Oxygen vacancies and V co-doped Co3O4 prepared by ion implantation boosts oxygen evolution catalysis∗
2021-10-28BoSun孙博DongHe贺栋HongboWang王宏博JiangchaoLiu刘江超ZunjianKe柯尊健LiCheng程莉andXianghengXiao肖湘衡
Bo Sun(孙博), Dong He(贺栋), Hongbo Wang(王宏博), Jiangchao Liu(刘江超),Zunjian Ke(柯尊健), Li Cheng(程莉), and Xiangheng Xiao(肖湘衡)
Department of Physics,Hubei Nuclear Solid Physics Key Laboratory,Wuhan University,Wuhan 430072,China
Keywords: ion implantation,oxygen vacancy,oxygen evolution reaction,heteroatom doping
1. Introduction
In the face of the rapid growth of energy demand and the consumption of traditional fossil fuels,it is urgent need to develop sustainable energy sources.[1,2]As is well known, hydrogen has long been considered as an ideal energy carrier to replace traditional fossil fuels because of its high energy density and carbon neutral.[3,4]As one of the most promising methods for hydrogen production,electrochemical watersplitting mainly includes hydrogen evolution reaction (HER)and oxygen evolution reaction(OER).However,as a sluggish kinetics process, OER involves a four-electron and four protons transfer process, which impedes the energy conversion efficiency of hydrogen production by water electrolysis.[1,5–8]Currently,the commercial catalysts for water splitting are noble metals and their compounds such as ruthenium dioxide(RuO2)/iridium dioxide (IrO2) for OER in practical application, but their high price and instability limit the large-scale commercial application of those electrocatalysts.[9–11]Thus,it is urgent to develop earth-abundant and high-efficient catalysts to replace noble metal-based catalysts for OER catalysis.
Recently, transition metal oxides (TMO) such as Ni/Co/Fe-based oxides have been widely concerned by researchers, considered to be the promising OER catalysts. Among TMO materials, Co3O4, B-doped Co3O4,CoO/Co3O4, CoFe2O4,etc., as potential non-precious metal OER electrocatalysts have been intensively investigated.[12–15]Specifically, Co3O4have been widely regarded as the wellstudied OER catalysts due to low cost, multivalence oxidation states, and high stabilities.[16,17]However, the relatively poor conductivity and unfavorable absorption ability to oxygen-containing reaction intermediates of Co-based TMOs impede their practical application.[18]Therefore, improving the conductivity and properly adjust the absorption behavior of Co3O4during the OER process is expected for a long time.[19,20]Recently,defects and oxygen vacancies(Ov)have been intensively explored as effective method to coordinate the electronic structure of Co3O4toward improving catalytic activity.[18,21]Creating oxygen vacancies are effective strategy to enhances the adsorption of intermediates and promote the electronic conductivity of Co3O4, and oxygen vacancies filled with OH• first could facilitate the pre-oxidation has been confirmed by operando spectroscopy and electrochemical measurement for Co3O4with oxygen vacancies (Ov–Co3O4).[12,22]Furthermore, doping suitable heteroatoms is widely used to improve conductivity and further enhance its OER performance of Co3O4. Although it has been reported that doping heteroatoms into cobalt oxide can improve the OER activity, but more work has focused on cation doping with some atomic whose size similar to Co atoms,limited by conventional chemical doping methods.[23]So far, compared with iron, nickel and manganese, there are rarely studies on vanadium as a dopant for electrocatalysts,so it is very meaningful to develop cobalt oxides with V-doping and oxygen vacancies.[24–26]Meanwhile, it is very important and meaningful to analyze on the synergistic interaction of V dopant and oxygen vacancies. However, limited by methods that simultaneously generate oxygen vacancies and V doping, the mechanism of the synergistic interaction still needs to be studied in depth. The development and application of simple, efficient and controllable doping and defect generated methods have always been the focus and deficiencies of research.
