Differentiation of gestational diabetes mellitus by nuclear magnetic resonance-based metabolic plasma analysis
2021-10-15LipingLiuLenanLiuJunsongWangQiZhengBaiJinLizhouSun
Liping Liu, Lenan Liu, Junsong Wang, Qi Zheng, Bai Jin,✉, Lizhou Sun,✉
1Department of Obstetrics, the First Affiliated Hospital of Nanjing Medical University, Nanjing, Jiangsu 210029,China;
2Center for Molecular Metabolism, Nanjing University of Science and Technology, Nanjing, Jiangsu 210094, China.
Abstract This study aimed to investigate the metabolic profile of gestational diabetes mellitus (GDM) at both antepartum and postpartum periods. Seventy pregnant women were divided into three groups: the normal glucose-tolerant group (NGT, n=35), the abnormal glucose-tolerant groups without insulin therapy (A1GDM, n=24) or with insulin therapy (A2GDM, n=11). Metabolic profiles of the plasma were acquired by proton nuclear magnetic resonance (1H-NMR) spectroscopy and analyzed by multivariate statistical data analysis. The relationship between demographic parameters and the potential metabolite biomarkers was further explored. Group antepartum or postpartum showed similar metabolic trends. Compare with those of the NGT group, the levels of 2-hydroxybutyrate, lysine, acetate, glutamine, succinate, tyrosine, formate, and all three BCAAs (leucine, valine,isoleucine) in the A2GDM group were increased dramatically, and the levels of lysine, acetate, and formate in the A1GDM group were elevated significantly. The dramatically decreased levels of 3-methyl-2-oxovalerate and methanol were observed both in the A1GDM group and A2GDM group. Compare to the A1GDM group, the branched-chain amino acids (BCAAs) of leucine, valine, and isoleucine were increased dramatically in the A2GDM group. The levels of aromatic amino acids (AAAs), tyrosine and phenylalanine, were significantly increased in GDM women, consistent with the severity of GDM. Interference of amino acid metabolism and disturbance in energy metabolism occurred in women with different grades of GDM. Metabolic profiles could reflect the severity of GDM. Plasma BCAA concentrations showing strong positive correlations with weight and pre-delivery BMI. This study provides a new perspective to understand the pathogenesis and etiology of GDM,which may help the clinical management and treatment of GDM.
Keywords: gestational diabetes mellitus, metabolomics, biomarkers, 1H-NMR
Introduction
Gestational diabetes mellitus (GDM) is defined as hyperglycemia for the first time during pregnancy,without pre-existing type 1 or type 2 diabetes[1].Poorly controlled GDM could cause severe short-term complications and long-term consequences for both mothers and their fetus[2–3]. Pregnant women suffering from GDM may have complications including gestational polyhydramnios, hypertension, infection,increased risks of type 2 diabetes[4], cardiovascular diseases[5], and female malignancies[6]in later life. As a clinical feature of the metabolic syndrome, GDM also has long-term effects on the metabolic profile and future risk of diabetes in the offspring. It may increase the risk of macrosomia[7], respiratory distress syndrome[8], hypoglycemia, hyperbilirubinemia[9], and other adverse health outcomes in neonates[10].
Considering the great risk of GDM for type 2 diabetes in women and their children, metabolic features of GDM can not only illuminate potential new biological mechanisms underlying diabetes in pregnancy, but also help the prevention and optimal treatment of GDM.
By systematically studying the small-molecule metabolites, metabolomics has shown great potential in the identification of key metabolites associated with the pathogenesis of several metabolic diseases including gestational diabetes[11–12]. Among the various techniques used in metabolomics, proton nuclear magnetic resonance (1H-NMR) has its inherent advantages: non-bias for a wide range of metabolites, easy for quantification, non-destructive to samples, minimal sample processing, and high throughput[13].
Women with gestational diabetes are usually treated first by diet control and lifestyle changes (A1GDM).But sometimes these means fail to achieve optimal glycaemic control, and then insulin therapy (A2GDM)has to be applied[14]. Many metabolic studies on GDM have been conducted by profiling fluid and tissue samples, which gives new insights into the development of GDM[15], but differentiation of the two subtypes of GDM was seldom investigated[15]. The complex pathogenesis of GDM makes the prediction of GDM difficult and no single indicator has been found for high-specific screening in patients who meet the GDM diagnostic criteria. In this study, we analyzed the metabolic profiles of plasma of pregnant women with (A1GDM and A2GDM) or without GDM to characterize the metabolic profile of GDM from antepartum to postpartum with different severity at different stages, which might be helpful for the understanding of GDM pathogenesis and prediction of its severity.
Materials and methods
Patient population and experimental design
The study was conducted according to the guidelines in the Declaration of Helsinki, and approved by the Ethics Committee of the First Affiliated Hospital of Nanjing Medical University,China (No.2013SR061). All patients signed a written consent and fulfilled a detailed survey. A routine oral glucose tolerance test (OGTT) was performed between 24 and 28 weeks of gestation, following the criteria of the International Association of Diabetes and Pregnancy Study Groups. The study cohort,composed of 70 pregnant women, was divided into three groups: patients with negative OGTT (NGT,n=35); those with positive OGTT treated with diet alone during pregnancy (A1GDM,n=24), or with insulin (A2GDM group,n=11). Women with multiple pregnancy, maternal age <18 or >45 years, gestational age <36 weeks, or accompanying diseases (preeclampsia, heart disease, asthma,etc.) were excluded.
