A nomogram for predicting lymph node metastasis in superficial esophageal squamous cell carcinoma
2021-10-13WeifengZhangHanChenGuoxinZhangGuangfuJin
Weifeng Zhang, Han Chen, Guoxin Zhang, Guangfu Jin
1Department of Gastroenterology, the First Affiliated Hospital of Nanjing Medical University, Nanjing, Jiangsu 210000, China;
2The First Clinical Medical College of Nanjing Medical University, Nanjing, Jiangsu 210000, China;
3Department of Epidemiology, Center for Global Health, School of Public Health, Nanjing Medical University,Nanjing, Jiangsu 211166, China.
Abstract Superficial esophageal squamous cell carcinoma (SESCC) is defined as carcinoma with mucosal or submucosal invasion, regardless of regional lymph node metastasis (LNM). The lymph node status is not only a key factor to determine the training strategy, but also the most important prognostic factor in esophageal cancer. In this study,we establish a clinical nomogram for predicting LNM in patients with SESCC. A predictive model was established based on the training cohort composed of 711 patients who underwent esophagectomy for SESCC from December 2009 to June 2018. A prospective cohort of 203 patients from June 2018 to January 2019 was used for validation. Favorable calibration and well-fitted decision curve analysis were conducted and good discrimination was observed (concordance index [C-index], 0.860; 95% confidence interval [CI], 0.825–0.894)through internal validation. The external validation cohort presented good discrimination (C-index, 0.916; 95%CI, 0.860–0.971). This model may facilitate the prediction of LNM in patients with SESCCs.
Keywords: superficial esophageal cancer, squamous cell carcinoma, lymph node metastasis, nomogram,prediction model
Introduction
Superficial esophageal squamous cell carcinoma(SESCC) is defined as carcinoma with mucosal or submucosal invasion, regardless of regional lymph node (LN) metastasis[1]. The conventional curative treatment for SESCC is esophagectomy, although it is associated with considerable postoperative mortality[2].Recently, endoscopic submucosal dissection (ESD),firstly reported by Japanese scholars to treat early gastrointestinal cancers[3], has become an alternative of esophagectomy for most non-metastatic SESCC cases of T1a stage. Several recent studies showed that the 3- and 5-year overall survival rates exceeded 90%in patients undergoing endoscopic resection with T1a-EP (epithelium) to T1b-SM1 (submucosal) cancer[4–5].Thus, the indications of ESD for esophageal cancer have been gradually broadened considering the effectiveness and safety of endoscopic therapy[6–7].According to the National Comprehensive Cancer Network (NCCN) guideline (Version 1, 2019),endoscopic resection is only applicable to T1a stage cancer while esophagectomy is indicated if the tumor infiltrates into the submucosal layer (T1b) or deeper[8].But guidelines of the Japanese Esophageal Society(JES) and the Chinese Society of Clinical Oncology(CSCO) suggest if invasion depth is confined to the upper submucosal layer (T1b, SM1), ESD is still considered as the main strategy[9–10]. Currently, most Chinese patients with a fairly good general condition prefer minimally invasive endoscopic resection to surgical treatment, and therefore, ESD enjoys wider popularity in China[5]. However, ESD is deemed applicable only in patients without LN metastasis(LNM) because endoscopic treatment could not replace lymphadenectomy[11]. The LN status is not only a key factor to determine the training strategy,but also the most important prognostic factor in esophageal cancer[12]. Therefore, it is necessary for clinicians to establish a model for predicting the risk of LNM in SESCC patients so as to decide the therapeutic indication of endoscopic treatment.
Although such models have been reported in recent years, the methodology applied was usually suboptimal. Most previously established models were nonparametric[13–14]and/or insufficiently validated[13–17].And some were established with a less-than-300 sample size[14–17]. The aim of this study is to establish an updated and validated nomogram for predicting LNM in patients with SESCC, which can not only help evaluate cancer prognosis but also provide guidance for intervention selection.
