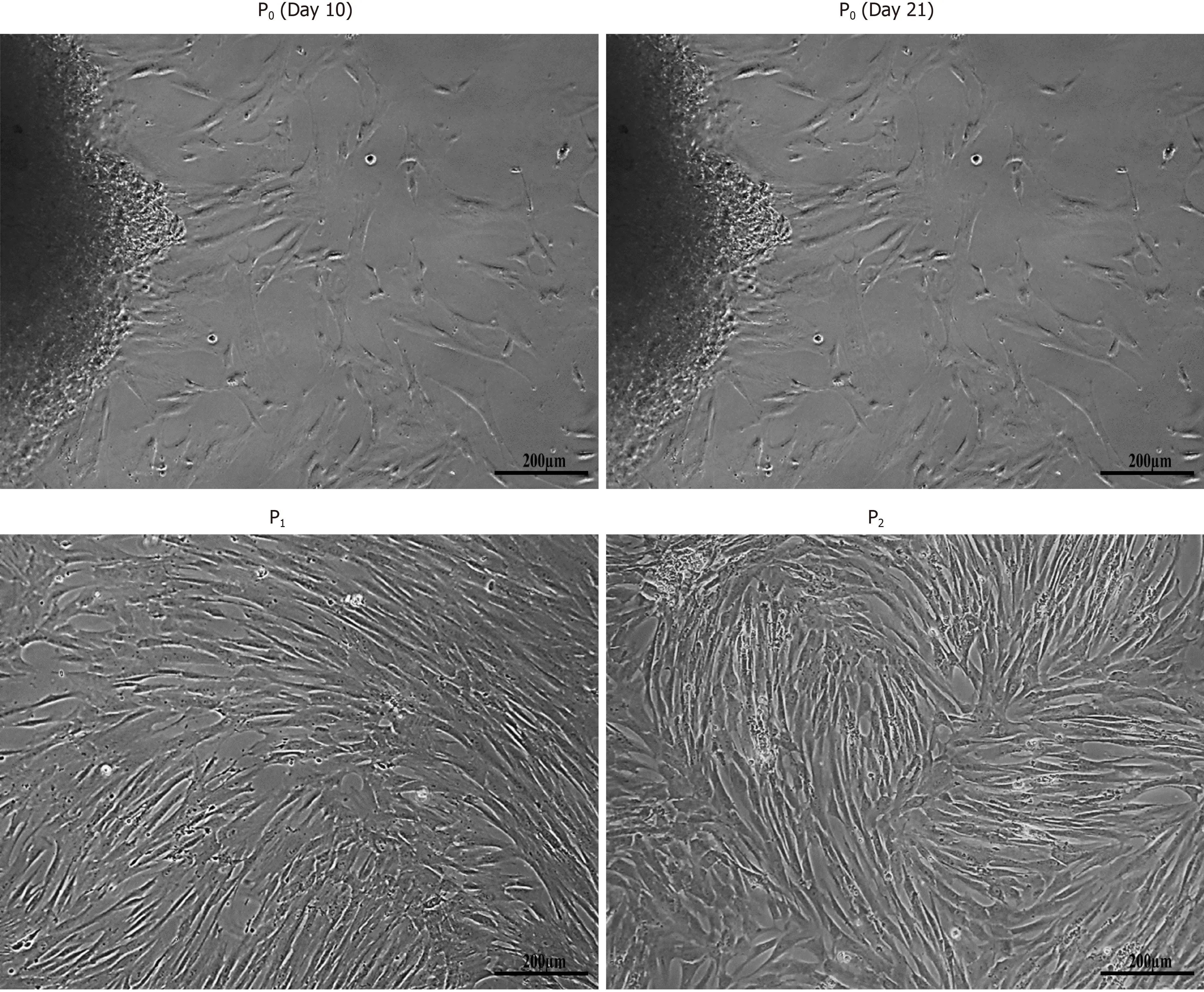
Figure 1 Isolation of human umbilical cord-mesenchymal stem cells. Morphological observations of human umbilical cord-mesenchymal stem cells showing passage 0 (P0) cells at day 10 and day 21 with cells migrating towards the periphery of the adherent tissue, and passage 1 (P1) and passage 2 (P2) cells with homogeneous population showing fusiform- and fibroblast-like appearance after sub-culturing. All images were taken under phase contrast at 10× magnification.
Phenotypic characterization of hUC-MSCs
Cultured hUC-MSCs were phenotypically characterized through immunocytochemistry and flow cytometry which confirmed increased number of cells expressing MSC-specific markers including CD73, CD105, and vimentin, while negative expression of hematopoietic marker, CD45 was observed (Figure 2).