Clinical sample collection
Maternal weight, height, blood pressure, and fetal weight were measured using calibrated apparatus following standard procedures. Gestational age was calculated based on the last menstrual period and verified by ultrasound examination during early pregnancy. Before the beginning of delivery(antepartum) and on the 2ndday after cesarean section(postpartum), after an overnight fasting, venous blood was collected into EDTA-prepared tubes. The plasma samples were obtained by centrifugation (1200g,10 minutes, 4 °C), and stored at -80 °C before measurement.
1H-NMR spectroscopy
In order to obtain a clear NMR spectrum with good quality, 300 μL of plasma was added with 600 μL of methanol to remove the soluble protein in the plasma.After vortex, the mixtures were kept at -20 °C for 20 minutes. The supernatant was taken into the centrifuge tube after centrifugation at 1330gat 4 °C for 15 minutes, and then the solvent was removed with a nitrogen blower complemented with freezedryer to afford freeze-dried sample powder. The freeze-dried plasma samples were dissolved in 600 μL of phosphate buffer in D2O, vortexed, and then centrifuged at 1200gat 4 °C for 10 minutes to afford supernatant (550 μL), which was taken out and transferred into a 5 mm NMR tube for NMR measurement.
1H-NMR spectra were recorded on a 500 MHz flow-injection NMR system (Bruker AV500 spectrometer, Germany) at 300 K. In order to remove the influence of macromolecular on the small molecular signals, we used Call–Purcell–Meiboom–Gill sequence [90°–(τ–180°–τ)n–acquisition] with a total spin-echo delay (2 nτ) of 10 milliseconds. The1H-NMR spectrum was measured by 64 scans of 32 K data points in the spectrum width range of 10 000 Hz.The Fourier transform after free magnetic induction attenuation was calculated by an exponential weighting function of line broadening at 0.5 Hz.
Data processing and analysis
The Bruker Topspin 3.0 software (Bruker GmbH,Germany) was used to manually adjust the spectral phase and correct the baseline. The zero point calibration was determined by the chemical shift of the trimethylsilylpropanoic acid (1H, 0.00 ppm).Resonances were assigned according to literature,database query (Madison, [http://mmcd.nmrfam.wisc.edu/]; HMDB, [http://www.hmdb.ca/],etc.), and Chenomx NMR suite (Version 7.5, Chenomx,Canada)[16–17].1H-NMR spectra were converted to ASCII format by MestReNova software (Version 8.0.1, Mestrelab Research SL, Spain) and then were processed with R language (http://cran.r-project.org/).The residual water signal and the affected neighboring regions in 4.7 to 5.4 ppm were excluded, and the remaining integrated spectral regions were binned using an adaptive binning approach. To account for the difference in sample dilution effect, all the data were normalized by probability quotient normalization, and then mean centered followed by Paretoscaling before further analysis.
As an unsupervised multivariate statistical method,principal component analysis (PCA) does not need the input of group information. PCA was first used to outline metabolic patterns and metabolic trends, and can be used to detect any outliers in the sample. The unsupervised nature of PCA makes its group resolving ability poor. Therefore, to better find patterns among groups, supervised orthogonal partial least squares discriminant analysis (OPLS-DA) method was used,which placed changes contributing to grouping to the first component, and the remaining unrelated changes in subsequent components. Repeated two-fold crossvalidation and mutation test (n=2000) were used to obtain R2 and Q2 values to verify the validity and predictability of the model. TheP-value of the permutation test was less than 0.05, indicating that the OPLS-DA model was reliable. Variables differentiating groups were identified and displayed by S-plot and color-coded loading plot.
Statistical analysis
The score plot showed the classification of groups and the color-coded loading plot with the absolute value of the coefficient values revealed variables that contributed to group differentiation. In the loading plot, the warm-colored (e.g., red) metabolites were more significant to grouping than the cold-colored(e.g., blue) ones. Parametric Student'st-test or nonparametric Mann-Whitney test (according to the conformity of the data to normal distribution) were used to compare the differences between groups.AP-value less than 0.05 was considered statistically significant.
Results
Demographic and biochemical analysis among patient groups
Demographic data and biochemical analysis of the three groups are summarized inTable 1. Women in older ages were more likely to incur GDM. Slim women were healthier according to parameters of height, pre-pregnancy weight, pre-delivery weight,pre-pregnancy body mass index (BMI), pre-delivery BMI, and BMI gain, despite the insignificance. The levels of fasting blood glucose, 1-hour OGTT, and 2-hour OGTT in the A2GDM group were significantly higher than those in the A1GDM and NGT groups. No statistically significant differences were detected regarding systolic and diastolic blood pressure, among the three groups. The gestational weeks in the A2GDM group were significantly lower than those in the NGT and A1GDM groups. With the severity of the disease increased, neonatal weight and weight ratio increased but without statistical significance.