Herein, we report an oxygen vacancy and V co-doped Co3O4(V–Ov–Co3O4)as an OER catalyst,prepared by novel and controllable V ion implantation. The increased of low valence state of Co species (demonstrated by XPS results) in V–Ov–Co3O4is higher than that of pristine Co3O4(Co3O4)and V–Co3O4represent the generated of oxygen vacancies by V ions implantation. At the contributed of the vanadium doping and the generation oxygen vacancies for the optimization of electronic properties,the overpotential of V–Ov–Co3O4on Ti foil was reduced to 329 mV, compared with Co3O4(403 mV) and V–Co3O4(381 mV). The Tafel slope of V–Ov–Co3O4is much smaller, 74.5 mV·dec−1, which is obviously better than that of Co3O4(172 mV·dec−1)and V–Co3O4(152.9 mV·dec−1). The density functional theory(DFT)simulations confirm synergistic interaction between V doping and oxygen vacancies generated by ions implantation. Apart from improving conductivity,another factor for the significantly improved OER activity is due to the energy barrier from O∗to HOO∗was decreased, caused by the increase of the charge density around Co atoms and the improvement of HOO∗absorption.
2. Results and discussion
Cobalt hydroxide is prepared on Ti foil substrate using a general electrochemical deposition method, and it was converted to Co3O4after a thermal annealing process.[27]And then, Co3O4was subjected to vanadium ion-implantation at 40 kV (V–Ov–Co3O4) to the dosage of 4×1016ions·cm−2,and V−Co3O4was prepared from annealed V–Ov–Co3O4(Fig. 1(a)) in air atmosphere. The x-ray diffraction (XRD)patterns of Co3O4, V–Co3O4, and V–Ov–Co3O4were firstly tested as show in Fig. 1(b). The diffraction peaks of Co3O4sample located at 31.2°,36.7°,59.2°,and 65.2°,can be corresponded to(200),(211),(321),and(400)planes of the spinel Co3O4phase (JCPDS No. 43-1003), apart from diffraction peaks belongs to Ti foil. Meanwhile, the V–Ov–Co3O4(irradiated Co3O4) and V–Co3O4(annealed V–Ov–Co3O4) exhibited the similar diffraction pattern as Co3O4,indicating no obvious phase changes occurred during the implantation process. The morphology change of the high-energy vanadiumion implantation Co3O4was analyzed by scanning electron microscope (SEM). Figure 1(c) showed that the Co3O4sample was consisted of smooth and continuous two-dimensional(2D) nanosheets oriented and interconnected other. After the high-energy vanadium-ion implantation, broken surface and porous framework was observed for V–Ov–Co3O4(Fig.1(d))which was constructed by ion sputtering,and the similar morphology was also found in the sample of V–Co3O4(Fig.S1).Energy dispersive spectrometer(EDS)reveals that Co,O,and V elements homogenously distribution throughout the V–Ov–Co3O4(Fig.1(e)). Compared to Co3O4(Fig.S2),the elemental mapping images of V–Ov–Co3O4and V–Co3O4(Fig. S1)suggested that V element was successfully homogenously doped into Co3O4after the V-ion implantation. The vanadium content in V–Ov–Co3O4is 0.34 wt%.In short,V-ion implantation realized the doping of V element and increases the surface area in Co3O4nanosheets,which was supported by SEM and XRD results.