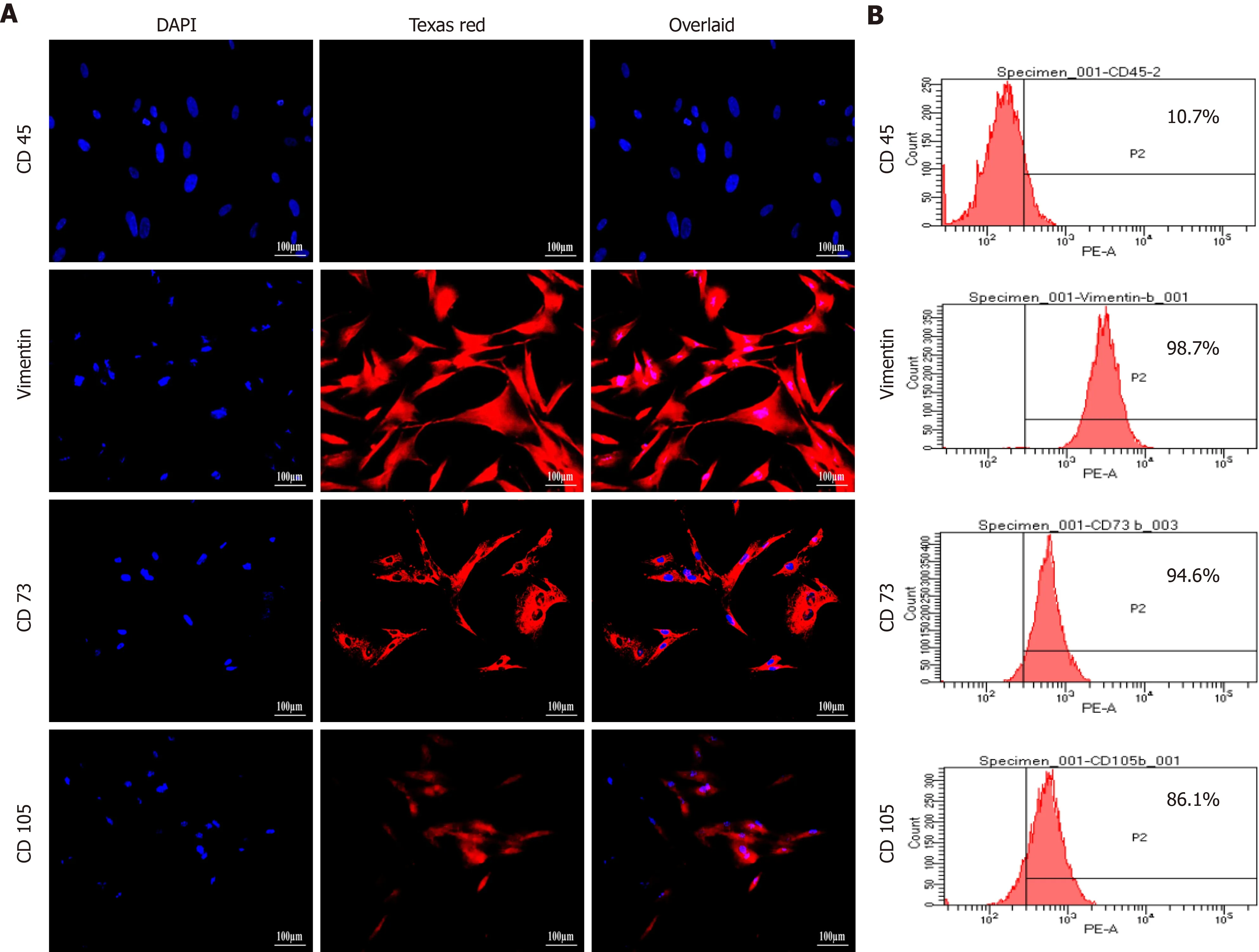
Figure 2 Characterization of human umbilical cord-mesenchymal stem cells by immunocytochemical analysis and flow cytometry. A:Immunocytochemical analysis showing positive expression of mesenchymal markers, CD73, CD105, and vimentin, and negative expression of hematopoietic marker CD45. Fluorescent micrographs were captured at 20 × magnification; B: Flow cytometric analysis showing mesenchymal markers CD73, CD105, and vimentin having more than 85% positive cells. Data were analyzed using Cell Quest Pro software.
Cell viability
Viability of hUC-MSCs was decreased in a concentration-dependent manner when cells were subjected to different concentrations of GA, GT and their combination.Concentration of 10 µM of both compounds was non-cytotoxic and therefore selected for cell treatment in all groups (Figure 3).
