Current knowledge on the multiform reconstitution of intestinal stem cell niche
2021-11-02ZiYanXuJinJianHuangYeLiuYunZhaoXiuWenWuJianAnRen
Zi-Yan Xu, Jin-Jian Huang, Ye Liu, Yun Zhao, Xiu-Wen Wu, Jian-An Ren
Zi-Yan Xu, Xiu-Wen Wu, Jian-An Ren, Research Institute of General Surgery, Jinling Hospital,Medical School of Nanjing University, Nanjing 210002, Jiangsu Province, China
Jin-Jian Huang, Ye Liu, Medical School, Southeast University, Nanjing 210009, Jiangsu Province, China
Yun Zhao, Department of General Surgery, BenQ Medical Center, Nanjing 210019, Jiangsu Province, China
Abstract The development of “mini-guts” organoid originates from the identification of Lgr5+ intestinal stem cells (ISCs) and circumambient signalings within their specific niche at the crypt bottom. These in vitro self-renewing “mini-guts”, also named enteroids or colonoids, undergo perpetual proliferation and regulated differentiation, which results in a high-performance, self-assembling and physiological organoid platform in diverse areas of intestinal research and therapy. The triumphant reconstitution of ISC niche in vitro also relies on Matrigel, a heterogeneous sarcoma extract. Despite the promising prospect of organoids research, their expanding applications are hampered by the canonical culture pattern, which reveals limitations such as inaccessible lumen, confine scale, batch to batch variation and low reproducibility. The tumor-origin of Matrigel also raises biosafety concerns in clinical treatment. However, the convergence of breakthroughs in cellular biology and bioengineering contribute to multiform reconstitution of the ISC niche. Herein, we review the recent advances in the microfabrication of intestinal organoids on hydrogel systems.
Key Words: Intestinal organoids; Reconstitution; Stem cell niche; Bioengineering;Hydrogel
INTRODUCTION
In mammals, intestinal epithelium hosts diverse cell types at different stages of differentiation and possesses high self-renewal efficiency regulated by an sophisticated extracellular niche environment with a renewal cycle of 4-5 d, to ensure absorption of nutrients and defense against microorganisms[1,2]. As the inner layer of the intestinal wall, the intestinal epithelium and inferior lamina propria, which line the lumen,display a specific organization of crypt and villus structures to maximize the absorption surface especially in the small intestine[3].
Over the last 4 decades, efforts have been focused on identifying proliferative intestinal stem cells (ISCs) and reconstituting anin vitromodel of the intestine[4]. Due to the complexity of the intestinal microenvironment and deep-seated residence of stem cell, the most available research tools are genetically variable cell lines derived from colorectal cancers, such as Caco-2 and HT-29[5,6]. However, Caco-2 cell-based culture lacks precise 3D architecture, interactions among different cell types and biochemical gradients, which are essential for the ISC niche[5,7]. Hence, stable culture and long-term expansion of ISCsin vitrowas thought to be unattainable until 2005,when Wnt signaling was found to play a key role in maintaining the stemness and proliferative status of ISCs for the first time[8,9]. Subsequently, leucine-rich repeatcontaining G-protein coupled receptor 5 (Lgr5) was recognized as the marker gene of ISCs[4]. In addition, Satoet al[10] creatively applied Matrigel, which was extracted from Engelbreth-Holm-Swarm (EHS) sarcoma and resembles basal membrane in terms of its components, to provide biochemical support and construct a 3D niche environment, thus establishing the first generation of organoids. Conventional organoids cultured within Matrigel exhibit micron-sized 3D aggregates with projecting crypt-like buds and sealed-off lumen lined by epithelium[10-12]. ISCs interspersed in the crypt-like region are surrounded by Paneth cells, transit-amplifying cells (TA cells) and adhesion sites of matrices, which constitute the stem cell niche along with biochemical signaling gradients and mechanical cues[1,13].
In recent years, organoids have represented a biomimic platform for human development and physiological research and disease modeling including infectious,malignant, inherited and nutritional diseases, serving as an alternative to conventional animal models[14-16]. Even though stem cell research and organoid techniques have made remarkable breakthroughs, limitations have now surfaced such as low operability, uncertain biosecurity, poor plasticity and immunity deficiency[17,18].Efforts have been made to develop alternative materials to Matrigel and enrich conventional organoid culture systemsvia3D printing or microengineering[19,20].
This review focuses on integrant keys to reconstitute the stem cell niche microenvironment in organoid, the numerous candidate materials for culturing matrices and bioengineered models of the intestinal organoids.
NICHE CUES WITHIN THE MICROENVIRONMENT OF ISCS
Long term establishment of the novel intestinal organoid culture system depends on the preservation of self-renewal and self-organizing properties of the ISCs, which are regulated by various external environmental cues. So far, stemness-relevant biochemical signaling, niche cells, mechanical cuesetc.have been identified as optimal niche cues for the reconstitution of ISCsin vitro.
