Dynamic regulation of anti-oxidation following donation repairing after circulatory determined death renal transplantation with prolonged non-heart-beating time
2021-10-13XinningWangChangchengZhouJingyuLiuRuipengJia
Xinning Wang, Changcheng Zhou, Jingyu Liu, Ruipeng Jia,✉
1Department of Urology, Nanjing First Hospital, Nanjing Medical University, Nanjing, Jiangsu 210006, China;2Center for Renal Transplantation, Nanjing First Hospital, Nanjing Medical University, Nanjing, Jiangsu 210006,China.
Abstract Donation after circulatory-determined death (DCD) is an important part of renal transplantation. Therefore,DCD renal transplantation animal model should be established to study the mechanism of organ injury. Here, we established a stable DCD rat renal transplantation model and investigated the dynamic regulation of graft selfrepairing and antioxidant capacities with different non-heart-beating times (NHBTs). Male Sprague-Dawley rats were randomly divided into four groups with the NHBT of the donors from 0 to 15, 30, and 45 minutes.Recipients in long NHBT groups had a significantly lower survival rate and poorer graft function than those in short NHBT groups. Grafts from the 15-minute and 30-minute NHBT groups showed light and severe injury respectively at an early stage after transplantation and recovered within 7 days after transplantation, whereas the self-repairing of the grafts in the 45-minute NHBT group was delayed. The expressions of proliferating cell nuclear antigen (PCNA) and von Willebrand factor (vWF) were dependent on NHBT. The expression of antioxidant proteins paralleled graft recovery. In conclusion, the recipients can up-regulate antioxidant capacity to enhance graft self-repairing in DCD renal transplantation. Prolonged NHBT can delay the self-repairing and antioxidation of grafts.
Keywords: renal transplantation, rat model, donation after circulatory-determined death, ischemia-reperfusion injury, antioxidant
Introduction
The shortage of donated organs is a global issue and restricts renal transplantation[1–2]. Donation after circulatory-determined death (DCD) increases the supply of solid organs for transplantation and shortens the waiting list for renal transplantation[3]. However,DCD renal transplantation has a higher risk of complications, including delayed graft function (DGF),primary graft non-function (PNF), or acute rejection,which affects short- and long-term graft function and survival, and thus limits the application of DCD[4–5].The incidence of DGF is reported to be 50%,compared with 20% in donation after brain death[6].
The kidney is a hypertransfusion organ, making it very sensitive to ischemia. Non-heart-beating time(NHBT) can result in insufficient kidney blood flow,which will induce warm ischemia in the kidney and clinically present as DGF or PNF[7]. Hence, the warm ischemia during prolonged NHBT in DCD is responsible for the high discard rate of abdominal kidneys[8]. For example, the odds of discard with total donor warm ischemia time (WIT) >48 minutes were higher in the United States[9]. Moreover, severe warm ischemia could sensitize DCD grafts to cold storage and aggravate cold ischemia injury[10–11]. Ischemiainduced injury to a transplanted organ during procurement followed by reinitiation of blood flow through the organ after the transplant is referred to as ischemia-reperfusion injury (IRI)[12]. IRI intensifies oxidative stress by increasing the production of reactive oxygen species (ROS), which leads to parenchymal cell necrosis, apoptosis, inflammation,and other disorders[13]. The ROS would overload and overwhelm the ability of the recipient's body to scavenge them, resulting in an imbalance in the oxidation-reduction system[14]. Activation of the antioxidant system could protect against IRI[15]. Thus,dynamic regulation of the antioxidant system plays an important role in the self-repairing of grafts in the early post-transplantation period. A better understanding of the association between NHBT, selfrepairing, and antioxidant capacity is critical to the prevention of initial dysfunction in DCD renal transplantation. For this purpose, we established a stable experimental DCD renal transplantation model and analyzed the effect of prolonged NHBT on the dynamic regulation of the self-repairing and antioxidant capacity of grafts.