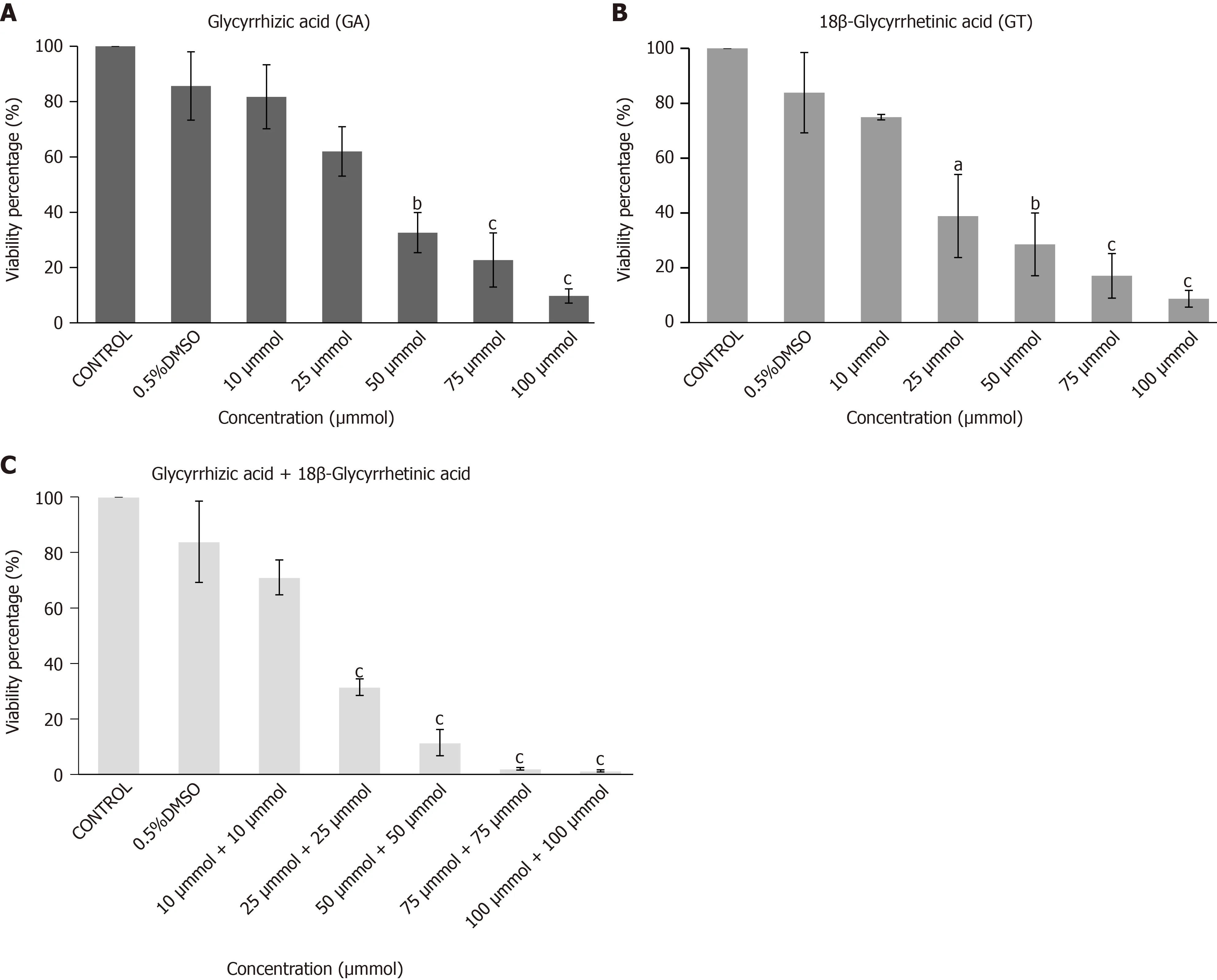
Figure 3 Cytotoxicity analysis of human umbilical cord-mesenchymal stem cells by MTT Assay. Bar graphs showing percent viability of human umbilical cord-mesenchymal stem cells treated with A: glycyrrhizic acid (GA), B: 18β-glycyrrhetinic acid (GT), and C: their combination (GA + GT) at different concentrations. A decrease in percent (%) cell viability is observed with increasing concentrations of GA, GT and their combination. Statistical analysis was conducted by One-way ANOVA and Post-Hoc Bonferroni tests. Results are expressed as mean ± SEM (n = 3); level of significance aP ≤ 0.05 (where aP ≤ 0.05, bP ≤ 0.01, and cP ≤ 0.001).
Morphological observation of preconditioned MSCs
hUC-MSCs were observed for the morphological changes at three different time pointsi.e.,day 7, 14 and 21. Cells lost their fusiform / spindle shaped morphology and progressed towards polygonal morphology of hepatocytes in a time-dependent manner. At day 7, GA- and GT-treated hUC-MSCs became slightly shorter, flattened,and broader in appearance with retracted ends, while in the combination group, cells also began to change their morphology and appeared as polygonal. Changes were more apparent at day 14, where cells decreased in size and transformed into an irregular or polygonal shape. By day 21, majority of the cells became more compact and polygonal in shape exhibiting tight interactions between the cells in all treatment groups (Figure 4).
Temporal gene expression analysis
Gene expression pattern of GA and GT-treated hUC-MSCs showed significant expression of early hepatocyte markerAFPat all time points. Highest expression was observed at day 7 but it decreased in a time-dependent manner. Significant expression ofALBandCK-18was detected at all time points. Late hepatic markers, TAT,CYP450-2B6, andHNF-4αwere not detectable at day 7 but their expression levels were significantly up-regulated at day 14 and 21, as compared to the untreated control(Figure 5A and B). Gene expression pattern of the combination group showed similar results forAFP. Significant expression ofALB, CK-18, TAT, CYP450-2B6,andHNF-4αwas observed at all time points and their expression increased with time of differentiation as compared to the untreated control (Figure 5C). These results suggest that MSCs were differentiated into hepatic lineage after treatment with these compounds separately or in combination.