Biochemical signaling within the ISC niche
Wnt/R-spondin signaling is the dominant regulator in the proliferation of ISCs[8].Secreted mainly by Paneth cells and subepithelial fibroblasts, Wnt ligands bind to Frizzled and Lrp5/6 receptor complexes on ISCs and TA cells[21,22]. After binding,the complexes induce translocation of β-catenin into the nucleus to stimulate the expression of target genes that preserve the proliferating and undifferentiated status of stem cells, such as Axin2, Lgr5, Rnf43 and Znrf3[23]. Moreover, such activation is sustained and enhanced by the binding between R-spondin, a subepithelial fibroblast secreted protein, and its ligand Lgr5, which blocks negative feedback from the Rnf43 gene[23]. In human derived small enteroids or colonoids and mouse derived colonoids, exogenous Wnt and R-spondin are both entailed (Table 1). R-spondin alone is sufficient for mouse small ISC propagation[24-26].
Constituting the largest subdivision of the transforming growth factor-β (TGF-β)family, mesenchymal-derived bone morphogenetic proteins (BMPs) induce epithelial stem cell differentiation towards enterocytes and goblet cells by activating downstream phosphorylated SMAD1/5/8[24,27]. As BMP antagonists, Noggin, Gremlin1/2 and DMH-1 are involved in the maintenance of ISC numbers and proliferation status in long-term organoid culture, which play determinant roles on the avoidance of ISC exhaustion[28,29]. Therefore, exogenous Noggin is added as an essential cue to regulate ISCs in most organoid cultures[10].
Notch ligands such as Delta-like 1 (Dll1) and Dll4 are mainly expressed on Paneth cells interspersed among ISCs[30]. Once bound to Notch receptors at the surface of neighboring ISCs, the Notch intracellular domain (NICD) will be released and transported into the nucleus, where NICD undergoes enzymatic shedding by ADAM10 and γ-secretase to activate the transcription of Hes1/3/5. Hes1 in turn blocks Atoh-1 mediated differentiation toward the secretory lineage[31]. Knockout of Dll1 and Dll4 in intestinal epithelium could lead to complete exhaustion of ISCs and TA cells[32]. Dll1/Dll4-mediated Notch signaling is required for long-term homeostasis of intestinal epithelium. In the prometaphase of organoid culture,constant binding between Dll1/4+ Paneth cells and Notch+ ISCs contributes to the commencement of symmetry breaking and formation of proliferating buds,highlighting the pivotal role of Paneth cells in the ISC niche and organoid development[33,34].
Epidermal growth factor (EGF) is a canonical determinant that facilitates intestinal epithelial cells’ self-renewal including ISCs and TA cells, and is mostly secreted by Paneth cells[35]. Activated EGF receptor (EGFR) initiates downstream RAS kinase and PI3K pathways, resulting in the translocation of ERK1/2 and boosting the mitotic signal[36]. As a significant factor in the organoid culture medium, EGF can be replaced by mesenchymal cell-derived insulin-like growth factor 1, which indicates the complementary effect of Paneth cells and the mesenchyme in constituting the ISC niche[37](Figure 1).
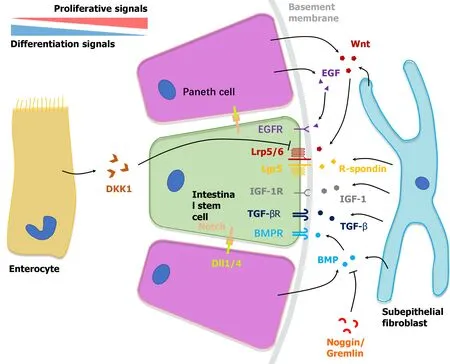
Figure 1 Niche microenvironment of intestinal stem cells at the crypt bottom. Bidirectional gradients of biochemical signals established by neighboring cells including Paneth cells, subepithelial cells and enterocytes regulate the self-renewal and differentiation of intestinal stem cells synergistically. TGF-β:Transforming growth factor-β; TGF-α: transforming growth factor-α; EGF: Epidermal growth factor; EGFR: Epidermal growth factor receptor; IGF: insulin-like growth factor; BMPR: Bone morphogenetic protein receptor; BMP: Bone morphogenetic protein; DKK1: Dickkkopf-1; Dll1/4: Delta-like 1/4.
Niche cells around ISCs
After being encapsulated into matrices, single Lgr5+ ISCs experience mitosis and end up with a cystiform sphere around day 3[24]. During the 4-cell stage and 16-cell stage,asynchronous cell division and alterations in extracellular matrix (ECM) density drives the variability in mechanosensor Yes-associated protein 1 (YAP1) subcellular localization, which is a cellular proliferating signaling activated by biophysical cues within the ISC niche and leads to variability in Dll1 expression in primitive Paneth cells[33,38]. It was also observed that ISCs cluster which failed to produce Paneth cells turned into ISC-absent enterocysts with a limited life-span, which contain only enterocytes[33]. In addition to Dll1-induced inhibition against secretory differentiation, Paneth cells generate endogenous growth factors including Wnt-3, EGF and TGF-α, facilitating niche environment reconstitution and budding organoid maturation[30,39]. Accordingly, Paneth cells are regarded as essential niche cells for the ISC niche.