Materials and methods
Animals
The experimental protocol was approved by the Institutional Animal Care and Use Committee of Nanjing First Hospital, Nanjing Medical University.The investigation was performed in strict conformity with the Institutional and National Guidelines for Laboratory Animals. Male Sprague-Dawley (SD) rats with an initial weight of 250 to 300 g were used in this study and bred in the Experimental Animal Center Affiliated with Nanjing First Hospital. The animals are randomly divided into four groups according to the different NHBTs: 0-minute NHBT (group with NHBT of 0 minutes;n=15), 15-minute NHBT (group with NHBT of 15 minutes;n=15), 30-minute NHBT(group with NHBT of 30 minutes;n=15), and 45-minute NHBT (group with NHBT of 45 minutes;n=15). In each group, 5 animals were sacrificed on day 1, 3, and 7 post-transplantation for further assay.
Donation
The donor rats were anesthetizedviaisoflurane inhalation, followed by intraperitoneal administration of ketamine (100 mg/kg). All donors were administered intravenous heparinization (300 U/animal). After systemic heparinization in the donors, cardiac arrest was induced by opening the chest. After 3 to 4 minutes, cessation of the heartbeat was observed, and the cadavers were kept on a 37 °C pad for 0, 15, 30, or 45 minutes. Then the left kidneys were perfusedin vivowith UW solution (Viaspan,USA), harvested immediately, and placed in cold storage for 1 hour.
Orthotopic renal transplantation
The anesthetization of the recipient rats was similar to that of donors, and left nephrectomy was then performed. The recipient renal artery and renal vein were occluded with vascular clamps. Two end-to-end anastomoses were performed with 9/0 monofilament sutures. After removing the vascular clamps, the graft was reperfused. The ureter was anastomosed to the bladder. The grafts and serum were harvested for further analysis on day 1, 3, and 7 after transplantation, respectively.
Reperfusion damage index
The reperfusion damage index (RDI), developed by Lopez-Neblina[16], was used to quantitatively evaluate the grafts in the first 10 minutes after reperfusion in transplantation. This index was applied to evaluate the injury of DCD grafts in the first 10 minutes. Four parameters (graft color, timing of reperfusion, urinary response, and macroscopic findings such as edema or hemorrhagic fat) were measured numerically (Table 1),and the scores were statistically compared.