Subjects and methods
Patients and study design
A retrospective cohort was established in patients receiving surgical resection and lymphadenectomy for SESCC between December 2009 and June 2018 in the First Affiliated Hospital of Nanjing Medical University (Nanjing, China). Inclusion criteria were:(1) histopathological diagnosis of esophageal squamous carcinoma on surgical specimens; (2) pT1 stage carcinoma (no tumor invasion beyond the submucosa); (3) surgical resection and at least twofield lymphadenectomy. Exclusion criteria were:(1) history of previous malignancies and anticancer therapies; (2) histopathological diagnosis of esophageal adenocarcinoma or other types of esophageal cancer; (3) mixed type of esophageal cancer; (4) tumor with undefined pathological origin and metastatic esophageal cancer; (5) esophagectomy after endoscopic resection; (6) aged <18 years;(7) perioperative mortality; and (8) no contrastenhanced computed tomography (CT) scans, endoscopic ultrasonography (EUS) or fluorodeoxyglucose positron emission tomography (FDG-PET) before surgery. Following the same inclusion and exclusion criteria, an independent validation cohort of 203 patients was prospectively studied from June 2018 to January 2019. The study was approved by the Institutional Ethics Committee of the First Affiliated Hospital of Nanjing Medical University (the register number, 2018-SR-152). Informed consent of surgical risk and anesthesia services was obtained before surgery.
Data collection
All participants undergoing endoscopic biopsy were assessed histopathologically to define esophageal cancer or high-grade intraepithelial neoplasia before surgery. And all must have at least one of the following radiographic results for preoperative LN status evaluation: contrast-enhanced CT scans, EUS or FDG-PET. Two experienced radiologists assessed the results of CT and FDG-PET scans, and two sonographers reviewed EUS results. Interpretations were given independently and disagreements were resolved by consensus. Positive radiographic results were defined as CT-, EUS-, or PET-reported positive LNM.
General clinical features, including age and sex,were collected. All the participants were divided into two age groups: 18 to 59 years and ≥60 years. The pathologic diagnosis of esophageal squamous cell carcinoma was performed by two experienced pathologists who assessed the specimens independently. In case of discordant outcome, the specimen was evaluated by a third independent expert pathologist.The diagnosis was determined only if at least two pathologists agreed.
Pathologic features, including tumor size, invasion depth, microscopic type, histologic grade, lymphvessel invasion (LVI), and LN status (LNM, location of LNM, the total number of resected LN, and the number of metastatic LNs during surgery), were collected. The tumor size (the maximum diameter)was measured in two dimensions by Vernier's calipers. Tumor location is defined by the position of epicenter of tumor. If the statement of epicenter was unavailable, the following measurements were applied: (1) upper: 15 to 24 cm from incisors;(2) middle: 25 to 29 cm from incisors; (3) low: 30 to 40 cm from incisors. Histologic grade (G) was categorized as well-differentiated (G1), moderately differentiated (G2), and poorly differentiated (G3)[18].The invasion depth was classified into four categories:epithelium (EP)/lamina propria mucosa (LPM),muscularis mucosa (MM), submucosal (SM)1, and SM2 or deeper. Macroscopic tumor type was classified by using the 2016 Japanese Classification of Esophageal Cancer (11thedition): superficial type,protruding type, ulcerative and localized type,ulcerative and infiltrative type, diffusely infiltrative type, and unclassified type[19].
Statistical analysis
Significant predictors for establishing nomogram were identified by IBM SPSS Statistics, Version 20.0(SPSS, USA). The univariate logistic regression and chi-square analyses were applied to identify the significance of each variable in the training cohort.Variables significantly associated with LNM (P≤0.05)were identified as candidate predictors for multivariate logistic regression analysis. The optimal cutoff value was assessed by Youden index in the receiver operating characteristic (ROC) curve. The area under receiver operating characteristic curves (AUC) was calculated to evaluate the diagnostic accuracy and compared with DeLong's test. The net reclassification improvement (NRI) and the integrated discrimination improvement (IDI) were further applied to quantify the refinement in predictive accuracy[20].