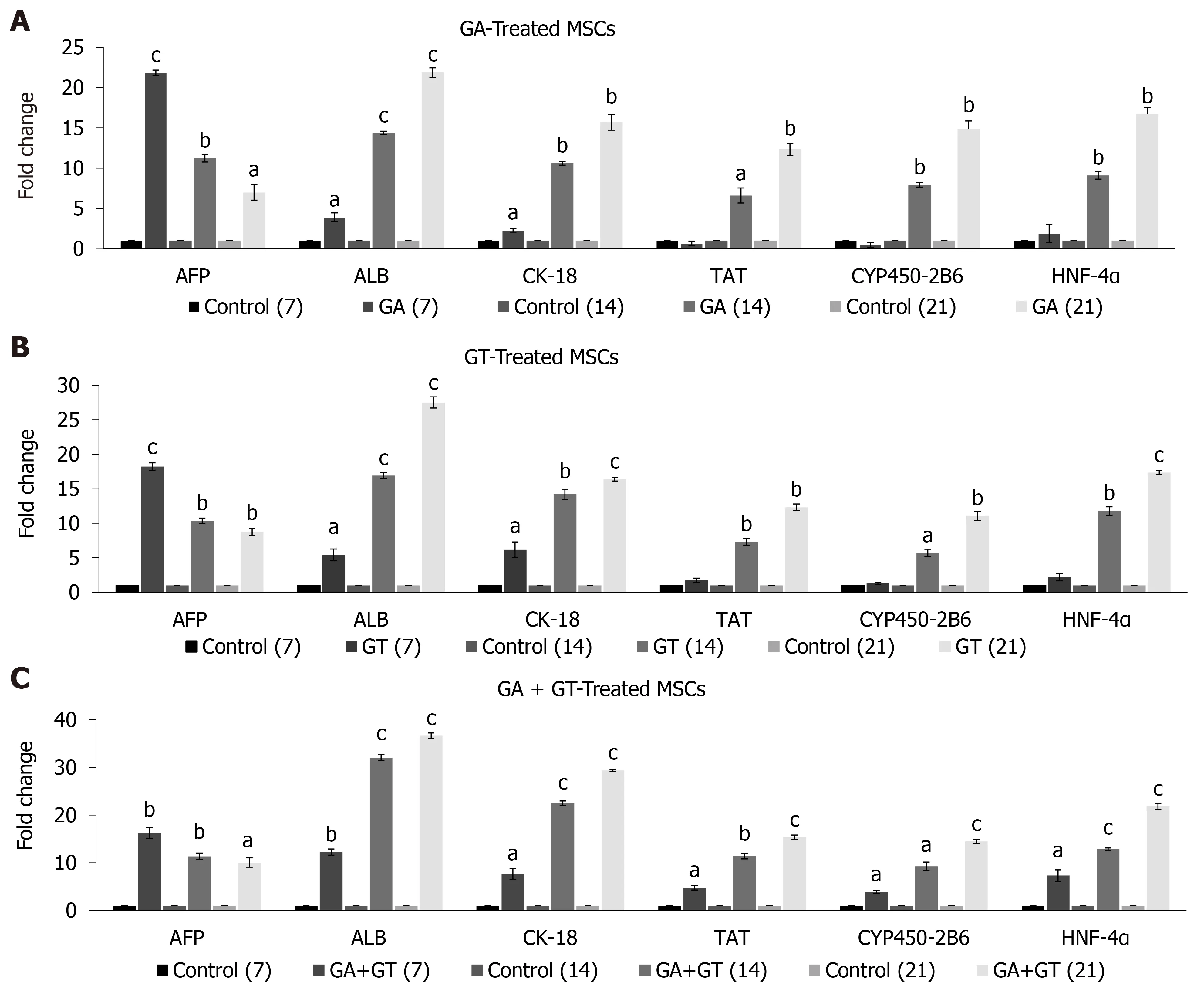
Figure 5 Expression of hepatic genes in human umbilical cord-mesenchymal stem cells treated with glycyrrhizic acid, 18β-glycyrrhetinic acid, and their combination. A and B: Glycyrrhizic acid (GA)- and 18β-glycyrrhetinic acid (GT)-treated mesenchymal stem cells showed significant expression of early hepatocyte marker AFP at all time points. Highest expression was observed at day 7 but it declined in a time-dependent manner. Significant expression levels of ALB and CK-18 were detected at all time points. Late hepatic markers, TAT, CYP450-2B6, and HNF-4α were not detectable at day 7 but were significantly upregulated at day 14 and 21, as compared to the untreated control; C: GA + GT-treated MSCs revealed significant up-regulation of AFP at all time points. Highest expression was observed at day 7 but it declined with treatment time. Significant expression levels of ALB, CK-18, TAT, CYP450-2B6, and HNF-4α were observed at all time points and their expression increased with time of differentiation, as compared to the untreated control. Statistical analysis was conducted by independent sample t-test. Results are expressed as mean ± SEM (n = 3); level of significance aP ≤ 0.05 (where aP ≤ 0.05, bP ≤ 0.01, and cP ≤ 0.001).