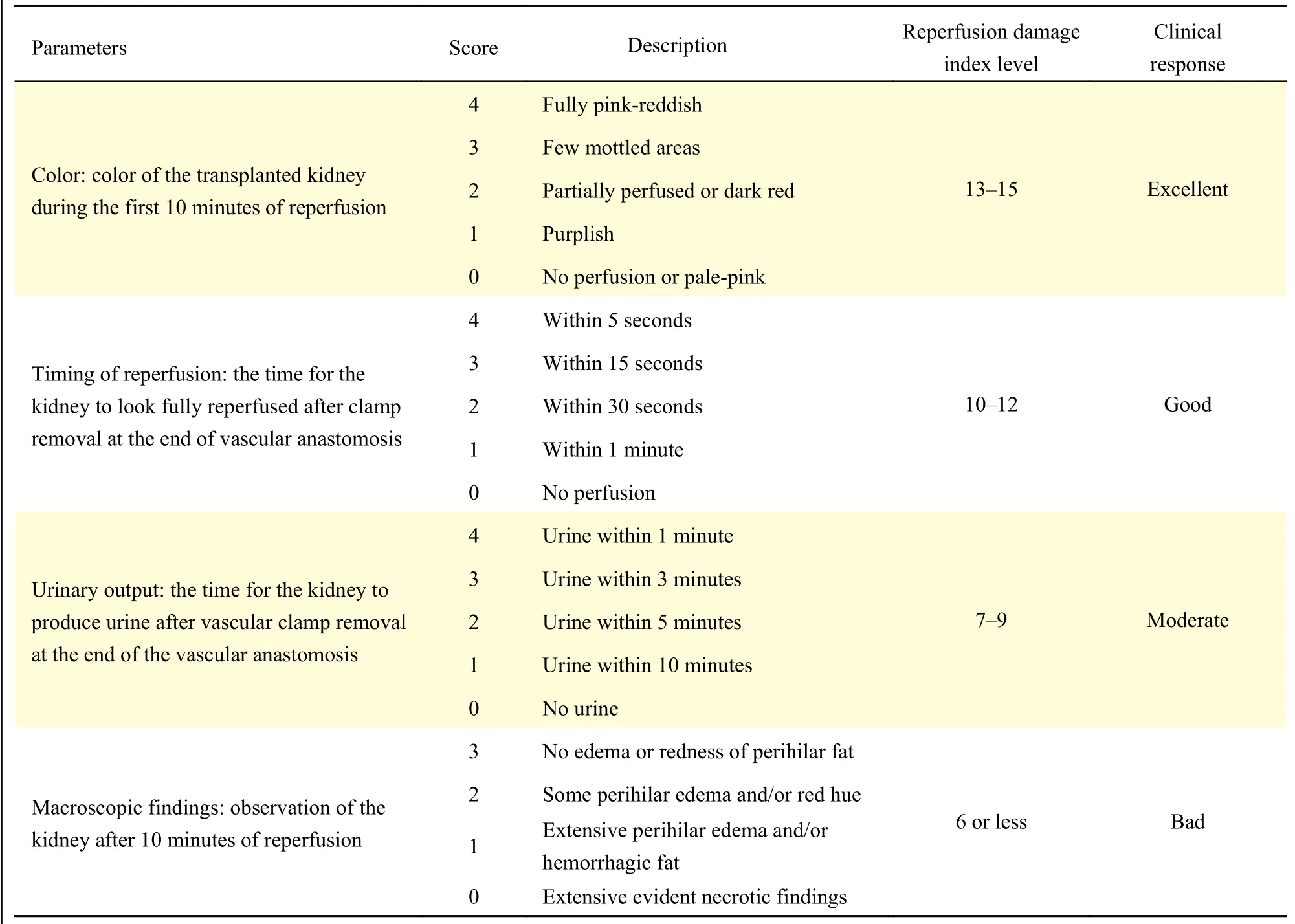
Table 1 Parameters associated with reperfusion damage index in kidney transplantation
Renal function assays
The blood samples were gained from all four groups of rats on day 1 after transplantation and centrifuged at 350 g for 10 minutes. The supernatants were subsequently collected to assess the blood serum creatinine (SCr) level by using clinically automated analysis methods (Hitachi 7600-10; Hitachi High-Technologies Corp, Japan)
Lipid peroxidation
Malondialdehyde (MDA), the product of reductionduring lipid peroxidation[17], can reflect the level of membrane lipid peroxidation. First, 0.1 g graft tissue was weighed to prepare a 10% tissue homogenate.MDA levels were measured in the supernatant of the graft homogenate using the thiobarbituric acid (TBA)method with a commercially available kit(Elabscience, China) according to the manufacturer's instruction, and the maximum absorption peak at 532 nm was measured using a spectrophotometer.
Western blotting
Total protein was extracted from the kidney specimens with RIPA lysis buffer containing a protease inhibitor cocktail (Roche, Switzerland).Equal amounts of proteins were separated by electrophoresis on 12% SDS-polyacrylamide gels and then transferred to polyvinylidene difluoride membranes (Millipore, USA). The membranes were blocked with 5% skim milk for 2 hours and incubated at 4 °C overnight with anti-superoxide dismutase 1(SOD-1) antibody (Bioworld, China), anti-heme oxygenase 1 (HO-1) antibody (Roche), or anti-nuclear factor erythroid-related factor 2 (Nrf2) antibody(Roche). The following day, membranes were extensively washed with tris-buffered saline with tween (KeyGEN Biotechnology, China) incubated with horseradish peroxidase-conjugated anti-rabbit secondary antibody (Biosharp, China) for 2 hours, and developed with an enhanced chemiluminescence system (ECL kit; KeyGEN Biotechnology, China).The images were then captured on the light-sensitive imaging film.