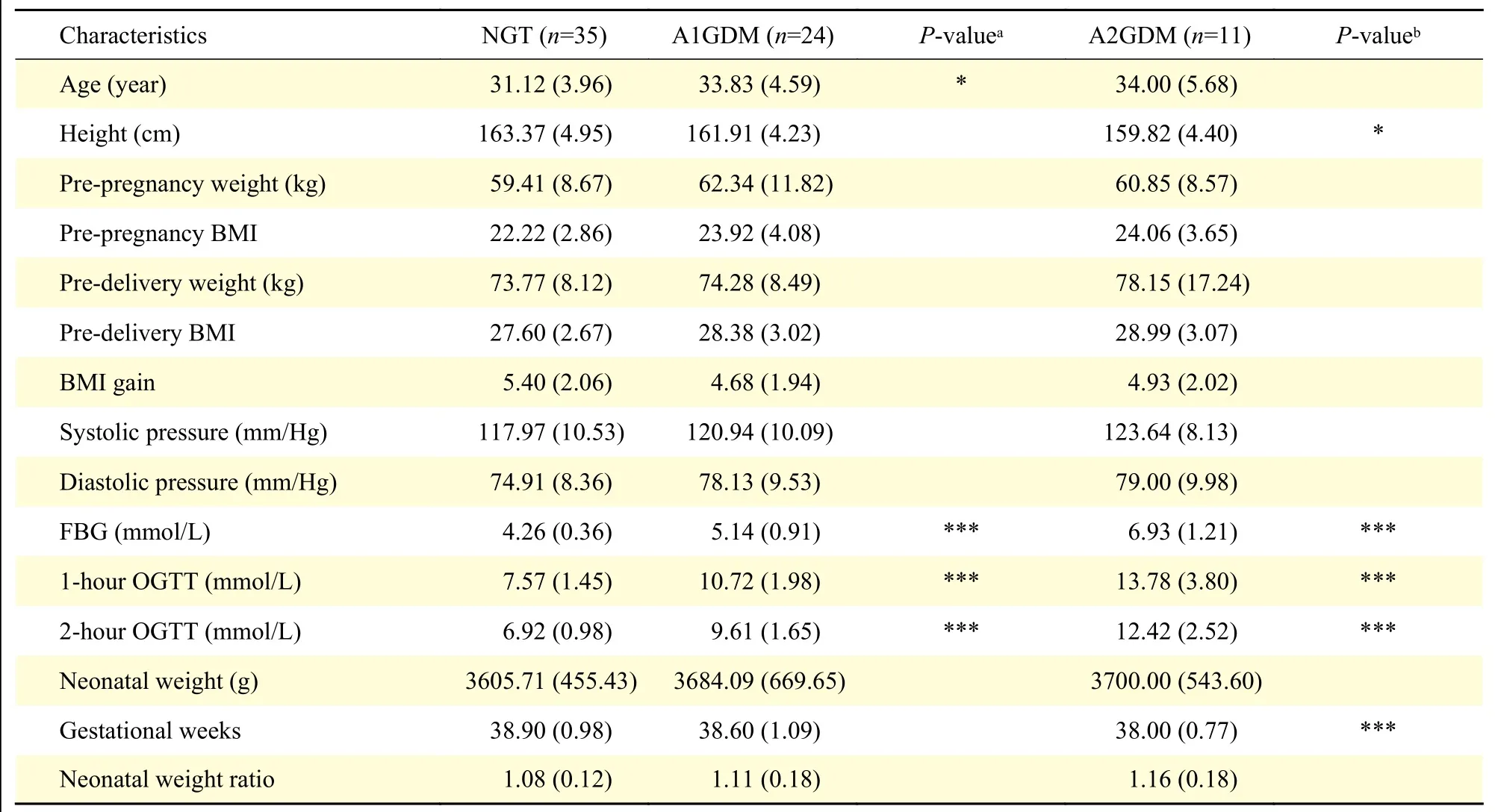
Table 1 Baseline clinical characteristics of the NGT, A1GDM, and A2GDM groups
1H-NMR spectra analysis
Representative1H-NMR spectra of plasma samples at both antepartum and postpartum periods are shown inFig. 1with major metabolites labeled. A total of 25 metabolites in plasma were identified.
Metabolic changes in the three groups
The important differential metabolites were visualized in heatmap (Fig. 2) aided by hierarchical cluster analysis to disclose the pattern of endogenous metabolites across groups intuitively.
To explore metabolic differences, we firstly compared the GDM (A1GDM+A2GDM) group and NGT group (Fig. 2A). Compared with the antepartum NGT group, the levels of sarcosine, lactate glutamine,formate, succinate, lysine, acetate, creatine, and all three branched-chain amino acids (BCAAs: leucine,valine, isoleucine) were increased dramatically in the antepartum GDM group, while the levels of hypoxanthine, 3-methyl-2-oxovalerate, 3-hydroxybutyrate,and methanol were dramatically decreased. Comparedwith the postpartum NGT group, phenylalanine,glucose, glutamine, formate lysine, tyrosine, acetate,and all three BCAAs were increased in the postpartum GDM group, while levels of 3-methyl-2-oxovalerate,3-hydroxyisobutyrate, and methanol were dramatically reduced.