Immunofluorescence analysis of hepatocyte specific proteins
Differentiation of treated hUC-MSCs towards hepatic lineage was further confirmed by immunocytochemical analysis of hepatocyte specific proteins. hUC-MSCs treated with GA, GT and their combination exhibited positive expression of hepatocyte specific proteins including, AFP, ALB, HNF-3α, and TAT as compared to the untreated control at day 21, demonstrating that hUC-MSCs successfully differentiated towards hepatic lineage (Figure 6A).
Quantification of fluorescence intensity
Fluorescence intensity of MSCs treated with GA, GT, and their combination was also measured and validated statistically. Significant up-regulation of hepatocyte specific proteins including, AFP, ALB, HNF-3α, and TAT was observed in all treatment groups as compared to the MSCs with no treatment (control) at day 21, demonstrating that MSCs differentiated towards hepatic lineage (Figure 6B).
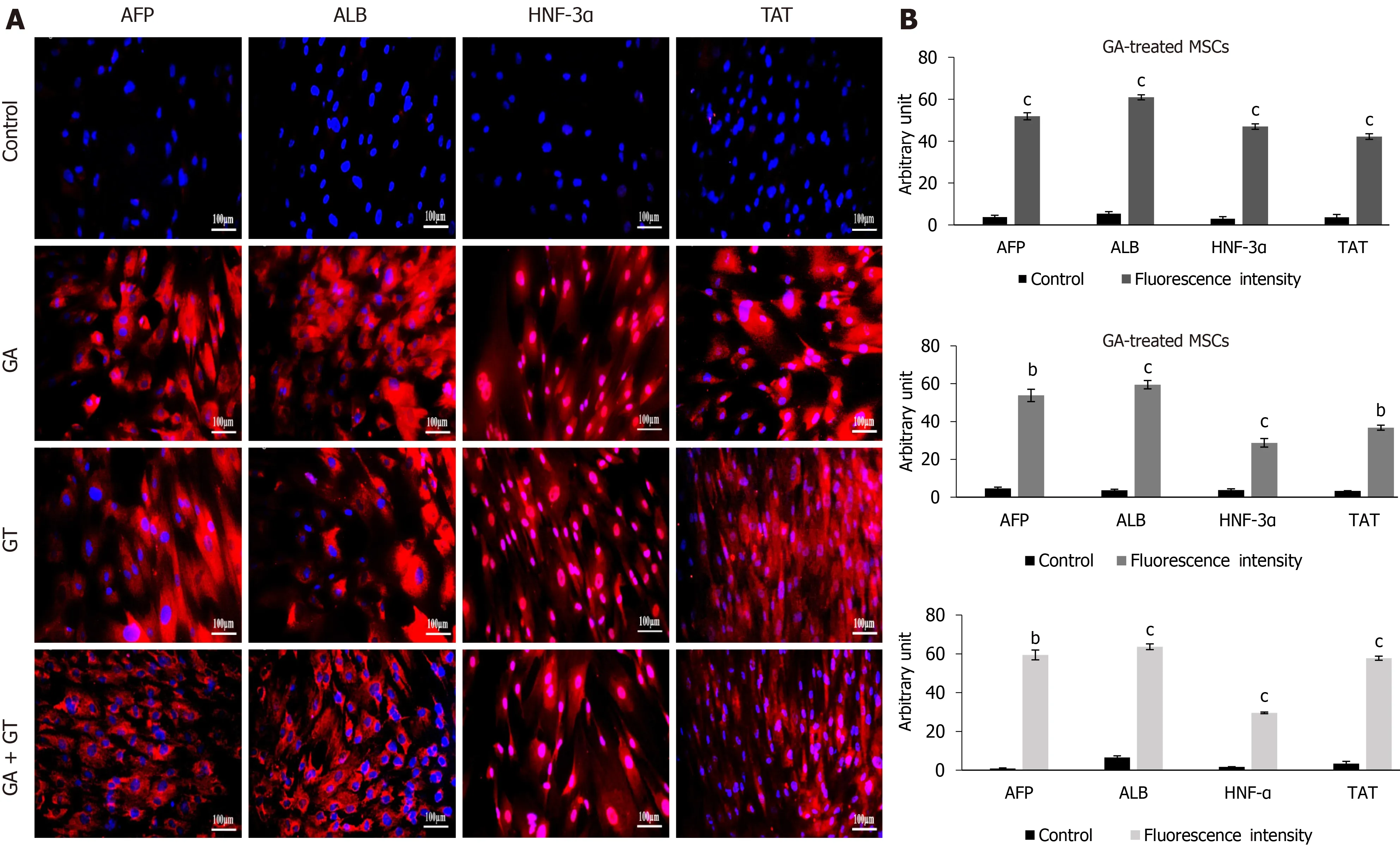
Figure 6 Immunocytochemical analysis and quantification of hepatic proteins in human umbilical cord-mesenchymal stem cells treated with glycyrrhizic acid, 18β-glycyrrhetinic acid and their combination. A: At day 21, treated mesenchymal stem cells exhibited positive expression for hepatocyte specific proteins including, alpha-fetoprotein, albumin, hepatocyte nuclear factor-3α, and tyrosine-aminotransferase in all treatment groups as compared to the untreated control; B: Fluorescence intensities of all treatment groups were measured using Image J software. Statistical analysis was conducted by independent sample t-test. Results are expressed as mean ± SEM (n = 3); level of significance aP ≤ 0.05 (where bP ≤ 0.01 and cP ≤ 0.001).