Microscopy and graft tubular damage evaluation
The graft samples were fixed with 10%formaldehyde, dehydrated, and embedded in paraffin and prepared into 5-μm sections. Tissue sections were stained with hematoxylin and eosin to evaluate the tubular damage. The microscopic assessment was performed in a blinded manner, and at least 20 random fields from each slide were evaluated. The acute tubular necrosis was scored on a 5-point scale (grade 0 to 5) as previously described[18]. The severity of tubular cell injury was semi-quantified by using the following scoring system: 0, normal kidney; 1, ≤10%;2, 11%–25%; 3, 26%–45%; 4, 46%–75%; and 5, ≥76%.
Immunohistochemical analysis
The proliferation of tubular cells was visualized by staining with anti-proliferating cell nuclear antigen(anti-PCNA) antibody (Roche). Peritubular capillaries were assessed by staining with anti-rat von Willebrand Factor (anti-vWF) antibody (Roche). Antioxidant capacity was assessed by staining with HO-1 antibody(Roche). Apoptosis was evaluated using the terminal transferase-mediated deoxyuridine triphosphate nickend-labeling (TUNEL) assay (Roche). TUNEL staining was performed according to the manufacturer's instructions (Roche). Eight randomly chosen fields from each slide were observed in a blinded manner by two experienced renal pathologists.
Statistical analysis
All data were expressed as the mean±SEM.Statistical comparisons of the data among the groups were evaluated by analysis of variance (ANOVA).When ANOVA showed a significant difference,further comparison between the groups was conducted by using the post hoc Tukey test. Statistical significance was set at a value ofP<0.05.
Results
Donation after circulatory-determined death induced poorer graft performance, renal function and recipient survival
,
The RDI could reflect the graft reperfusion performance[16]. We observed that the RDI could reflect the severity of warm ischemia injury. The mean RDI scores of the 0-minute, 15-minute, 30-minute, and 45-minute NHBT groups were 14.33,10.13, 7.60, and 7.27, respectively (0-minute NHBTvs.15-minute NHBT:P<0.001; 15-minute NHBTvs.30-minute NHBT:P<0.001; 30-minute NHBTvs.45-minute NHBT:P=0.870;Fig. 1A). The grafts in the 0-minute NHBT group showed better performance than other groups, whereas the RDI of DCD grafts with prolonged NHBT was lower than that of DCD grafts with a NHBT of 15 minutes. No urine production was observed in 15-minute NHBT, 30-minute NHBT and 45-minute NHBT groups during surgery.
We assessed the graft function by evaluating SCr.In the 0-minute NHBT group, the SCr on day 1 after transplantation was the lowest ([1.12±0.22] mg/dL)compared to that of the 15-minute ([1.97±0.27]mg/dL), 30-minute ([2.05±0.24] mg/dL), and 45-minute ([2.01±0.18] mg/dL) NHBT groups (P<0.001).No significant difference in SCr was found among the other three groups (15-minute NHBTvs.30-minute NHBT:P=0.93; 30-minute NHBTvs.45-minute NHBT:P=0.99) (Fig. 1B). These results showed the existence of DGF in this DCD renal transplantation rat model.
DGF, which exhibits irreducible SCr, is not an uncommon complication in DCD renal transplantation[4]. Dialysis is a common treatment of DGF.However, it is difficult to achieve this treatment in rat model. Hence, the survival rate would present the recovery of graft function, when the recipient might die from acute renal failure. The survival rates of the recipient rats on day 7 post-transplantation were 100%, 60%, 0%, and 0% for the 0-minute, 15-minute,30-minute, and 45-minute NHBT groups, respectively(Fig. 1C). The survival rates of the four groups were significantly different and showed a significant dependence on the NHBT of DCD grafts (Fig. 1C).However, few recipient rats with both kidneys removed and received grafts from donors with more than 30 minutes NHBT could survive more than 7 days after transplantation.