Stromal production of Wnts in the submucosa is necessary for maintaining murine and human intestinal epithelium homeostasis[17,40]. Fibroblasts and myofibroblasts are known to be a source of Wnt ligands, R-spondin and TGF-β around the ISC nichein vivo. In experimentsin vitro, organoid formation was rescued by the co-culture with embryo fibroblasts in the absence of supplemental Wnts and R-spondin, which indicates the fundamental role of stroma cells in intestinal homeostasis and crypts proliferation[22]. In addition, a recent publication also revealed endothelial cells and macrophages as Wnt ligand sources[22]. Although stroma cells especially fibroblasts and myofibroblasts can synergistically generate niche factor gradients and support the ISC microenvironment, single cell or crypt-based organoid culture lack submucosal components. In induced pluripotent stem cells (iPSCs) oriented induced human intestinal organoid, iPSCs can partially differentiate into mesodermal cells under activin A stimulation[41,42]. These mesodermal cells can further generate a fibroblastenriched mesenchymal layer surrounding the epithelium[24,43-47] (Table 1).
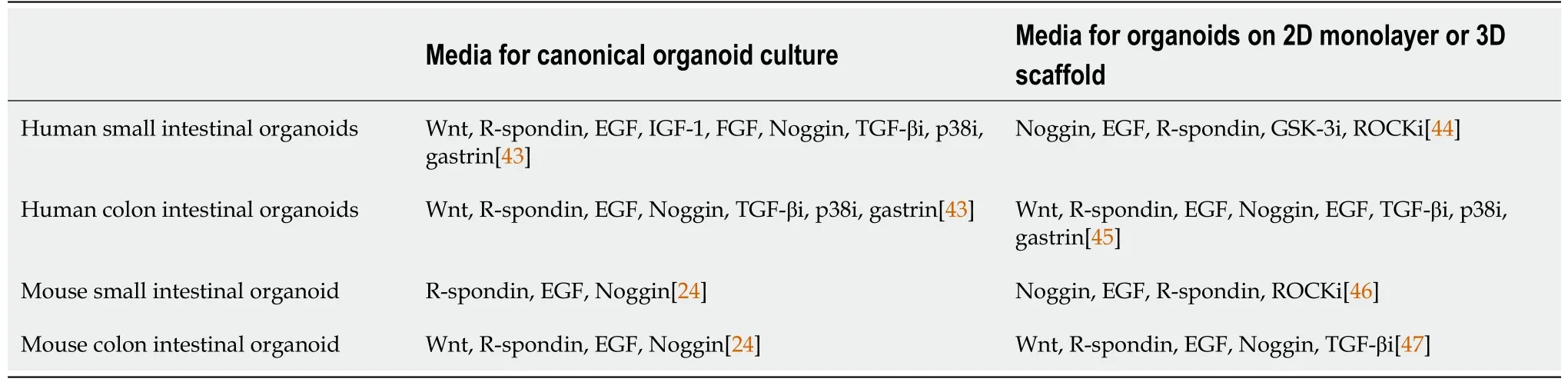
Table 1 Organoid culture conditions for multiform reconstitution of the intestinal stem cell niche
Mechanical cues
Substantial research has proved that the mechanical properties of ECM or the stiffness and elasticity of biomaterials are key parameters impacting cell behaviors[48].Organoid formation and ISCs growth, proliferation, differentiation and migration are impacted by such mechanical cues in organoid-ECM or cell-ECM interactions[49]. The incorporation of physiological relevant mechanical cues can contribute to ISCs proliferation and maturation. Stiffness refers to the degree of flexibility of the tissue microenvironment. Plastic dishes used in traditional cell culture provide a hard surface which is much stiffer than native tissues. However, Matrigel (approximately 100 Pa) provides organoids and ISCs with a softer microenvironment than intestinal tissue (approximately 800 Pa). High stiffness (approximately 1 kPa) of the matrix was demonstrated to facilitate ISC expansionviathe YAP1 molecule[33,50]. In contrast, a soft matrix promoted cell differentiation and organoid maturation. By inserting compressed nitinol springs in transplanted human organoids to introduce strain forces, Polinget al[51] observed enlarged tissue size, complexity and more similarities to native intestine. In addition, native ECM and synthesized matrix biomaterial also exhibit complex viscoelasticity, which describes a time-dependent response to loading or deformation[52]. Studies carried out using 2D and 3D culture systems indicated that matrix viscoelasticity might influence gene expression and differentiation pattern.
Epithelium of the intestinal tract especially the small intestine is constantly immersed in digestive juicein vivo. Dynamic fluid cues are often ignored in intestinal tissue models at the early stages. It was unrealistic to investigate such luminal mechanical force in structure-lacking cell line culture and canonical sealed-off organoids. Using the organoid culture system on monolayer or chips, luminal stream added through constant shaking was found to be an inductive cue for villus formation[53]. However, colon-derived organoids failed to exhibit similar sensitivity to dynamic fluid cues. This fluid cue stimulation is related to activation of an inherent villusforming program within the small intestine, which markedly improves villus formation and increases villus density[54].
CULTURE MATRICES
The novel organoid culture systems rely on hydrogels as matrix to provide soft mechanical support to facilitate the proliferation and differentiation of ISCs. However,in consideration of the appearing limitations, tissue-mimicking hydrogels with tunable properties based on well-defined natural biomaterials or customized synthetic polymers are needed. Among different kinds of biomaterials, hyaluronic acid (HA),silk protein, collagen gels, and various polyethylene glycol (PEG)-based hydrogels are found to have potential in organoid cultures.