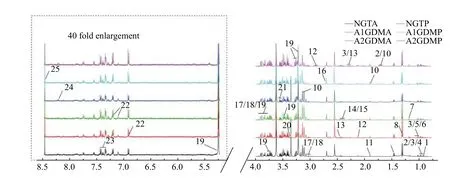
Fig. 1 Typical 500 MHz 1H-NMR spectra of plasma samples obtained from six groups. A total of 25 metabolites were identified in plasma with database query and software. 1: 2-hydroxybutyrate; 2: leucine; 3: valine; 4: isoleucine; 5: 3-hydroxyisobutyrate; 6: 3-methyl-2-oxovalerate; 7: 3-hydroxybutyrate; 8: lactate; 9: alanine; 10: lysine; 11: acetate; 12: N-acetylcysteine; 13: glutamine; 14: glutamate; 15:succinate; 16: sarcosine; 17: creatine; 18: creatine phosphate; 19: glucose; 20: methanol; 21: glycine; 22: tyrosine; 23: phenylalanine; 24:hypoxanthine; 25: formate. 1H-NMR: proton nuclear magnetic resonance; NGT: normal glucose-tolerant group; A1GDM: abnormal glucosetolerant group without insulin therapy; A2GDM: abnormal glucose-tolerant group with insulin therapy; NGTA: antepartum NGT group;NGTP: postpartum NGT group; A1GDMA: antepartum A1GDM group; A1GDMP: postpartum A1GDM group; A2GDMA: antepartum A2GDM group; A2GDMP: postpartum A2GDM group.
The differences of metabolic profile among the three groups were further explored (Fig. 2B). Subtle changes were found indicating both antepartum and postpartum changes in metabolite abundance among the three groups of NGT, A1GDM, and A2GDM. As compared to the antepartum NGT group, trends of metabolite changes in the A1GDM and A2GDM groups were similar. Compared with the antepartum NGT group, the levels of 2-hydroxybutyrate, lysine,acetate, glutamine, succinate, tyrosine, formate, and all three BCAAs were increased dramatically in the antepartum A2GDM group, but only three metabolites(lysine, acetate, and formate) were significantly elevated in the antepartum A1GDM group.Dramatically decreased levels of 3-methyl-2-oxovalerate and methanol were observed in the antepartum A1GDM and A2GDM groups compared with the antepartum NGT group. All three BCAAs were increased dramatically in the antepartum A2GDM group compared with the antepartum A1GDM group.
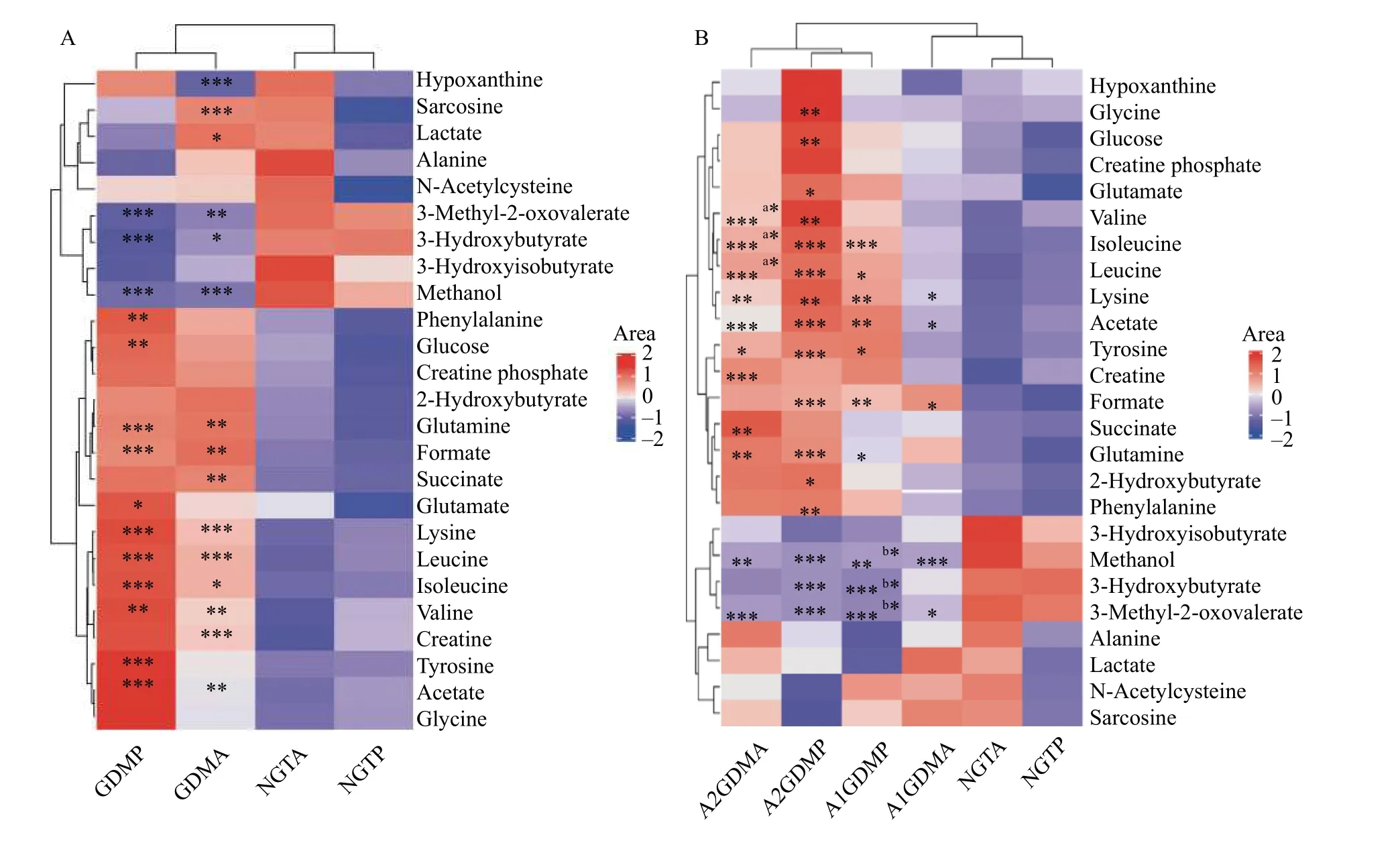
Fig. 2 Heat map visualization of clustering results of the metabolites in plasma for gestational diabetes mellitus patients of different groups. Rows represent metabolites and columns represent groups. Color key indicates metabolite quantities: reddish means increase and bluish means decrease of metabolites. A: Comparisons between the GDM (A1GDM [n=24] + A2GDM [n=11]) group and NGT group(n=35) at antepartum and postpartum periods: GDMP vs. NGTP, GDMA vs. NGTA. *P<0.05, **P<0.01, ***P<0.001. B: Comparisons between the A1GDM group (n=24), A2GDM group (n=11), and NGT group (n=35) at antepartum and postpartum periods: A2GDMA vs. NGTA,(a)A2GDMA vs. A1GDMA, A2GDMP vs. NGTP, A1GDMP vs. NGTP, (b)A1GDMP vs. A1GDMA, A1GDMA vs. NGTA. *P<0.05, **P<0.01,***P<0.001. Parametric Student's t-tests were used for calculating the P values. NGT: normal glucose-tolerant group; A1GDM: abnormal glucose-tolerant group without insulin therapy; A2GDM: abnormal glucose-tolerant group with insulin therapy; NGTA: antepartum NGT group; NGTP: postpartum NGT group; A1GDMA: antepartum A1GDM group; A1GDMP: postpartum A1GDM group; A2GDMA:antepartum A2GDM group; A2GDMP: postpartum A2GDM group.