Multivariate analysis was performed by using the"glmnet" package in R software (version 3.5.1,http://www.R-project.org/). The results of multivariate analysis were integrated into R software to formulate a nomogram by using the "rms" package. The validation of nomogram was measured by concordance index (Cindex)[21–23], calibration curve, and decision curve analysis (DCA)[24–26]. NRI and IDI were calculated by using the "PredictABEL" package. The statistical significance level was two-sided and statistical significance was considered asP<0.05.
The establishment of the model consisted of five steps: (1) analyzing logistic regression for candidate predictor selection; (2) developing a parametric model(nomogram); (3) evaluating model performance by calibration plot, discrimination, and decision-curve analysis; (4) establishing a prospective cohort for external validation; (5) comparing different models by multiple statistical methods.
Results
Baseline characteristics
Fig. 1shows the flowchart of generating the training and validation cohorts. A total of 8984 LNs were resected from 711 patients (510 [71.7%] males;median age, 61.2 years; range, 36 to 83) by surgery.The number of patients receiving CT, FDG-PET, and EUS is 711 (100%), 78 (11.0%), and 63 (8.9%),respectively. The baseline characteristics of both training and validation cohorts are shown inSupplementary Table 1(available online). The overall incidence of LNM was 13.8% (112/711) in the training cohort and 17.2% (35/203) in the validation cohort, respectively. Of the 112 patients with metastatic LN in the training cohort, 1546 LNs were resected (average, 16.8; range 8 to 51) and 186 LNs were found positive for metastasis. The average tumor size was (1.50±1.2) cm. A total of 265 (37.3%)patients were defined as T1a stage and 446 (62.7%)patients were T1b stage. In T1a, the number of patients with EP/LPM and MM invasion was 110 and 155, respectively. In T1b, the number of patients with SM1 and SM2 or deeper invasion was 352 and 94,respectively.
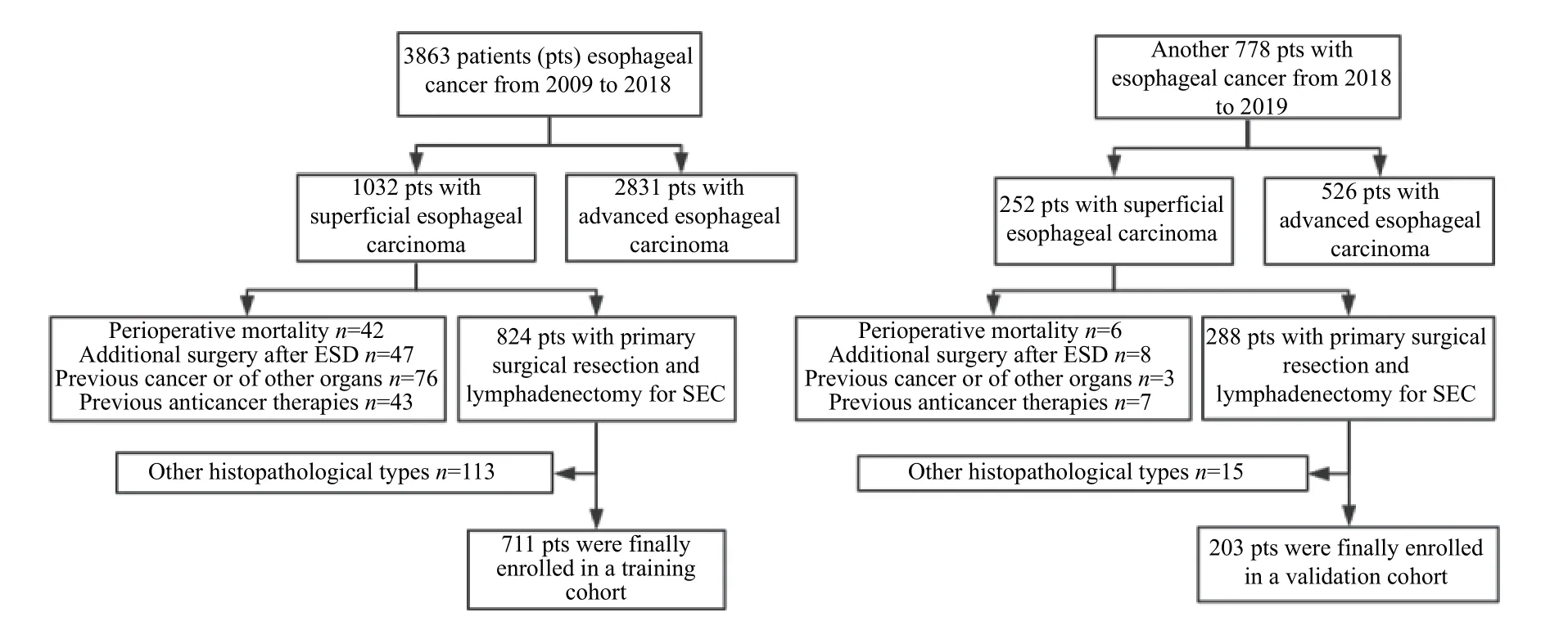
Fig. 1 Flowchart of generating training and validation cohorts. ESD: endoscopic submucosal dissection; SEC: superficial esophageal cancer.