Matrigel
As a basement membrane extract from mouse EHS tumor and a natural ECM analogue, Matrigel (also named Cultrex or EHS matrix) has been applied in the majority of cellular experiments for nearly half a century, such as cell culture, tumor invasion, lineage differentiation and gene expression[9]. Matrigel also serves as a vital and seemingly exclusive matrix that has been used in organoid culture since the early 2010s[55].
Canonical Matrigel consists of 4 major ECM proteins, which include approximately 60% laminin, approximately 30% collagen Ⅳ, approximately 8% nidogen and approximately 2% heparin sulfate proteoglycan perlecan (Figure 2A)[56]. Moreover, Matrigel contains several tumor-derived growth factors, such as TGF-β and fibroblast growth factor as well as enzymes such as matrix metalloproteinases (MMPs), which synergistically lead to its first-class bioactivity. In the temperature range of 25-37 ℃, Matrigel undergoes rapid gelation driven by entactin-mediated strong crosslinking between laminin and collagen Ⅳ as well as relatively weak hydrogen bonds within collagen molecules. Gelated hydrogel in the form of a dome or coating provides concise and optimal 3D or 2D platforms, which are suitable for multi-type cell culture. Although they vary from batch to batch, the poor mechanical properties of Matrigel (approximately 100 Pa) facilitate stem cell differentiation and organoid maturation rather than stem cell proliferation[20,57].
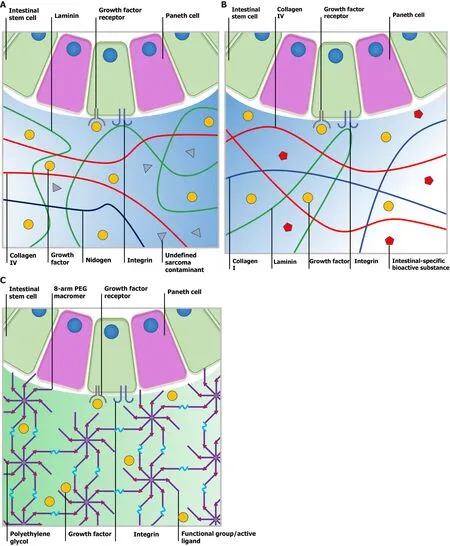
Figure 2 Comparison of Matrigel, decellularized extracellular matrix and a synthetic matrix. A: Ill-defined Matrigel is variable in composition.Although certain bioactive factors exist, Matrigel still contains undefined contamination from the tumor microenvironment, which leads to low reproducibility and biosecurity; B: Submucosa-derived decellularized extracellular matrix (dECM) provides intestinal stem cells (ISCs) with a natural microenvironment closest to native tissue and the crypt niche. Tissue-specific bioactive substances could help maintain the physical phenotype of cultured ISCs. However, the composition of dECM may vary from batch to batch and be affected by the age, gender and health status of source animals; C: Polyethylene glycol is one of the most frequently used synthetic material for cell culture as it is bioinert and tunable. Following modification with diverse functional groups or active ligands, researchers can manipulate the physical and chemical parameters to further support organoid formation. PEG: Polyethylene glycol.
When ISCs or other epithelial cellsin vitrolose intimate integrin attachment to the basal membrane, cells easily cease propagation and undergo anoikis. Different laminin subtypes within Matrigel offer a great number of adhesion sites for integrins in the ISC niche, which is mostly made up by α2β1, α7β1, and α5β1 subtypes[50]. Upon binding to laminin binding peptides, anoikis of ISCsin vitroinduced by Rho-ROCK kinase activation is markedly inhibited. The uniqueness and ubiquitousness of bioactive Matrigel make it the most preferred culture matrix for organoid, based on which canonical intestinal organoids culturing pattern is fully established. After being encapsulated in Matrigel, disassociated intestinal crypts or ISCs form cyst structures in the first 3 d. During days 4-5, the first batch of Paneth cells develop, thus forming heterogeneous Wnt activation among cysts and proliferating buds[41].
Although commonly used, Matrigel has limitations in the application of organoid techniques. Firstly, variations exist in component contents and biochemical properties from batch to batch or even within a single batch of Matrigel, which have caused a lack of homogeneity and reproducibility in cell culture results. Proteomic analyses of Matrigel are consistent between each other along with the growing number of identified different proteins (> 2000) and peptides (> 14000)[58]. Secondly, the mechanical properties of Matrigel also vary from batch to batch even between regions within a single dome. Heterogenicity among cross-linked materials such as multitype laminin and collagen peptides in part leads to uneven distribution of material density,causing variations in the stiffness of median and marginal areas. Thirdly, the tumor origin and natural resource of Matrigel restrict the promising potential of the organoid technique in tissue repair and regeneration. Although transplanted intestinal organoids carried by designed scaffolds show robust viability and origin-specific functions in animal experiments, concerns have arisen about Matrigel’s uncertain tumorigenicity when it comes to clinical use[59]. Finally, Matrigel is not amenable to chemical modification or manipulation to adjust mechanical properties, which is attributed to the inconsistencies in the concentrations of its contents. As an ill-defined complex, the infinite variety of cues within Matrigel cannot be split to elaborate their specific function in different stem cell niches. These undesirable characteristics of Matrigel require further research on synthetic or natural alternatives with highly tunable biochemical and biophysical properties to reconstitute the ISC niche[60].