As compared with the postpartum NGT group, the levels of 2-hydroxybutyrate, lysine, acetate, glutamine,glutamate, glucose, glycine, tyrosine, phenylalanine,formate, and all three BCAAs were increased in the postpartum A2GDM group, while the levels of leucine, isoleucine, lysine, acetate, glutamine,tyrosine, and formate were increased in the postpartum A1GDM group. Dramatically reduced levels of 3-methyl-2-oxovalerate, 3-hydroxyisobutyrate, and methanol were observed in the postpartum A1GDM and A2GDM groups, compared with the postpartum NGT group. There were no significant metabolites changes between the postpartum A1GDM and A2GDM groups. The levels of 3-hydroxyisobutyrate,3-methyl-2-oxovalerate, and alanine were significantly decreased in the postpartum A1GDM group compared with the antepartum A1GDM group.
Metabolic pattern of the three groups
The PCA model was performed on the binned1HNMR metabolites from the study groups. The PCA score plot for1H-NMR data of plasma exhibited partial separation in the scores plots among the three groups. Relatively good separation was achieved between the NGT and A2GDM groups for both antepartum and postpartum periods, but severe overlapping of other groups was found. To eliminate the variations irrelevant to the grouping, OPLS-DA was performed to disclose the metabolic differences among groups. Score plots were used to show the clusters between classes and loading/S-plot were used to identify differential metabolites between groups.Differential metabolites were those in the upper right and lower left quadrant of the S-plot.
In the score plot (Fig. 3), the antepartum GDM groups were completely separated from the antepartum NGT group (Fig. 3A), which suggested that severe metabolic perturbation happened in antepartum women of both the A1GDM and A2GDM groups. The partial overlapping of the antepartum A1GDM group and the antepartum A2GDM group suggested subtle severity difference of the diseases.The postpartum A1GDM and A2GDM groups were separated from the postpartum NGT group (Fig. 3C),with severe overlapping between the postpartum A1GDM and A2GDM groups. Metabolites with significant contribution to the clustering were showcased in the score plot (Fig. 3BandD).
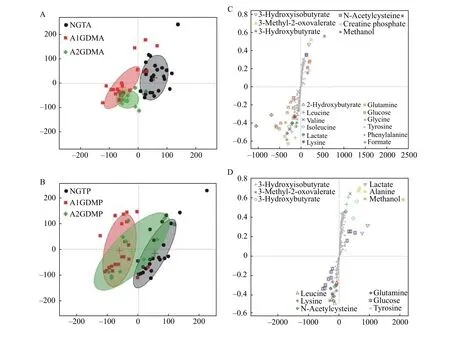
Fig. 3 OPLS-DA score plot and mean score trajectory of plasma 1H-NMR spectra obtained from antepartum and postpartum NGT/A1GDM/A2GDM patients. A: The OPLS-DA score plot between NGTA (n=35), A1GDMA (n=24), and A2GDMA (n=11) groups.B: The OPLS-DA score plot between NGTP (n=35), A1GDMP (n=24), and A2GDMP (n=11) groups. C and D: Metabolites with significant contribution to the clustering were showcased in the score plot. NGTA: antepartum NGT group; NGTP: postpartum NGT group; A1GDMA:antepartum A1GDM group; A1GDMP: postpartum A1GDM group; A2GDMA: antepartum A2GDM group; A2GDMP: postpartum A2GDM group; OPLS-DA: orthogonal partial least squares discriminant analysis.