The LNM rate of T1a and T1b tumors were 2.3%(6/265) and 23.8% (106/446), respectively. Positive radiographic results were as follows: 245 were CT positive (including 4 CT+PET/CT+EUS positive, and 9 CT+PET/CT positive), and 3 were PET/CT positive.
The sensitivity and specificity of positive radiographic results (CT-, FDG-PET- or EUSreported positive LNM) were 59.0% and 59.1%,respectively. The incidences of the upper, middle, and lower thoracic esophagus cancer were 9.8%, 40.1%,and 50.1%, respectively.Supplementary Table 2(available online) summarizes the LN status of the resected LNs according to regions and N Stage.
Candidate predictors of lymph node metastasis
Table 1summarizes the characteristics of patients according to lymph node status in both training and validation cohorts. In the training group, patients' age,sex, tumor location, and macroscopic type were not significantly associated with LNM. The optimal applicable cut-off value of tumor size was defined as 1.55 cm by calculating Youden's index. The area under the ROC curve was 0.70 (95% confidence interval [CI], 0.651–0.747) by using univariate logistic regression.
The multivariate logistic regression were applied and confirmed that tumor size ≥1.55 cm (odds ratio[OR], 3.75; 95% CI, 2.265–6.2;P<0.001), submucosal invasion (OR, 9.03; 95% CI, 3.78-21.551;P<0.001),poorly-differentiated histologic type (OR 2.81; 95%CI, 1.731–4.5556;P<0.001), positive lymph-vessel invasion (OR, 11.64; 95% CI, 5.271–25.72;P<0.001),and positive radiographic results (OR, 2.3; 95% CI,1.412–3.735;P=0.001) were significantly associated with LNM in SESCCs (Supplementary Fig. 1andSupplementary Table 3, available online).
Next, estimated through stratification of significant risk factors, the probability of LNM with one, two,three, and more than three risk factors were 5.1%,12.1%, 37.9%, and 78.7%, respectively(Supplementary Table 4, available online).
Development and validation of nomogram
A nomogram was established with five significant candidate predictors: tumor size ≥1.55 cm,submucosal invasion, poorly-differentiated histologic type, positive lymph-vessel invasion, and positive radiographic results(Fig. 2).