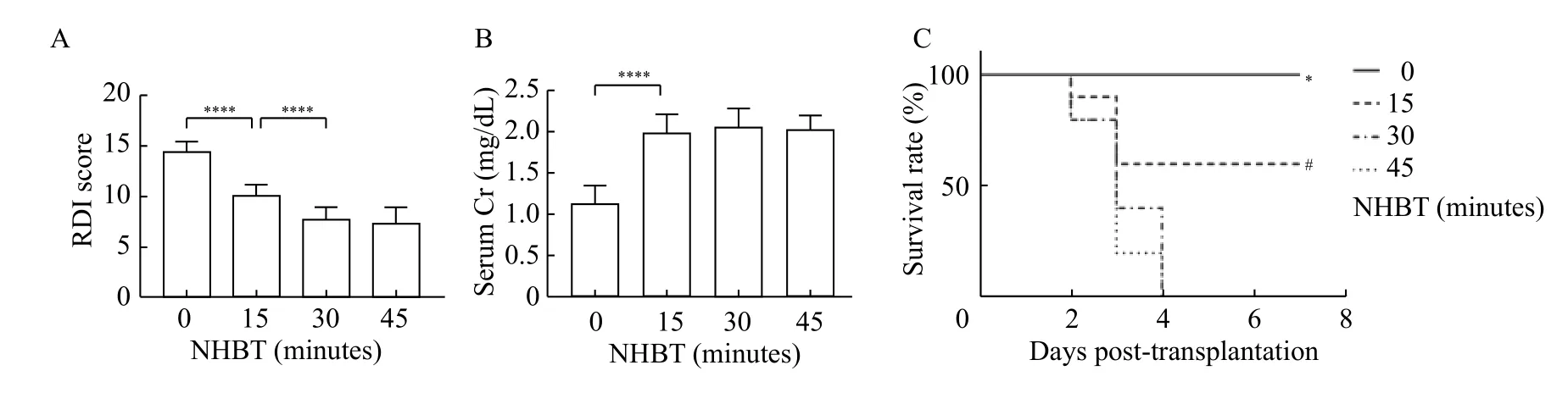
Fig. 1 The early graft function and short-term survival rate of the recipient rats were affected by different non-heart-beating times.Recipient rats accepting grafts from donation after circulatory-determined death rats with different non-heart-beating times (NHBTs). A: The reperfusion damage index (RDI) was evaluated for all of the groups in the first 10 minutes after reperfusion. Data are expressed as mean±SEM. Statistical analyses were performed by two-way ANOVA for comparisons between multiple groups with multiple variables.****P<0.0001; n=15. B: The serum creatinine level of the recipients on day 1 post-transplantation was evaluated to represent the grafts function among different groups. Data are expressed as mean±SEM. Statistical analyses were performed by two-way ANOVA for comparisons between multiple groups with multiple variables. ****P<0.0001; n=5. C: The survival rate of all groups before day 7 posttransplantation. Statistical analyses were performed by survival curve analyses. *P<0.001 vs. 15-minute NHBT; #P<0.001 vs. 30-minute NHBT.
Apoptosis and necrosis were significantly enhanced in prolonged NHBT donation
We compared the difference of acute renal tubular necrosis between different NHBTs in different posttransplantation time-points to evaluating the selfrepairing of grafts in the early post-transplantation period. There was a significant difference in the histological assessment among the four groups after transplantation (Fig. 2). Cell damage was hardly observed in the 0-minute NHBT group, whereas light acute tubular injury was present in the 15-minute NHBT group on day 1 after transplantation, which was reduced over time. Severe cell damage and delayed recovery were observed in the 30-minute NHBT and 45-minute NHBT groups, and on day 7 the cell damage over a large area was only found in the 45-minute NHBT group. Similar results were found in apoptosis and necrosis of renal tubular epithelial cells.The number of TUNEL-positive cells in the renal sections was significantly increased with the increase of NHBT but reduced over time (Fig. 3). The severe damage in DCD grafts could be attributed to DGF and stagnated self-repairing of grafts.