Analysis of glycogen content
Treated hUC-MSCs were further evaluated for their functionality by periodic acid Schiff staining (PAS) to corroborate their hepatic differentiation. hUC-MSCs treated with GA, GT and their combination, showed strong PAS positive signals at day 21,indicating their capability to store glycogen as compared to the untreated control. Binucleated cells were also observed, which is one of the prominent characteristics of functional hepatocytes (Figure 7).
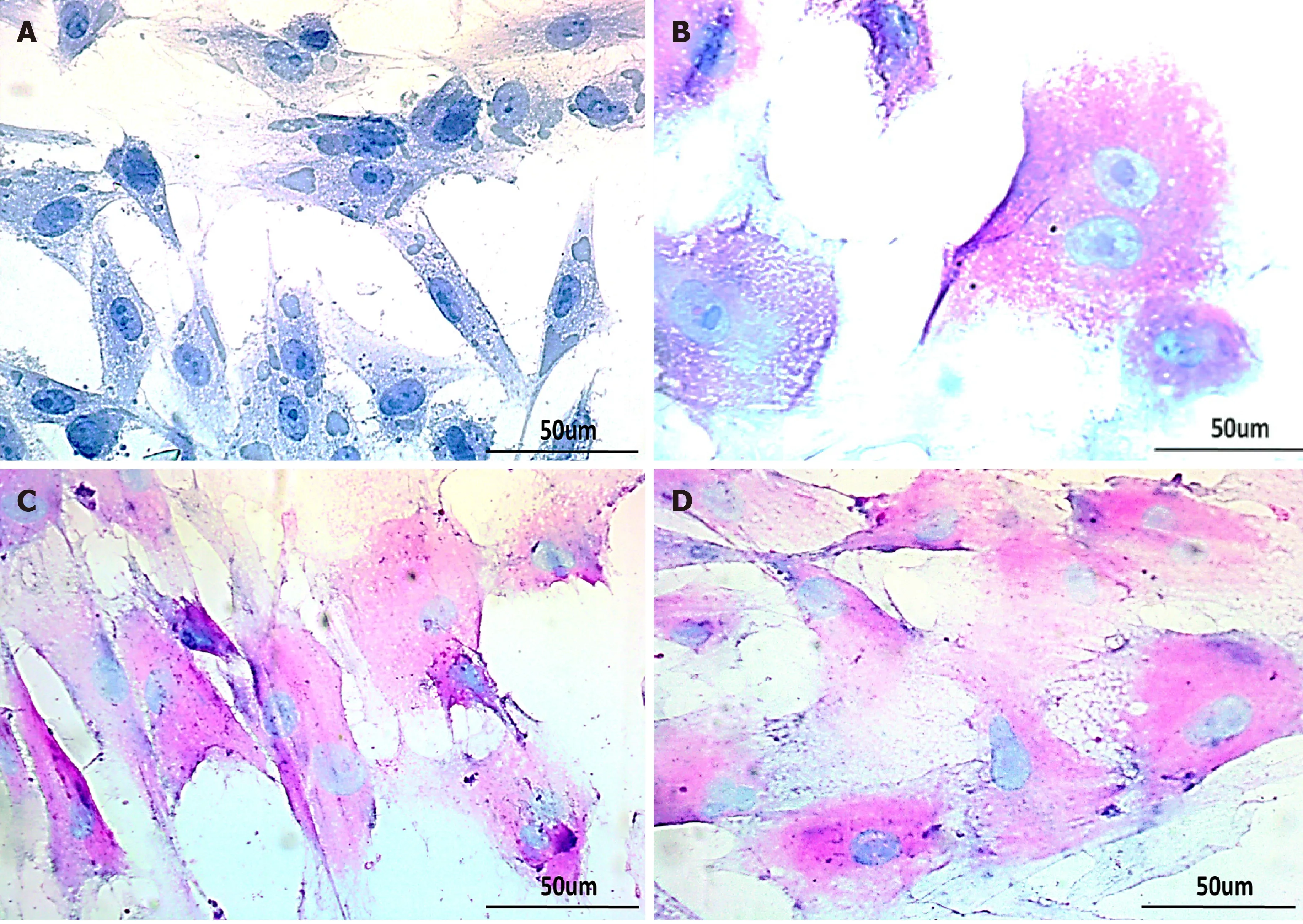
Figure 7 Glycogen detection in treated human umbilical cord-mesenchymal stem cells by periodic acid Schiff staining. Bright-field images of A: untreated control showing negative periodic acid Schiff staining (PAS); B: MSCs treated with glycyrrhizic acid (GA); C: 18β-glycyrrhetinic acid (GT); and D: GA+GT(combination group) at day 21 showing PAS positive cells stained as purple-pink, indicating the presence of glycogen in their cytoplasm. Bi-nucleated cells (a hepatocyte characteristic) were also observed. Images were taken at 40× magnification.