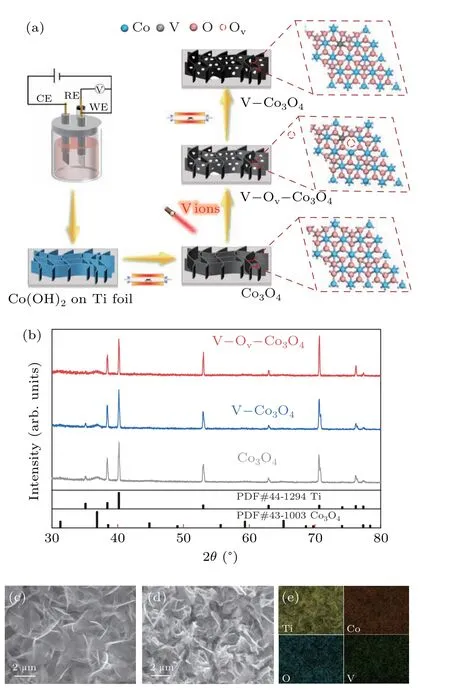
Fig. 1. Illustration of fabrication, structural, and morphology characterization. (a) Schematic diagram of preparation Co3O4, V–Co3O4, and V–Ov–Co3O4. (b) XRD patterns of Co3O4, V–Co3O4, and V–Ov–Co3O4. SEM images of(c)Co3O4. SEM images(d)and elemental mapping images(e)of V–Ov–Co3O4.
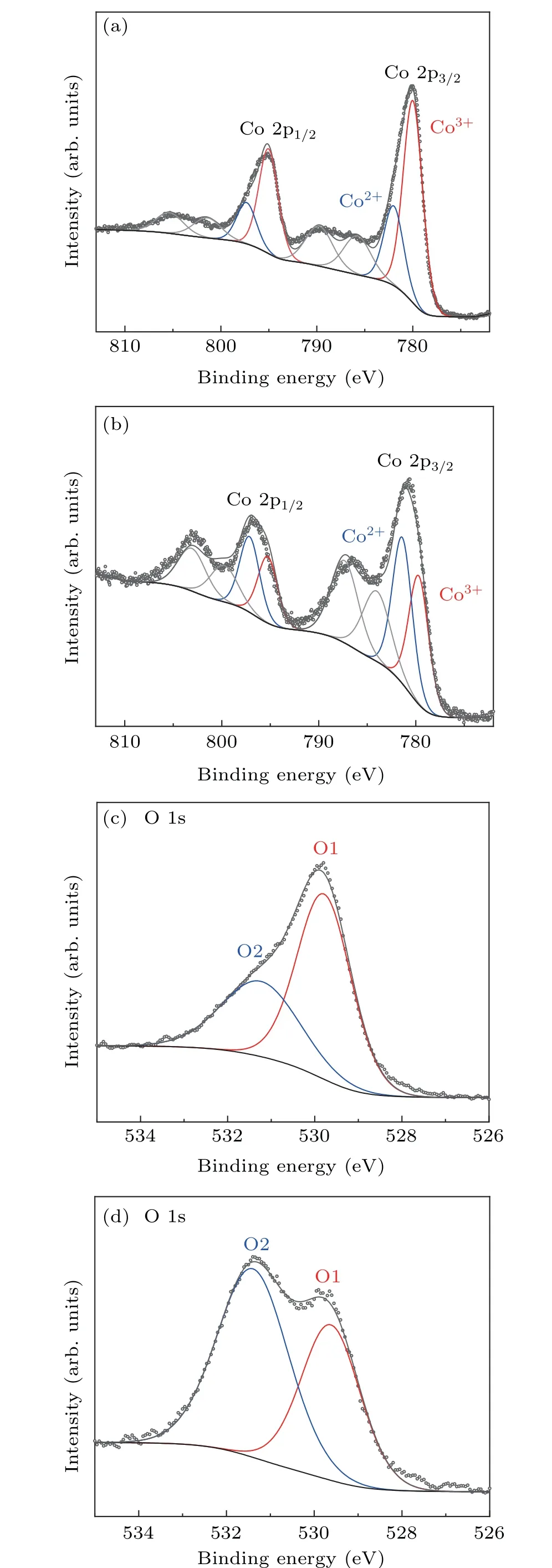
Fig. 2. Electronic structure characterization: Co 2p XPS spectra of Co3O4 (a) and V–Ov–Co3O4 (b). The O 1s XPS for Co3O4 (c) and V–Ov–Co3O4 (d).