Decellularized ECM
The ideal matrix for cell culture should mimic the native microenvironment and provide original tissue-specific ECM contents, sites for adhesion, stiffnessetc.[61]. This concept initiated research into decellularized ECM (dECM), which has emerged as a promising material for regenerative medicine and tissue engineering. Recent studies have also highlighted dECM as a promising natural hydrogel material for organoid culture[62].
dECM is a biological scaffold derived from native tissues in which cellular components, preserved structural components, functional enzymes and partial factors have been removed[63]. Three decellularizing strategies are commonly used to produce dECM based on different natural tissues such as cartilage, liver, kidney,breast, intestine, prostate and heart. (1) Physical strategies include freeze-thaw,agitation and rinsing to break up the cell membrane and strip off cells; (2) By using chemical agents such as acid (acetic acid), base (sodium hydroxide), chelating agent(EDTA), hypotonic detergents (Tris-HCL), ionic detergents (sodium dodecyl sulfate) or non-ionic detergents (Triton-X-100), cytoarchitecture is easily disrupted and undergoes disaggregation; and (3) Biological strategies utilize enzymes to break up ribonucleotide and deoxyribonucleotide chains specifically[64,65]. After multistep decellularization, dECM eliminates most xenogenic antigens and acquires minor immunogenicity and assured biosecurity. As an FDA approved biosafe material and medicinal product in use, different types of dECM vary greatly in major constituent concentrations such as collagens, fibronectin, elastin, laminin, proteoglycan and glycoprotein according to the tissue source and decellularization strategy. Despite these differences,the dECM material provides a truly biomimetic environment which retains native structural, signaling components and specific cell-ECM interactions (Figure 2B).Digested dECM powder can undergo collagen-based gelation in response to external conditions such as temperature, ionic concentration and pH with tunable contents and stiffness, which shows more intelligence and maneuverability over Matrigel[66].
A study by Giobbeet al[67] identified that dECM gel from porcine small intestine mucosa/submucosa enables the formation and growth of multiple types of endodermderived human organoids with equivalent efficiency. In dECM gel, gastric, hepatic,pancreatic and small intestinal organoids showed regular proliferation and differentiation capacities. However, transcriptomic analysis of small intestinal organoids in dECM gel revealed a higher expression of ISC and Paneth cell markers, such as OLFM4, SMOC2 and LYZ, and a reduction in the expression of differentiation markers, such as EZR, VIL1 and MUC12, compared to Matrigel. This discrepancy was caused by culture matrices possessing a differential biochemical signature and environmental niche, which lead to varied effects on cellular behavior and experimental results[68]. Interestingly, the dECM scaffold carrying small intestinal organoids survived two months after transplantation, which was superior to Matrigel[67]. In addition, application of dECM in organoid culture promotes physiological function.Saheliet al[69] seeded human hepatocarcinoma (Huh7) cells, human umbilical cord vein endothelial cells (HUVEC) and human bone marrow-derived mesenchymal stem cells (MSCs) in sheep live-derived dECM to produce liver organoids. Mixed cells not only formed self-organized liver organoids, but also exhibited enhanced hepatic functions with significant upregulation of transcripts of albumin, CYP3A4 and CYP3A7 compared to Matrigel and collagen Ⅰ. A recent study also used dECM as a tool to determine the exact interactions between environmental cues and stem cell behavior. By applying rat pancreatic ECM gel, the study identified collagen Ⅴ as the key cue within the dECM that boosted the formation of cultured human pluripotent stem cells (iPSCs) towards islet-like organoids and functional α, β, δ type pancreatic endocrine cells[70]. Similarly, dECM may act as the bridge between poorly-defined Matrigel material and pinpoint biochemical contents that are adequate for the propagation of ISC. In addition, chemically defined dECM gel can be tailored to have tunable mechanical properties and viscoelasticity by chemical modification or appending a compound hydrogel system[71]. By slightly enhancing viscosity or utilizing multi-step crosslinking, organoid-laden dECM ink may be used directly to fabricate biomimetic crypt-villus structures or a sophisticated bioreactorvia3D printing[72].
PEG
The great potential of organoids in research and therapy remains restricted due to illdefined matrices derived from animals. Therefore, efforts have been made to design and synthetize chemically defined hydrogel networks that enable ISC propagation and organoid formation by recapitulating key cues from the ECM[73]. To fully recognize the key cues that dominate ISC expansion, Gjorevskiet al[74] created a well-defined 3D matrix based on PEG and peptides from fibronectin, laminin and collagen IV, which were enriched within the ISC microenvironment. As a biocompatible and enzymatically biodegradable polymeric substance, after reaction with diverse nucleophiles,PEG can bond to reactive groups such as vinyl sulfone (VS) or acrylate, to form multiarmed-PEG macromers. A subsequent Michael-type addition with thiol-reactive peptides allows the formation of PEG-based hydrogel networks (Figure 2C)[75].