DISCUSSION
In our study, we determined the effects of two triterpenes, GA and GT on differentiation of human umbilical cord-MSCs towards hepatic lineage. These compounds exhibit remarkable pharmacological characteristics. GA has a significant role in the treatment of liver fibrosis, hepatic steatosis, viral hepatitis, acute and chronic liver injury, and myocarditis[19-21]. In liver injury, GA exerts anti-inflammatory response by inhibiting TNF release, myeloperoxidase activity, and translocation of nuclear factor-κB in the nuclei. It also increases the expression of proliferating cell nuclear antigen, promoting the regeneration of damaged hepatic cells[22]. Pretreatment with GA significantly increases cytochrome P450 enzymes in liver to regulate oxidative metabolism of dietary toxins, drugs, mutagens and carcinogens, which lowers the risk of carcinomas[23]. GT exhibits numerous biological activities including anti-oxidative,anti-apoptotic, anti-inflammatory and anti-allergic effects[24]. GT treatment showed anti-diabetic effects by elevating plasma insulin levels, and reducing the levels of gluconeogenic enzymes in liver. Previously, a study showed hepato-protective effects of GT on drug-induced hepatotoxicity, by down-regulation of HMGB1-TLR4 (High-Mobility Group Protein B1- TollLike Receptor 4), Nrf2, PPARγ signaling pathway[25,26].
On the basis of their therapeutic potential and beneficial role in modulating several physiological pathways in liver regeneration, we hypothesized that these chemical compounds may have the ability to either directly differentiate MSCs into hepatocytes or aid in the process of differentiation. For this purpose, MSCs were harvested from human umbilical cord tissue (hUC) using explant method[27]. The isolated cells expressed MSC markersi.e.,CD73, CD105, and vimentin, but were negative for hematopoietic marker, CD45. Therefore, the isolated cells possess the basic characteristics of MSCs in accordance with the specifications suggested by the Society of Cellular Therapy[28]. Next, we identified the non-toxic concentration of GA and GT by conducting MTT assay. For both compounds, 10 µM concentration was selected for subsequent experiments as this concentration was found to be non-toxic to cells. hUCMSCs were treated with GA and GT separately and in combination for 24 h, and then examined at three different time points; day 7, 14, and 21. We observed fibroblast-like and fusiform-shaped morphology of MSCs in all treatment groups in comparison with the control group. This eventually progressed towards irregular or hepatocyte-like polygonal morphology with time of differentiation, in accordance with earlier findings[29,30].
To evaluate hepatic differentiation, we determined gene expression levels of hepatocyte specific markers such asAFP, ALB, CK-18, TAT, CYP450-2B6, andHNF-4αby qRT-PCR, as their expression is predominately regulated at the transcription level.The transcriptional profile of treated hUC-MSCs of GA, GT, and the combination group showed positive expression of early to mid-late hepatic markers includingAFP,ALB,andCK-18at all time points. The expression ofAFPwas higher at day 7,indicating initiation of cell differentiation, however, it decreased at later time points;whereas the expression ofALBandCK-18enhanced with time of differentiation. The late hepatic markers,HNF-4α, TAT,andCYP450-2B6were detectable at day 14 and 21 in GA-, and GT-treated MSCs, while in the combination group, their significant up-regulation was observed at all time points.
AFP is an endodermal marker as well as early developmental marker of hepatoblast formation; its level gradually reduces with time indicating that the immature hepatocytes progress towards adult phenotype[31]. ALB is the most abundant protein produced by adult hepatocytes; its synthesis begins in early fetal hepatocytes and reaches the maximum level in the mature hepatocytes[32]. Similarly, cytokeratin-18(CK-18) is a hepatic cytoskeletal protein and epithelial surface marker; it is weakly expressed during hepatoblast formation, while up-regulated at the maturation stage[33]. HNF-4α, a liver enriched transcription factor, maintains the hepatic differentiation status of MSCs, and is considered as a key regulator of hepatic functions and metabolic enzymes[34,35]. TAT and cytochrome P450-2B6 (CYP450-2B6) are enzymes,largely expressed in adult hepatocytes[36]. The gene expression pattern of all treatment groups suggests that GA, GT and their combination was able to direct hUCMSCs towards hepatic lineage. Interestingly, in the combination group, positive expression of early and late hepatocyte markers at the initial days of treatment may indicate their synergistic effect in an early switch to hepatic differentiation.
Additionally, hepatic markers were evaluated at the protein level, where hUCMSCs of GA, GT, and the combination groups showed positive immunostaining of AFP, ALB, TAT, and HNF-3α proteins at day 21 of treatment. HNF-3α and HNF-4α are transcription factors essential for hepatic specification, and primarily involved in hepatic metabolism[37,38]. To further validate the hepatic differentiation, we performed PAS to assess glycogen storage ability of the treated cells. In comparison to the control group, hUC-MSCs treated with GA, GT and their combination, stained purple-pink at day 21 of treatment, indicating the presence of glycogen in their cytoplasm, which is in line with earlier findings[39]. Furthermore, bi-nucleated cells were also seen in all treatment groups, which is the typical feature of functional hepatocytes[40].