Since electrochemical reaction usually occurs on the solid–liquid interface between the surface of the electrocatalyst and the electrolyte, probing the surface electronic structure of the prepared catalyst samples is crucial to study the OER reaction. Hence, x-ray photoelectron spectroscopy(XPS),as a universal and super-sensitive instrument,was used to study electronic states on prepared catalyst surfaces. Figures 2(a), 2(b), and S3 show that the Co 2p XPS spectra of Co3O4,V–Co3O4,V–Ov–Co3O4. Two typical peaks at around 795 eV and 779.9 eV should be specified to Co 2p1/2and Co 2p3/2for Co3O4and V–Co3O4,respectively. After V-ion implantation, it could be distinctly observed that the peaks of the two new satellites are located at 787 eV and 803 eV, respectively, and the Co 2p of V–Ov–Co3O4peaks shift negatively(about 0.2 eV)(Fig.2(b)),which indicates the increase of electron density in the Co species. In order to explore the differences in the chemical valence of Co atoms before and after V-ion implantation,Co 2p spectra were fitted. There are two fitted peaks,located at 779.8 eV and 781 eV,which belong to Co3+oxidation state and Co2+oxidation state for Co 2p3/2,respectively.[27,28]From the fitted result,it is obviously exhibited that atomic ratios of Co2+/Co3+of V–Ov–Co3O4(1.1)is significantly higher than that Co2+/Co3+of Co3O4(0.4). The higher atomic ratios indicate that there is a higher proportion of Co2+in the V-ion-implanted Co3O4,and oxygen vacancies were created by sputtering effect of V-ion implantation. The atomic ratios of Co2+/Co3+of V–Co3O4are similar to that of Co3O4. The oxygen vacancies in V–Co3O4were also validated by fine-scanned O1s spectrum. Figures 2(c), 2(d), and S4 shows that there are two typically oxygen peaks located at 529.8 eV(O1)and 531.6 eV(O2)of Co3O4,V–Co3O4and V–Ov–Co3O4.[27,29]O1 is identified as a typical metal–oxygen bonds. The O2 with higher binding energy peak is considered to be oxygen defect species.[27]Comparing with O2 peak of Co3O4and V–Co3O4,the stronger intensity O2 peak of V–Ov–Co3O4(Fig.2(d))reveals a large number of oxygen defect sites with Co2+species are formed. The results shown in XPS for three samples indicated that Co2+species were produced with creating oxygen vacancies by V-ion implantation. Combined with the characterization of structure,morphology,and composition, it is confirmed that V-ion implantation can realize both of the doping of V element and the generation of oxygen vacancy in Co3O4without distinct phase change.
The role of oxygen defect and doped V sites on Co3O4nanosheets were investigated herein by carefully evaluating the as-prepared catalysts performance for OER activity. All electrocatalytic performance tests are performed in a threeelectrode cell with workstation (CHI 760E) at 25°C in 1-M KOH solution. Due to Co3O4, V–Co3O4, and V–Ov–Co3O4electrocatalysts were supported on Ti foil, which were tested directly as work electrodes. The OER electrocatalytic activity of the prepared samples were measured by polarization curves with a scan rate of 5 mV·s−1and the data were presented with IR corrected by 90%. Figure 3(a) illustrates OER polarization curves of the Co3O4, V–Co3O4, and V–Ov–Co3O4.The V–Ov–Co3O4catalysts displayed outstanding OER catalytic activity. To maintain a current density of 10 mA·cm−2,the V–Ov–Co3O4only needs overpotential of 329 mV. The V–Co3O4(381 mV) and Co3O4(403 mV) need higher overpotential, indicating the great OER catalytic performance of V-ion implantation Co3O4. Tafel slope is a commonly used descriptor for studying the catalytic mechanism of OER catalysis. According to the Tafel equation, Tafel slope was fitted from the LSV curves.