Synthetic inert and soft PEG scaffolds were not sufficient to maintain ISC expansion and organoid formation, similar to sodium alginate or gelatin methacrylate (GelMA)hydrogel. Interestingly, by replacing VS reactive groups on 8-arm PEG monomers with fibronectin-derived RGD (Arg-Gly-Asp) peptides to target integrins on ISCs,intestinal crypts embedded in such modified RGD-functionalized PEG gels (PEG RGD) exhibited long-term propagation and colony formation abilities, suggesting that both physical support and biochemical signals from the matrix are involved in ISC survival[50,74]. Thus, PEG RGD is regarded as a synthetic hydrogel with tunable mechanical, biochemical properties that promotes intestinal organoids growthin vitro.
In intestinal organoid culture, stiffness of the matrix has been depicted to play a critical role in ISC fate and organoid formation as described previously[76]. For PEG hydrogel formation, by blending 8-arm PEG macromers of 20- or 40-kDa at various ratios and modulating final PEG content, the storage modulus of PEG gel ranged between 110-1034 Pa[77]. Adjusting the network’s crosslink level also enabled tunable biophysical properties. By incorporating peptide sequences, which are sensitive to cellsecreted MMP, the PEG gel could acquire degradability and increase stiffness, which stimulates cell proliferation in the early phase of culture[78]. Following partially enzymatic gel degradation over time, softened PEG gel (approximately 300 Pa)promoted ISC and TA cell differentiation which is needed for organoid maturation.Compared to stable PEG gel, degradable PEG gel showed abundant expression of differential markers and higher organoid formation efficiency.
In addition, the 8-arm PEG monomer can be modified with customized peptides according to different integrin subunits[79]. The most frequently used fibronectinderived peptide Arg-Gly-Asp binds to αvβ3 and αvβ5 integrins. It was also reported that the presence of a collagen-derived peptide (GFOGER) targeting α2β1 integrin exhibited outstanding culture efficiency of human duodenal and colon enteroids[50].Also, collagen-like peptide grafted PEG gel promoted the spontaneous organization of other primary stem cells into clusters.
Although PEG-RGD gel has shortcomings such as inferior organoid culture efficiency (50%) compared with Matrigel, PEG materials fill the vacancies in the synthetic microenvironment by mimicking ECM composition which are devoid of unknown factors and enable standardized ISC culture[80]. Modification of 8-arm/4-arm PEG macromers with multiple chemical groups or peptides, which are sensitive to MMPs, pH, temperature or chemical irritation may endow the synthetic matrix with more interesting characteristics for organoid culture and achieve a fully-designed stem cell niche.
BIOENGINEERED MODELS OF THE INTESTINE
As described earlier, the first generation of organoid culture is characterized by encapsulated single ISCs, fresh crypts or disassociated organoids within a dome-like matrix. After days of proliferation and self-assembly, cell clusters grow into sealed-off difform spheroids hosting 7 intestinal epithelial cell types. Although encapsulated organoids exhibit a crypt-villus structure and mesenchyme-free ISC niche, they still have a number of dissimilarities compared to intestinal tissue. To fully enhance the maneuverability and adaptability to high-throughput screening and microfabrication,many technical approaches have been established to reproduce the stem cell niche and intestine modelin vitro.
Two-dimensional monolayers
The monolayer system on a dish or porous transwell insert provides an intestinal tissue model that offers access to both luminal and basal sides, which enables the establishment of biochemical gradients of growth factors and observation of hostpathogen interactions (Figure 3A). By introducing extrinsic Wnt and BMP to the monolayer system, Thorneet al[19] was able to evaluate the contribution of epithelialintrinsic and extrinsic Wnt to epithelial homeostasis. In a colonoid monolayer, the lack of Paneth cells in crypt-like zones explained why extrinsic Wnt is an essential factor for colonoid culture. For diarrheal pathogen study, enteroaggregative Escherichia coli revealed aggregative adherence to enteroids from the duodenum and ileum, which revealed unique patterns of intestinal segment-specific adherence of various pathogens. It is also worth noting that the self-organized monolayer showed integrated and effective barrier function with a physiological transepithelial electrical resistance (55 ohms.cm2) and dextran permeability[46].
Although the monolayer system offers a culture platform that mimicsin vivo-like cell distribution and is compatible with high-throughput drug absorption or hostpathogen interactions, these simple systems lack crypt-villus architecture and mesenchymal components. In addition, static culture cannot provide ISCs with the dynamic mechanical forces of peristalsis in the native microenvironment which is believed to affect cellular behavior and organoids self-assembly[44,81].
Three-dimensional scaffolds
The intestinal epithelium is a highly polarized tissue containing crypt-villus topography, while canonical organoids within Matrigel are much more like heterogeneous and difform spheroids. Thus, 3D scaffolds mimicking physiologic morphology have been fabricated to study stem cell behavior influenced by complex architecture, in which organoids showed robust proliferation and differentiation(Figure 3B).
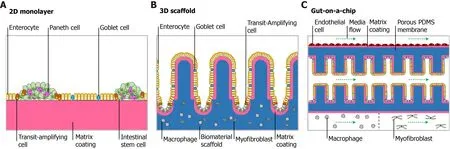
Figure 3 Multiform reconstitution of the intestinal stem cell niche. A: On matrix coating with relatively high substrate stiffness, intestinal stem cells (ISCs)generate an epithelial monolayer that recapitulates polarized cell distribution and barrier function; B: Micropatterned scaffold with suitable extracellular matrix coating enables ISC self-renewal, differentiation and epithelial cell migration and resembles distinct crypt-villus or multilayer architecture; C: Gut-on-a-chip allows incorporation of vasculature and lymph-vessels into the organoid technique and provides an effective platform for high-throughput drug screening.