Three key measurements were performed to evaluate model performance: calibration plot,discrimination, and decision-curve analysis. A new cohort consisting of 203 patients was formed for external validation. The calibration curve of nomogram presented good agreement in predicting probability and actual LNM rate in the training cohort,with a non-significant Hosmer-Lemeshow test statistic(P=0.476).Fig. 3shows the results of calibration curve in predicting model. The calibration curves of nomogram were highly consistent with actual observation for LN status. ROC curves were further constructed and the area under curve exceeded 0.8 in all established cohorts (Fig. 4). Internal validation presented good discrimination with a C-index of 0.855(95% CI, 0.820–0.890; sensitivity, 83.9%; specificity,71.3%). In external validation, good calibration and discrimination were also achieved with a C-index of 0.820 (95% CI, 0.782–0.859; sensitivity, 82.9%;specificity, 94.6%). Cross-validation was conducted in the training cohort, which was randomly divided into two testing groups: 494 (70%) and 217 (30%). The AUC was 0.923 (95% CI, 0.846–0.998) in testing group 1 (70%) and 0.872 (95% CI, 0.835–0.909) in testing group 2 (30%). The predicted accuracy was 87.2% in testing group 1 and 84.3% in testing group 2.
Subtracting predictive candidates for nomogram comparison
To simplify the model and further validate model accuracy, another model was set up by subtracting predictive candidates: a simplified model integrating invasion depth, tumor size, and LVI. The calibration curves for the probability of LNM in the training and validation cohorts demonstrated the agreement between prediction and observation in the simplified model (Fig. 4AandB). The simplified model presented discrimination with a C-index of 0.916(95% CI, 0.858–0.973; sensitivity, 78.6%; specificity,75.0%) in training cohort while a C-index of 0.827(95% CI, 0.748–0.906; sensitivity, 48.6%; specificity,98.2%) in validation cohort (Fig. 4).
According to DeLong's test, the training model was superior to the simplified model (Z=3.654,P<0.001)in the training cohort. To further quantify the refinement in predictive accuracy, NRI and IDI were applied. The results showed that the training model was significantly improved in prediction performance when compared with the simplified one (NRI, 19.3%,P<0.001; IDI, 6.75%,P<0.001).Table 2summarizes the results of the two models.

Table 1 Characteristics of patients according to lymph node status in the training and validation cohorts

Table 2 Risk factors for lymph node metastasis in SESCCs
Clinical use of the nomogram system
Well-fitted DCA was achieved in the training groupand better performance was found than that in another model (Fig.5). The results indicated that if the threshold probability of LNM ranged from 10% to 80%, the application of the nomogram could yield more benefit than either the treat-all-patients or the treat-none strategies. Net benefit between the training model overlapped at several points while net benefit of training model was superior to the simplified model almost within the whole range.

Fig. 2 Development of nomogram to predict lymph node metastasis in superficial esophageal squamous cell carcinoma. The nomogram was generated from the training cohort incorporating tumor size, invasion depth, histologic grade, lymph-vessel invasion, and preoperative radiographic results. LVI: lymph-vessel invasion; LNM: lymph node metastasis.
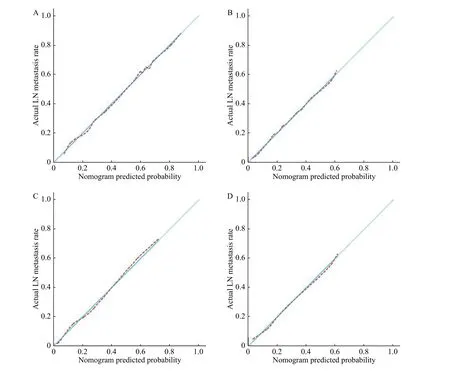
Fig. 3 Calibration curves of the training and simplified model. A and B: Calibration curves of training model in the training cohort (A)and validation cohort (B). C and D: Calibration curves of the simplified model in the training cohort (C) and validation cohort (D). The xaxis represents the predicted LN metastasis risk. The red dotted lines represent the performance of the prediction models. The 45-degree light blue solid lines represent a perfect prediction. The closer the red dotted line fits the light blue solid line, the better accuracy of the model shows. LN: lymph node.