Relationship between endogenous metabolites and demographic parameters
To further investigate the relationship between demographic parameters and the plasma biomarkers,PLS regression analyses of patients' data in three groups at antepartum and postpartum periods were performed using demographic parameters as Y variables and metabolite concentrations as X variables showing inFig. 4. The NGT women were shown on the left panel, while the GDM women on the right,with A1GDM closing to NGT women. Positive correlations between BCAAs and parameters of weight and pre-delivery BMI were observed. Lysine,2-hydroxybutyrate, acetate, and glutamine showed positive correlations with weight and pre-delivery BMI. In addition, negative correlations were presented from methanol, 3-hydroxyisobutyrate, 3-hydroxybutyrate , and 3-methyl-2-oxovalerate to weight and predelivery BMI (Fig. 4AandB).
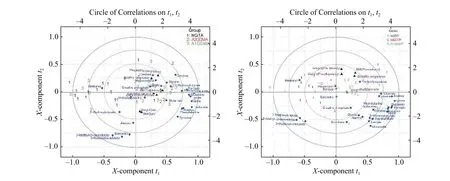
Fig. 4 Relationship between endogenous metabolites and demographic parameters. Correlation between endogenous metabolites and demographic parameters. A: Correlation circle (biplot, three components, R2=0.73, Q2=0.54) of the three group patients (antepartum NGT[n=35], antepartum A1GDM [n=24], and antepartum A2GDM [n=11]). B: Correlation circle (biplot, three components, R2=0.73, Q2=0.54) of the three group patients (postpartum NGT [n=35], postpartum A1GDM [n=24], and postpartum A2GDM [n=11]). The X- and Y-axis of the figure denote the first and second components of the PLS model; and the concentric circles represent the explained variance, respectively.NGT: normal glucose-tolerant group; A1GDM: abnormal glucose-tolerant group without insulin therapy; A2GDM: abnormal glucosetolerant group with insulin therapy; PLS: partial least squares.
Correlation network between metabolites and demographic parameters
Several important demographic parameters were filtered out, including risk factors such as BMI and weight. BMI and pre-delivery weight were in the center of the network, showing a positive correlation with most metabolites at antepartum period (Fig. 5A).BCAAs (such as leucine) played a vital role and were correlated positively with weight and BMI at both predelivery and pre-pregnancy periods. A negative correlation was presented between 3-methyl-2-oxovalerate and pre-pregnancy BMI. BMI gain and weight gain had positive correlations with two metabolites, 3-hydroxyisobutyrate and succinate (Fig. 5A). Negative correlations were exhibited from succinate to BMI and weight gain, and from 3-hydroxyisobutyrate to weight and pre-delivery BMI at postpartum period (Fig. 5B).Acetate, lysine, and formate show positive correlations with weight and BMI at both pre-delivery and pre-pregnancy periods (Fig. 5B).
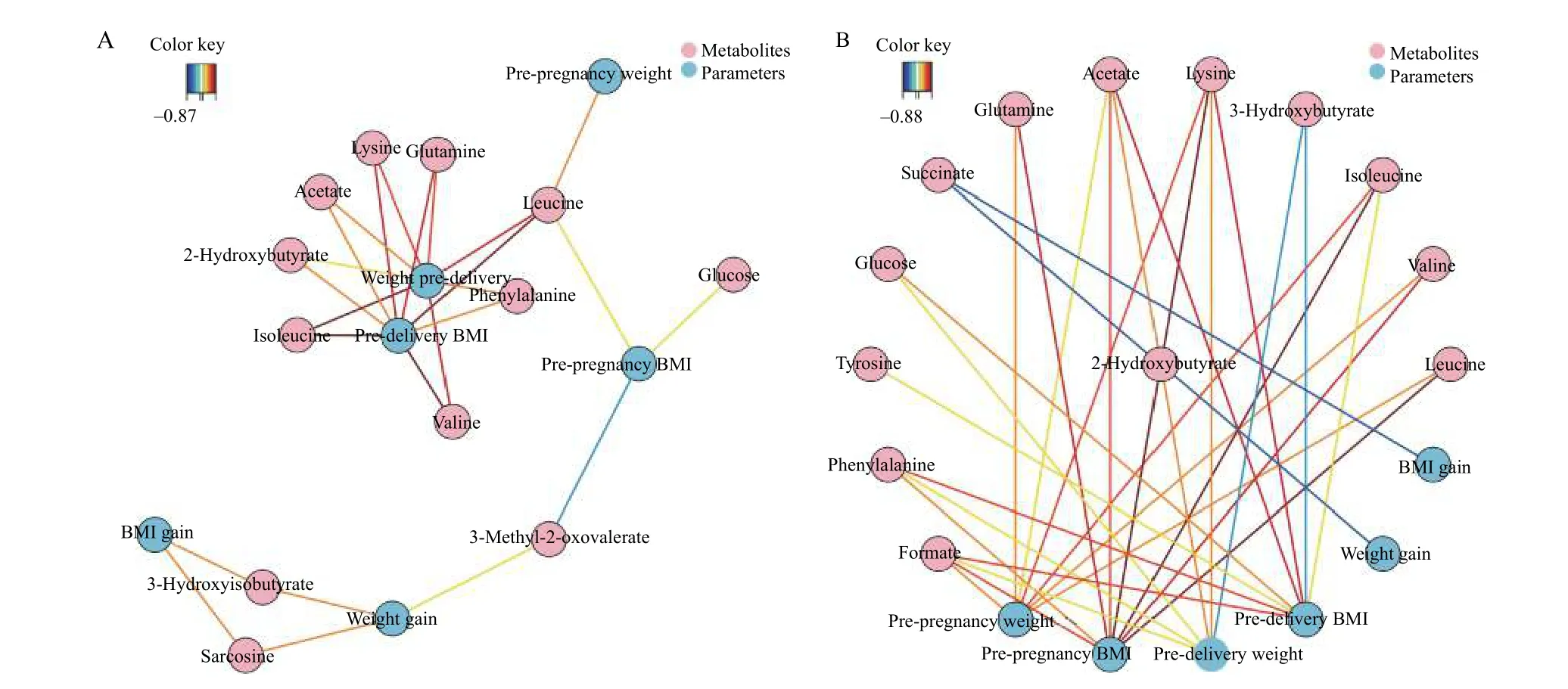
Fig. 5 Correlation network between metabolites and demographic parameters. The network is represented with metabolites and other parameters as nodes (framed in circle). The colored lines indicate correlations (red for positive and blue for negative correlations, color coded according to the correlation coefficients), and the dotted lines represent the compounds of a similar structure. Only correlations with absolute values of correlation coefficients above the threshold (0.65) were shown. A: Correlation network of metabolites with BMI and weight in antepartum period at the threshold of 0.65. B: Correlation network of metabolites with BMI and weight in postpartum period at the threshold of 0.65.
Discussion