In the first study, photolithography (5 μm resolution) was introduced into the microfabrication of 3D scaffold for enteroids, Costelloet al[82] used laser ablation to create an array of 500 μm deep holes in polymethyl methacrylate (PMMA). Through multistep casting, a porous villous poly-lactic-glycolic acid (PLGA) scaffold (villi height = 500 μm) was fabricated, which supported the propagation of primary intestinal crypts and Caco-2 cells. Wanget al[83] generated a polydimethylsiloxane(PDMS) stamp with both crypt and villi architecturesviatwo times of ultraviolet (UV)irradiation. Cross-linked collagen-Ⅰ hydrogel was used as the culture matrix[84]. After applying the PDMS stamp, micromolded collagen in a transwell insert resembled physical intestinal lamina propria structure and a crypt-villus micropattern (crypt depth = 132 μm, villi height = 477 μm). In addition, biochemical gradients of factors were established along with crypt-villus axis which promoted the formation of a stem cell-abundant zone at the crypt bottom and differentiated an enterocyte-abundant zone around the villi. ISCs also revealed tunable migration and differentiation capacities in response to changes in extrinsic biochemical gradients.
As a cutting-edge technique wide-spread in biomedicine, 3D printing can provide ISCs and organoids with a designed niche microenvironment and delicate architecture that restores intestinal epithelium. By horizontally slicing the digital objective with a computer aided-design and solidifying materials layer by layer, 3D printing can create intestinal models or culture scaffolds with arranged parameters, which include a degradable ink-based soluble microenvironment, an insoluble microenvironment,shape, external force and additive components[85]. In 3D printed tubular scaffolds composed of collagen or silk fibroin, multiple cell types such as like fibroblasts,myofibroblasts, macrophages and neurocytes were appended in the organoid system to build mesenchymal, immune and nerve components, respectively. Notably, adapted biomaterials such as described collagen, PEG-DA, dECM and silk have made possible the fabrication of 3D architecture directly from bioinks containing living ISCs, which is also called 3D bioprinting (200 μm resolution). In 2020, Brassard[86] and colleagues embedded human ISCs into a viscous Matrigel/collagen precursor solution and applied it to bioprinting. By controlling printing geometry and spatial deposition of cells, bioprinted ISCs within the Matrigel demonstrated spontaneous self-organization into centimeter-scale tubular tissue incorporating intestinal features such as continuous lumen, branched vasculature and crypt-villus domains. Bright-field images confirmed obvious growth and expansion over time. On day 6, printed tubes showed multiple differential markers like Lyz (Paneth cells) and L-FABP (enterocytes). In addition, Sox9+ ISCs were well enriched in the self-organized crypt-like region, which was not found in other areas[87].
Taken together, these studies indicate that 3D morphology obviously contributes to ISC differentiation and tissue function. Also, macro-scale organoid systems on scaffolds have huge therapeutic potential for short bowel syndrome and genetic intestinal diseases such as multiple familial polyposis coli or cystic fibrosisviatransplantation. On the other hand, 3D organoid scaffolds have emerged as promising bioengineering tools to construct multicellular systems comprising epithelium,mesenchyme, vasculature, lymph-vessels, nerves and smooth muscles, which may reproducibly direct the fate of ISC into a coordinated and collective behaviorin vitro[88]. For instance, small-diameter vascular grafts produced by non-degradable materials or decellularized vessels may assist in the construction and functionalization of large-scale organoid systems[89].
Gut-on-a-chip
The term organ-on-a-chip was first proposed in 2010, describing microfluidic devices containing designed micrometer sized chambers for cell culture[90]. Organ-on-a-chip uses channels tens to hundreds of micrometers wide, in which fluid flow generates gradients by passive diffusion. At the junctions of channels, chambers perfused continuously are seeded with cells (Figure 3C). This type of culture system has been used to create a continuous digestive epithelial tube composed of stem cells from different segments of the digestive tract and mimic dynamic fluid mechanical stimulation and peristaltic motions[91].
“Gut-on-a-chip” was first presented as a microdevice composed of two microfluidic channels which were separated by a 30 μm ECM-coated PDMS porous membrane and lined by the Caco-2 cancer cell line. When exposed to a low flow rate (30 μL/h) and low shear stress (0.02 dyne/cm2), Caco-2 cells not only commenced with villus morphogenesis and the expression of differentiation markers expression such as mucin and villin, but also formed a proliferative cell-enriched zone. By replacing seeded Caco-2 cells with primary ISCs, this dynamic condition induced selforganization and villus formation was amplified. In this field, Brandenberget al[92]and Nikolaevet al[93] and colleague have made outstanding contributions in attention to achieve full control of ISCs behaviors on designed chips. Shinet al[53] elaborated the mechanism behind villi morphogenesis induced by dynamic fluid flow by means of assisted computational simulation. They identified Dickkkopf-1 (Dkk1) as a regulator of ISC Wnt signaling activation. As an epithelial cell-secreted Wnt antagonist toward the basolateral direction, Dkk1 accumulates around basal cells and thus inhibits villi morphogenesis under static conditions. However, the cell chamber within chips allows constant removal of Dkk1 which established a transepithelial gradient of Dkk1 and corresponding spatially heterogeneous proliferation activation.