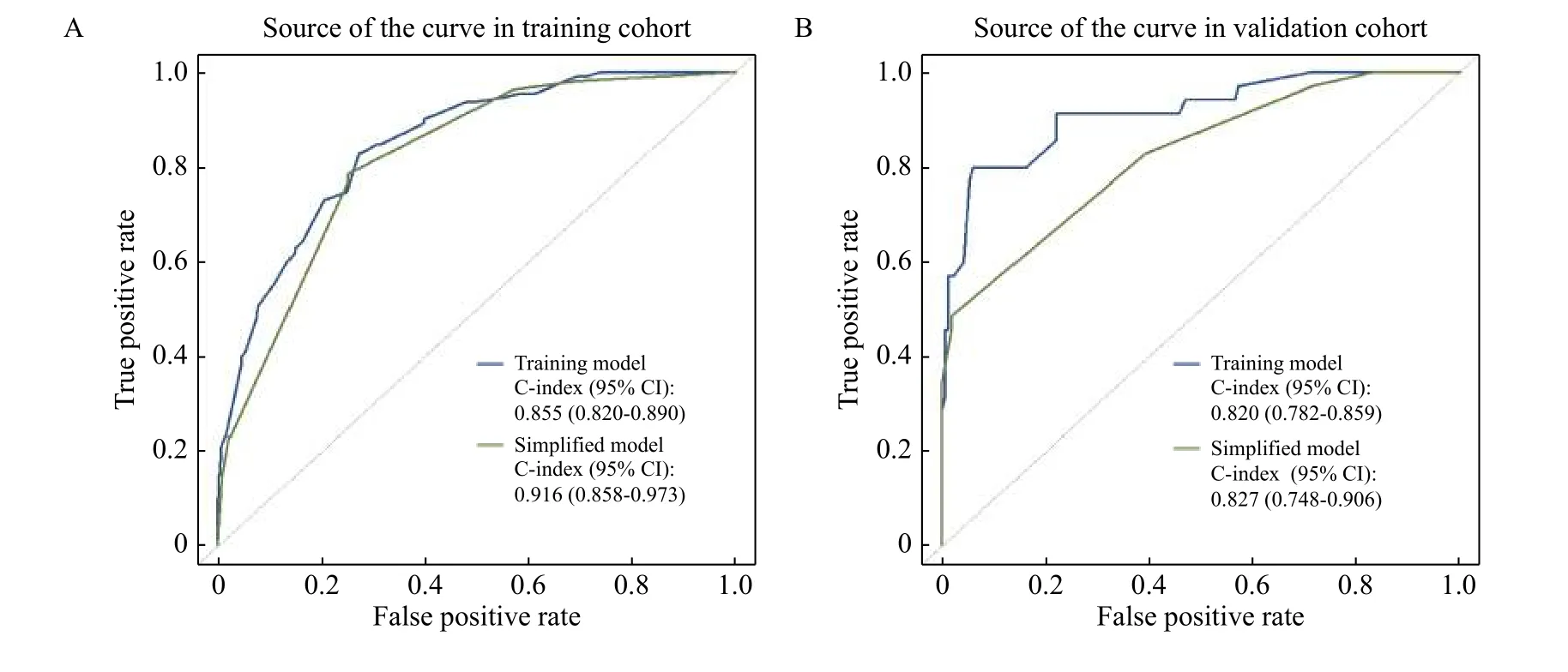
Fig. 4 ROC curves of the training and simplified models. A and B: ROC curves of the models in the training cohort (A) and the validation cohort (B). The blue curve represents the development data of the training model. The green curve represents the development data of the simplified model. ROC: receiver operating characteristic; C-index: concordance index.