In this study, we divided pregnant women with GDM into A1GDM and A2GDM groups according to the severity of the disease, then investigated the baseline clinical data and the changes in plasma metabolites by comparing with NGT group, at both antepartum and postpartum periods, using NMR and multivariate statistical analysis. The relationship between demographic parameters and the potential metabolite biomarkers was further explored.Significant differences in the blood glucose levels(fasting, 1-hour OGTT, and 2-hour OGTT) of pregnant women were found among the three groups,and the blood glucose levels of the A2GDM group were the highest. A2GDM, which needs insulin therapy, is usually considered more serious than A1GDM.
Interestingly, the height of pregnant women was gradually decreased as GDM became more severe: the height of women in A2GDM was significantly lower than those in NGT[18]. With the decrease of the mean gestational age at the time of delivery, the severity of maternal diabetes increased. The gestational age at delivery in A2GDM women was significantly smaller than normal pregnant women because they generally needed to terminate their pregnancy earlier due to drug therapy or other clinical instructions[19], leading to preterm delivery and birth of small for gestational age infant. However, neonatal weight and neonatal weight ratio showed a gradually rising trend as the disease severity increases, but without statistical significance, suggesting the overgrowth of the fetuses in A2GDM women[8,18].
A1GDM and A2GDM groups at both antepartum and postpartum periods shared similar metabolic changes as compared with the NGT group. Most of these metabolites were related to amino acid metabolism, energy metabolism, and oxidative stress.
Amino acids metabolism
BCAAs, consisting of leucine, isoleucine, and valine, are the most frequently studied metabolites in GDM[15]. BCAAs are essential amino acids that cannot be produced by the body, and must be primarily obtained from the diet. In our study, we found a steady elevation of BCAAs as the disease became more severe: their levels in A2GDM were significantly higher than those in NGT, which is inconsistent with the results reported in several literatures[20–21]. Higher concentrations of BCAAs are associated with insulin resistance in the transition from GDM to type 2 diabetes[21]and increased risk of incident type 2 diabetes[22]. Plasma BCAAs are useful markers to detect early type 2 diabetes and distinguish type 2 diabetes subtypes[23–24].
Recent studies found that elevated circulating BCAAs might lead to excess trans-endothelial fatty acid transport into skeletal muscle, promote lipid accumulation and blunt insulin signalingviasecreting 3-hydroxyisobutyrate (catabolic intermediate of the BCAA valine)[25], which was associated with insulin resistance[26]. As a metabolic product of isoleucine, 3-methyl-2-oxovalerate was closely associated with impaired fasting glucose tolerance. In our study, as compared with the NGT group, the two metabolic products of BCAAs were markedly decreased in both the A1GDM and A2GDM groups, and the decrease was even more significant at postpartum period,suggesting impaired catabolism of BCAAs.
In addition to BCAAs, elevated levels of plasma aromatic amino acids (AAAs) were also associated with insulin resistance, impaired glucose tolerance,and type 2 diabetes[24]. Many studies showed elevated plasma levels of AAAs before the manifestation of type 2 diabetes[24]. Compared with the NGT group, the levels of AAAs tyrosine and phenylalanine were significantly increased in both A1GDM and A2GDM groups before and after delivery[20].