[30,31]It can be seen from Fig.3(b)that the Tafel slope of V–Ov–Co3O4is 74.5 mV·dec−1, which is smaller than that of Co3O4(172 mV·dec−1) and V–Co3O4(152.9 mV·dec−1), suggesting the rapid reaction kinetics of V–Ov–Co3O4. As displayed in Fig.3(c),the overpotential and Tafel of among all as-prepared samples are put together to emphasize the improve of the OER activity of V–Ov–Co3O4.Furthermore,electrochemical impedance spectroscopy(EIS)test,a cogent method to analyses the electrode kinetics in electrocatalytic process,performed from high frequencies 100 kHz to low frequencies 0.1 Hz in 1.0-M KOH.The Nyquist diagram of V–Ov–Co3O4, V–Co3O4, and Co3O4were compared under same potential in Fig.3(d). Apparently, the semicircle of V–Ov–Co3O4at the high-frequency range in Nyquist diagram,is much smaller compared with Co3O4and V–Co3O4, which indicated a smaller charge transfer resistance(Rct).As show in Fig.S8,the charge-transfer resistance values of V–Ov–Co3O4is 2.2 Ω, which is far less than of V–Co3O4(10.3 Ω) and Co3O4(34.9 Ω). The smallerRctsuggest that high intrinsic activity performance can be ascribed to the great improvement interfacial electron-transfer kinetics and improved electrical conductivity. It is well known that the activity of a catalyst depends on the number of active centers and the inherent activity of each active centers. It is a widely recognized method to compare the electrochemical active surface area(ECSA)by measuring the electrochemical double-layer capacitance(Cdl).TheCdlwas obtained by measuring theC–Vcurve at different scan rates (Fig. S5).[32]As shown in Fig. 3(e), theCdlof V–Ov–Co3O4is 50.6 mF·cm−2,higher than that of the Co3O4(37.6 mF·cm−2).TheCdlof V–Co3O4(33.2 mF·cm−2)is similar than that of Co3O4,which due to the reduction in the number of active sites caused by defect repair during thermal annealing. This confirms that high-energy V-ion implantation produced and exposed more active sites. Moreover,the longterm stability is crucial factors of the OER catalyst. The stability measure of V–Ov–Co3O4was tested by chronopotentiometric curves at 10 mA·cm−2and 20 mA·cm−2for continuous 27 h, respectively. Indicating a good durability without appreciable increase in potential (Fig. 3(f)). The OER activity of three samples indicate that V-ion implantation is an efficient and stable method to generate oxygen vacancies and dope V element in Co3O4for improving electrochemical activity. From the point of view of the reaction process, it is essential to in-depth investigate the mechanism of oxygen vacancy and vanadium doping to enhance the activity of OER.
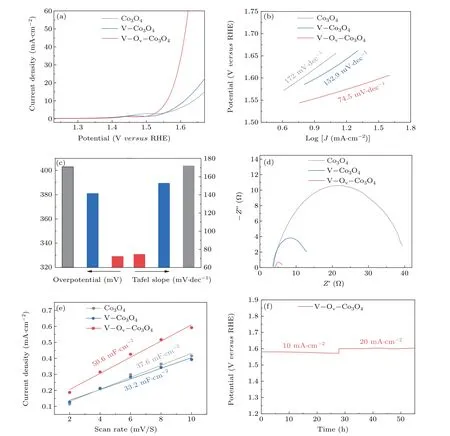
Fig.3. Electrochemical catalytic measurement: (a)LSV curves of Co3O4,V–Co3O4,and V–Ov–Co3O4;(b)corresponding Tafel slopes of Co3O4,V–Co3O4,and V–Ov–Co3O4;(c)overpotential and Tafel slope of Co3O4,V–Co3O4,and V–Ov–Co3O4;(d)Nyquist plots of Co3O4,V–Co3O4,and V–Ov–Co3O4;(e)ECSAs of Co3O4,V–Co3O4,and V–Ov–Co3O4;(f)chronopotentiometric curves at 10 mA·cm−2 and 20 mA·cm−2,respectively.