As the forefront of organoid research, gut-on-a-chip has emerged as a multicellular system to mimic organ-like features. Kimet al[94] isolated human peripheral blood mononuclear cells to seed the lower capillary channel and cocultured commensal microbes contacting epithelial cells. Analysis of epithelial inflammation indicated secretion of proinflammatory cytokines (IL-8, IL-6, IL-1β and TNF-α) induced by mixed immune cells and lipopolysaccharide. This human gut-on-a-chip microdevice resembled impaired villi and compromised intestinal barrier function, mimicking the pathophysiology observed in patients with inflammatory bowel disease and ileus.Thus, gut-on-a-chip can be used to investigate the interaction between intestinal epithelial cells, immune cells, microbe etc. in a tunable microdevice, which has major implications for intestinal disease research.
CONCLUSION
Identification of intestinal ISCs by exquisite and specific Lgr5 expression enables access to these stem cells through a minimal isolation process. As non-classical rapidcycling stem cells, ISCs undergo heterogeneous differentiation towards Paneth cells upon asymmetrical YAP1 activation. It seems that ISCs are inclined to build their own niche environment rather than depend on an exogenous one to guarantee lifelong proliferation, which has resulted in a revolutionary advance in basic science and translational therapy. In addition, it is now known that the biochemical signals especially Wnt ligands secreted by mesenchymal cells are crucial to transepithelial gradient establishment[2]. The influence of mesenchyme cell such as fibroblasts,myofibroblasts, smooth muscle cells, innate macrophages and nerve cells upon ISC and other epithelial cells fate and behavior is not yet fully elaborated, which highlights the development of an intestinal model containing mesenchyme, immune components,microbiome as well as epithelium. The advent of tissue engineering and microfabrication based on HA or PLGA hydrogels has enabled the development of multilayer or tubular co-culture systems[95]. 3D engineered scaffolds or chips composed of patient-derived ISCs and immune cells offer powerful models to study fundamental biochemical mechanisms or disease pathophysiology. Newly developed bioprinting approaches such as bioprinting-assisted tissue emergence contribute to macroscale organoid tissue that could be applied in regenerative therapy to treat short bowel disease[86]. However, the lack of standardization in culture conditions and interventions restricts its clinical application. The production of reproducible and easily manageable platforms that recapitulate the key features of native tissue is of great significance[96]. In addition, the organ-on-a-chip technique offers a new approach that can reproduce dynamic fluid cues and peristaltic forces and allows multicellular culture at the same time. Simultaneous control over cell distribution,biochemical gradients and mechanical cues can be achieved in such microfluidic systems, which have set a trend to reproduce the complexity of the digestive tract.
ISC proliferation and organoid generation entail appropriate mechanical support and adhesion sites. The conventional widely-used culture matrix Matrigel, extracted from native ECM, has gradually revealed its drawbacks. Matrigel cannot fully resemble intestinal ECM components or provide microenvironmental cues within the ISC niche, which may alter cellular behavior and limit the reliability of organoids as platforms for disease modeling and transplantation therapy. Well-defined or engineered materials have been established to replenish or replace conventional matrices. For instance, several natural polymer materials such as dECM, collagen and laminin also offer ISCs with a porous, fibrillar environment and structural properties of ECM proteins. Synthetized multiarmed-PEG macromers produce a structure with tunable adhesion sites and degradability to mimic ECM characteristicsin vivo. The user-defined tunability of engineered biomaterials allows intervention during organoid culture by changing their physicochemical properties to determine the interaction between organoid morphogenesis and adhesive ligand or physicochemical cues. Equipping the matrix with light sensitivity by incorporating light-sensitive moieties could enable external control over cell differentiation level or investigate matrix-stiffness relevant disease, such as fibrosis.
In conclusion, to dispel concerns regarding biosecurity and enable further drugscreening or transplantation therapy, the conventional Matrigel-based organoid system requires optimization. Reliability, reproducibility, culture effectiveness and biosecurity of the natural or synthetized hydrogel for ISC culture and organoid generation need to be tested and verified. The development of biomaterial-based bioink for ISCs is also significant, which requires specific viscosity and bioactivity.However, technique challenges need to be overcomed to reach the designed cell deposition and fabricate refined tissue modelsvia3D printing. Gut-on-a-chip incorporating blood and lymph vasculature and nerves may further advance organoid in future pathophysiological studies or functional intestine tissue reconstitutionin vitro.
杂志排行
World Journal of Stem Cells的其它文章
- Translational products of adipose tissue-derived mesenchymal stem cells: Bench to bedside applications
- Unveiling the morphogenetic code: A new path at the intersection of physical energies and chemical signaling
- Alternative RNA splicing in stem cells and cancer stem cells:Importance of transcript-based expression analysis
- SOX transcription factors and glioma stem cells: Choosing between stemness and differentiation
- Retina stem cells, hopes and obstacles
- Considerations for the clinical use of stem cells in genitourinary regenerative medicine