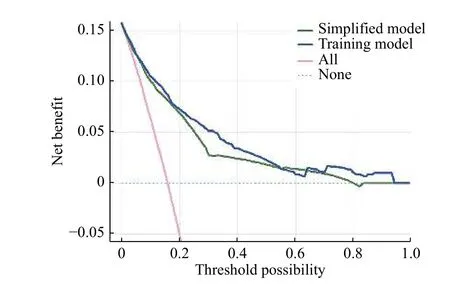
Fig. 5 Decision curve analysis: curve of the established models in training cohort. The y-axis represents the net benefit. The blue and green lines represent the performance of the training model and simplified model, respectively. The pink dotted line represents the hypothesis that all patients have positive lymph node metastasis(LNM) and the dotted light blue line represents the hypothesis that all patients are negative for LNM. The threshold probability stands for where the expected benefit of treatment is equal to the expected benefit of avoiding treatment. If a patient's possibility of LNM involvement is over the threshold probability, then a treatment strategy for LNM should be chosen.
Discussion
In the current study, a nomogram was developed and validated as an easy-to-use tool for clinicians to predict the possibility of LNM in SESCC patients.Our study showed the rates of LNM were 13.8% in the training cohort and 17.2% in the validation cohort,which was consistent with previously published data[27–28]. The sample size in the training cohort was twice larger than the previously published models,which ensured a higher precision of the present model[29–30].
T1 substage, LVI, tumor differentiation, and tumor size have been indicated as predictors of LNM in several studies[31], which were also verified in our study. The presence of LNM was observed in 28 of 43(65.1%) and 17 of 20 (85%) of superficial esophageal cancer with vascular invasion in the training and validation cohorts, respectively, which was significantly more frequent than in cancers without lymph-vessel invasion in these two cohorts (12.6%and 9.8%, respectively). Some other studies also suggested that patients with lymph-vessel invasion were more likely to present LNM[32]. Meanwhile,tumor lymph-vessel invasion was considered as an independent risk factor for LNM of superficial esophageal cancer (OR=4.06).
The pathological type of well-differentiated tumors is commonly considered to be close to mature tissues with slow growth and low risk of LNM, while the poorly differentiated tumors are featured by rapid growth and high risk of LNM. Thus tumor differentiation is also considered an independent risk factor for LNM of superficial esophageal cancer[12]. In our study, the rates of LNM in patients with well-,moderately-, and poorly-differentiated superficial esophageal cancer were 2.9% (2/70), 11.3% (50/441),and 30% (60/200) in training cohort, and 0% (0/29),11.7% (16/137), 51.4% (19/37) in validation cohort,respectively. In this study, only 2 cases with LNM were found in patients with well-differentiated tumors in the training cohort, and no LNM was found in the patients with well-differentiated tumors in validation cohort, which confirmed that tumor differentiation was an independent risk factor for lymph node metastasis (OR=3.2).
Submucosa is considered as the boundary layer of esophageal cancer cell metastasis[33]. The depth of tumor invasion was another independent risk factor associated with lymph node metastasis. Previous studies reported LNM rates in MM1, MM2, MM3,SM1, SM2 and SM3 were 0%, 1.5%–3.7%,5.3%–30.8%, 8.7%–42.1%, 12.7%–40.7% and 28.4%–66.7%, respectively[29,33–39]. Our results showed that the LNM rate of patients with superficial esophageal cancer confined to mucosa was much lower than that of tumors confined to submucosa (2.3% and 23.8%,respectively,P<0.001) in the training cohort, which was further confirmed in the validation cohort (4.0%and 21.6%, respectively,P<0.001). The current study also confirmed tumor infiltration depth as an independent risk factor for LNM (OR=8.71), which is in accordance with some other previous reports[15].
Wanget alfound that the probability of lymph node metastasis and the prognosis are closely associated with tumor size[40]. In this study, tumor diameter showed a significant association with LNM. The rates of LNM were 8.1% and 26.7% of tumors with diameter <1.55 cm and ≥1.55 cm in the training cohort, respectively, and similar results were obtained in the validation cohort (9.6% and 23.9%,respectively).