Glutamine and glutamine metabolism are known to be perturbed in diabetes[27].Glutamine levels are reduced in case of insulin resistance[28]. As compared with the NGT group, glutamine was increased in both the A1GDM and A2GDM groups, with more significant for A2GDM. Several studies have reported the increase of glutamine in GDM patients[29], which might be attributed to blood sugar control in GDM patients as both diet control and insulin treatment could inhibit gluconeogenesis: the latter could exert a greater impact. Increased gluconeogenesis rate is a key and very early pathological feature of diabetes and other states involving low insulin concentration.Excessive gluconeogenesis is also a contributing factor to hyperglycemia in diabetes because of impaired downregulation in response to insulin. As a gluconeogenic amino acid, glutamine is increased due to gluconeogenesis inhibition and thus decreases the consumption of glutamine. Glutamate can be synthesized from glutamine metabolized by the mitochondrial enzyme. The significant increase of glutamine in A2GDM could provide ample substrate for the synthesis of glutamate, leading to its increase in the A2GDM group.
Energy metabolism
In this study, the level of succinic acid, an important intermediate product of the Krebs cycle,was dramatically increased. It has been speculated that the utilization of succinic acid (i.e., the disturbance of Krebs cycle) is blocked, resulting in insufficient energy supply[30]. The cell needs to be replenished in other ways in order to gain energy. When energy cannot meet the body's needs, energy metabolism turns to anaerobic glycolysis as an energy remedy.
However, when anaerobic glycolysis is not sufficient to meet the body's energy need, it is necessary to activate other energy metabolic pathways,including the ketone bodies and the creatine/phosphocreatine (Cr/PCr) system[31]. Cr/PCr system,which produces ATP through the creatine kinase(CK), playing an important role in maintaining the ATP level. Cell high metabolic state and energy crisis can lead to changes in the level of Cr/PCr. The higher level of Cr/PCr in the A1GDM group and A2GDM group in our study showed that people with diabetes were in a high energy expenditure state.
As a kind of organic acid produced from 2-ketone butyric acid, 2-hydroxybutyrate is also produced when thioether is converted into cysteine for the biosynthesis of glutathione. Hence, it is speculated that 2-hydroxybutyrate is involved in insulin resistance and impair glucose homeostasis[32].Studies[33]have found that in late pregnancy, plasma 2-hydroxybutyrate concentration in GDM patients is higher than that in the control group. Plasma 2-hydroxybutyrate concentrations in the three groups were steadily increased, as shown in the heat map(Fig. 2B), indicating a progressive metabolic disorder of the disease. The increase of plasma 2-hydroxybutyrate could be detected 9.5 years before the diagnosis of hyperglycemia, demonstrating that 2-hydroxybutyrate could be an independent index of early prediction of human glucose abnormality.
We observed a significant decrease of 3-hydroxybutyric acid in both the A2GDM and A1GDM groups,suggesting that energy metabolism in the Krebs cycle was disturbed in GDM patients. In the absence of energy, 3-hydroxybutyric acid was used as the energy source for metabolism, leading to a decreased level of 3-hydroxybutyric acid[34].
Correlation network analysis
The relationships between metabolite changes and weight, and pre-pregnancy and pre-delivery BMIs were further investigated. Pre-pregnancy weight and BMI were significantly correlated with multiple metabolites, at both antepartum (Fig. 5A) and postpartum (Fig. 5B) periods. Strong positive correlations between plasma BCAAs concentrations and the levels of weight and BMI pre-delivery were observed. However, there was no strong correlation between prenatal weight and BMI gain, and metabolites. The possible reasons were that once pregnant women were diagnosed with GDM, they would receive intervention (such as diet and exercise)to control their weight.
Limitation
As compared with antepartum period, there were no significant metabolic differences among the three groups at postpartum period. One possible reason is that placental hormones related to insulin resistance usually fall back to normal 2 to 3 weeks after delivery,so its effect on metabolism remains till the second day after delivery, which is one limitation of our study.Another limitation is that the sample size is not large enough. Further studies should be done on a larger scale to increase the statistical power.
Conclusion
In this study,1H-NMR spectrometry was used to study the metabolic differences in GDM of different severity. The results showed the interference of amino acid metabolism and energy metabolism in women with GDM. Metabolic profiles could reflect the severity of GDM. Differences in metabolites in GDM patients persisted in a short time after delivery. The body weight and BMI of pregnant women were positively correlated with BCAAs and other metabolites: pregnant women of fatty-body type may be more prone to GDM. This study provides a new way for understanding of the pathogenesis of GDM,which is helpful for the prediction of GDM severity,as well as the management and treatment of GDM.
Acknowledgments
We are grateful to Prof. Junsong Wang for1HNMR technical assistance.
杂志排行
THE JOURNAL OF BIOMEDICAL RESEARCH的其它文章
- Regulatory role of sorting nexin 5 in protein stability and vesicular targeting of vesicular acetylcholine transporter to synaptic vesicle-like vesicles in PC12 cells
- A nomogram for predicting lymph node metastasis in superficial esophageal squamous cell carcinoma
- PUM1 represses CDKN1B translation and contributes to prostate cancer progression
- Dynamic regulation of anti-oxidation following donation repairing after circulatory determined death renal transplantation with prolonged non-heart-beating time
- Pathogenesis and drug response of iPSC-derived cardiomyocytes from two Brugada syndrome patients with different Nav1.5-subunit mutations
- Multiple pathological components in gliosarcoma