Collectively, our experimental results imply that triterpenes, GA and GT, have the potential to enhance hepatic differentiation of hUC-MSCs. The preconditioned MSCs may serve as an effective cell source for hepatic diseases in clinical applications.Nevertheless, the underlying mechanism still needs to be investigated to understand the process of cellular differentiation to ensure a better hepatic induction strategy.
CONCLUSION
It is concluded from this study that triterpenes, GA and GT at 10 µM concentration were able to induce hepatic differentiation of hUC-MSCs. The early and late hepatic genes were significantly up-regulated in a time-dependent manner. Moreover, the combination of both compounds has strong synergistic effect as shown by the induction of hepatic differentiation at early time points after treatment. The significant expression of hepatocyte markers, the ability to store glycogen and presence of binucleated cells, suggest hepatic differentiation in all treatment groups. However,further investigations are required to explore the molecular mechanism involved in the differentiation process and to assess the therapeutic effect of preconditioned hUCMSCs in thein vivomodel of end-stage liver disease. These findings will help in developing novel cell-mediated therapeutic strategies for regenerative medicine for end-stage liver disease.
ARTICLE HIGHLIGHTS
Re search background
End-stage liver disease (ESLD) causes extensive healthcare burden with limited treatment options. Cell-based therapies using mesenchymal stem cells (MSCs)emerged as a potential approach to support hepatic regeneration in ESLD. However,effective translation of this therapy requires MSCs to have maximum regenerative potential. In vitro preconditioning strategies have been employed to strengthen the regenerative and differentiation potential of MSCs. Chemical compounds of the class triterpenes; glycyrrhizic acid and 18β-glycyrrhetinic acid present diverse therapeutic features including hepato-protection and anti-fibrotic characteristics. They are capable of modulating several physiological pathways that are crucial in hepatic regeneration.Preconditioning with hepato-protective triterpenes may stimulate MSC fate transition towards hepatocytes.
Research motivation
Although mesenchymal stem cell-mediated therapy has proved as an effective approach for hepatic regeneration, the major challenge is low cell viability and resistance in the impaired tissue post-transplantation. To acquire a persistent therapeutic efficacy,in vitropreconditioning of MSCs with hepato-protective chemical compounds may facilitate their regenerative and differentiation potential towards hepatic lineage for better survival, homing, and migration ability at the site of injury.
Research objectives
Considering the characteristics of triterpenes, glycyrrhizic acid and 18β-glycyrrhetinic acid in hepatic anomalies, the objective of the study is to explore their role in hepatic differentiation of MSCs. Preconditioned cells may serve as a better source for tissue regeneration in liver injury.
Research methods
hUC-MSCs were harvested and characterized phenotypically by flow cytometry and immunocytochemistry for the expression of MSC associated surface molecules.Isolated cells were treated with glycyrrhizic acid, 18β-glycyrrhetinic acid, and their combination for 24 h, and then analyzed at three time points; day 7, 14, and 21. qRTPCR was performed to evaluate the expression of hepatic genes. On day 21, hepatic proteins were analyzed by immunocytochemistry. Periodic acid Schiff staining was performed to determine the functional ability of treated cells.
Research results
The transcriptional profile of preconditioned MSCs displayed significant expression of hepatic genes with increasing time of differentiation. Preconditioned cells showed positive protein expression of hepatocyte specific proteins. The results were further corroborated by positive periodic acid Schiff staining, indicating the presence of glycogen in their cytoplasm. Moreover, bi-nucleated cells which is the typical feature of hepatocytes, were also seen in the preconditioned cells.
Research conclusions
Our data suggest that preconditioning of hUC-MSCs with triterpene compounds,glycyrrhizic acid, and 18β-glycyrrhetinic acid or both, successfully differentiates these cells into hepatic-like cells. The study would serve as an attempt to develop new targeted therapies using triterpenes in combination with stem cells for the treatment of end-stage liver diseases.
Research perspectives
The present study is an endeavor to augment cell based therapeutic approach by preconditioning hUC-MSCs with glycyrrhizic acid and 18β-glycyrrhetinic acid to promote their therapeutic and differentiation potential towards hepatic lineage. The preconditioned MSCs may serve as an effective source for cell therapy for injured hepatic tissue in clinical applications.
杂志排行
World Journal of Stem Cells的其它文章
- Translational products of adipose tissue-derived mesenchymal stem cells: Bench to bedside applications
- Unveiling the morphogenetic code: A new path at the intersection of physical energies and chemical signaling
- Alternative RNA splicing in stem cells and cancer stem cells:Importance of transcript-based expression analysis
- SOX transcription factors and glioma stem cells: Choosing between stemness and differentiation
- Retina stem cells, hopes and obstacles
- Considerations for the clinical use of stem cells in genitourinary regenerative medicine