We also included the preoperative radiographic result as a predictor in the model. These modalities, as the principal non-invasive examination to determine clinical nodal status by regional lymph node imaging before interventions, have similar performance in clinical nodal status assessment[41]. Therefore, we defined preoperative radiographic results as CT-,EUS- or FDG-PET-reported positive LNM. Although the sensitivity and specificity were only 59.0% and 59.1%, this radiographic predictor was still statistically significant (P<0.001) as an independent risk factor for LN status. Thus, we included this factor in the final model.
The previous studies usually classify tumors into two categories: low-grade (well-differentiated)tumors, and high-grade (moderately- and poorlydifferentiated) ones[16–17]. We unexpectedly detected that the moderately-differentiated tumors were not statistically significant in multivariate logistic regression. Hence, we adopted a binary-tiered grading system: well-moderately differentiated (low grade)and poorly differentiated (high grade). This grading method improves precision of prognostic significance,especially for the poorly- and moderatelydifferentiated tumors.
To simplify the model, another model was set by subtracting the predictive candidates. Although calibration plot of the training model was not superior to the simplified model, the improved C-index and NRI/IDI analysis of the training model demonstrated a better performance in discrimination. In the external validation, adequate discrimination was achieved by using the comprehensive model (C-index, 0.860),which was then improved in the validation cohort (Cindex, 0.920). Considering the comparable baseline characteristics between the two cohorts, the enhanced discrimination implied good performance of this model by external validation results. However, after validation, we paradoxically detected that when compared with the simplified model, the training model in both training and validation cohorts presented a relatively poor calibration (potential of over-estimating the risk) but good discrimination.Since calibration and discrimination were both significant references in model evaluation, it was difficult to tell which one is more important. It is reported that calibration is more crucial in prognostic settings while discrimination is more meaningful in diagnosis[42]. Hence, we gave preference to good discrimination because patients with true LNM would always have higher predicted risks than those without.Although the relatively poor calibration plot might overestimate the risk, it could still distinguish the patients with LNM from those without, which could result in a stricter and more indicative ESD procedure as such risks would not be under-estimated. Thus, the training model is more clinically useful than the other two. Next, a decision curve has been applied to evaluate the clinical applicability of the nomogram system. The results indicated the net benefit of the training model is almost superior to that of the simplified model within the whole range of 10%–80%(the threshold probability).
The limitations of our study include: 1) this is a single-center retrospective study, and multi-center study with external validation is expected in the future; 2) a selection bias may exist; 3) the molecularbiological or immunohistochemical characteristics are not included in our model; 4) there are differences in some predictive indicators between validation and training cohort, necessitating a larger sample size to further validate the efficacy of this model.
In conclusion, we have developed a comprehensive nomogram integrating both clinically and statistically significant factors. This model may facilitate the prediction of LNM in patients with SESCC.
Acknowledgments
This work was supported by the National Natural Science Foundation of China for studies on earlystage GI neoplasms (Grant No. 81072032 and No.81470830).
杂志排行
THE JOURNAL OF BIOMEDICAL RESEARCH的其它文章
- Differentiation of gestational diabetes mellitus by nuclear magnetic resonance-based metabolic plasma analysis
- Multiple pathological components in gliosarcoma
- Pathogenesis and drug response of iPSC-derived cardiomyocytes from two Brugada syndrome patients with different Nav1.5-subunit mutations
- Dynamic regulation of anti-oxidation following donation repairing after circulatory determined death renal transplantation with prolonged non-heart-beating time
- PUM1 represses CDKN1B translation and contributes to prostate cancer progression
- Regulatory role of sorting nexin 5 in protein stability and vesicular targeting of vesicular acetylcholine transporter to synaptic vesicle-like vesicles in PC12 cells