Fig.4. DFT calculations of Co3O4 and V-implanted Co3O4. (a)The top view structures of Co3O4 (001)plane and V–Ov–Co3O4 (001). Slices of side view electron density differences of Co3O4 (c)and V–Ov–Co3O4 (d). (e)PDOS plots for Co3O4 and V–Ov–Co3O4. (f)The calculated OER Gibbs free energy diagrams.
Through density functional theory (DFT) calculations,the effects of oxygen vacancies and V doping on the electronic and catalytic properties of Co3O4at the atomic level were studied. As shown in Fig. 4(a), the Co3O4(001) crystal plane was first constructed, V-doped Co3O4(V–Co3O4,Fig. S5) and coexistence models of V and oxygen vacancies(V–Ov–Co3O4, Fig. 4(b)) were also constructed by V atom replacing and oxygen atom removing. As shown in Figs.4(c)and 4(d),due to the V doping and the oxygen vacancies caused by V ion implantation, the excess electrons generated by the oxygen vacancies are completely redistributed to the surrounding Co atoms, resulting in a decrease in the chemical state of the Co element compared with the original Co3O4, which is consistent with the result of XPS test. It can be clearly seen that the Millikan charge value of the exposed Co atom at the surface decreased from 0.87 to 0.84, which means that the charge density of the Co atom has increased. Therefore, its electronic structure can be effectively modulated to affect its catalytic activity. In addition, we found that the introduction of oxygen vacancies and V impurities can effectively adjust its band structure(Fig.4(e)).It can be seen that oxygen vacancies and V doping can introduce a large number of impurity states in the Co3O4band gap,thereby effectively enhancing the conductivity of the sample and accelerating its charge transfer process. The results are consistent with the EIS test data.
In order to further reveal the enhancement essence of Vion implantation on catalytic activity from the perspective of the reaction mechanism, we calculated the energy profile of OER reaction pathway as shown in Fig 4(f).[33–35]It can be found that the limiting energy barrier of OER reaction pathway is from O∗to HOO∗step for Co3O4(001)plane(Fig.S7).There is a 2.05 eV energy barrier for OER reaction. Interestingly, as a result of V-ions implantation, the adsorption capacity of the Co3O4surface for OOH groups increases as the charge density of Co atoms increases, so the OER reaction barrier is effectively reduced.The OER reaction barriers of V–Co3O4and V–Ov–Co3O4are reduced from 2.05 eV to 1.82 eV and 1.75 eV,respectively. This calculation result is fully coupled with the LSV test,which provides an in-depth analysis of the enhancement mechanism of ion implantation.
3. Conclusion
In summary, we have confirmed that introducing V dopant and oxygen vacancy can significantly improve the OER activity of Co3O4. XPS, EDS, and DFT consistently proved that V-doping and oxygen vacancy produced by V-ion implantation effectively regulated the electronic density of Co3O4,which increased the electrical conductivity of Co3O4and obviously decreased the energy barrier from O∗to HOO∗. The reducing of the reaction barrier is attributed to the improved HOO∗absorption.The optimized V–Ov–Co3O4shows a lower overpotential of 329 mV, compared with Co3O4(403 mV)and V–Co3O4(381 mV).Also, V–Ov–Co3O4has a low Tafel slope of 74.5 mV·dec−1. Therefore, ion implantation can be used precise approach to manipulating electronic properties of metal oxide based OER catalysts.
猜你喜欢
——机械厂)
杂志排行
Chinese Physics B的其它文章
- Physical properties of relativistic electron beam during long-range propagation in space plasma environment∗
- Heterogeneous traffic flow modeling with drivers’timid and aggressive characteristics∗
- Optimized monogamy and polygamy inequalities for multipartite qubit entanglement∗
- CO2 emission control in new CM car-following model with feedback control of the optimal estimation of velocity difference under V2X environment∗
- Non-peripherally octaalkyl-substituted nickel phthalocyanines used as non-dopant hole transport materials in perovskite solar cells∗
- Dual mechanisms of Bcl-2 regulation in IP3-receptor-mediated Ca2+release: A computational study∗