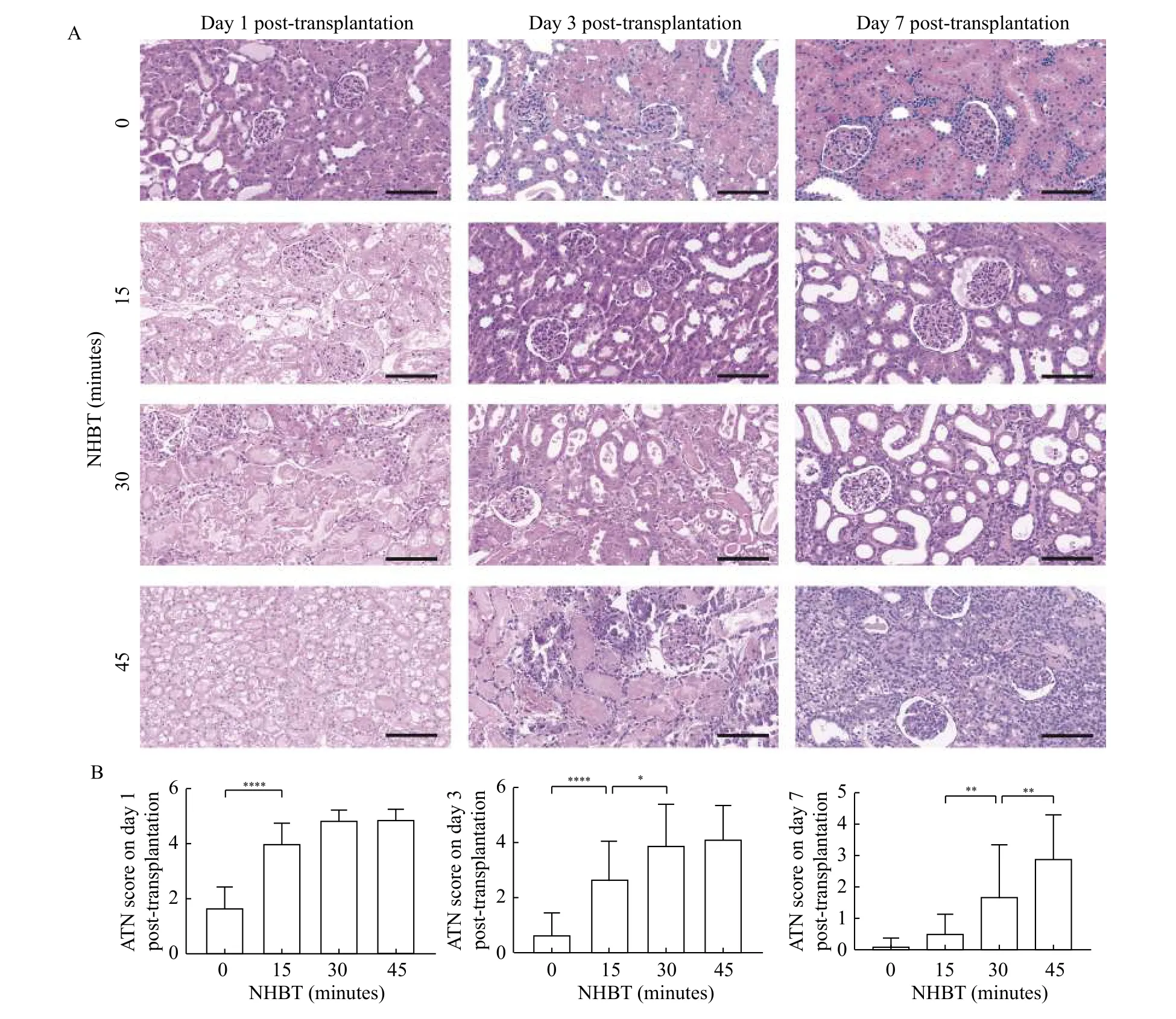
Fig. 2 Prolonged NHBT made severe graft acute tubular necrosis and delayed graft self-recovery. Representative example of renal histology at different time points after transplantation and corresponding ATN scores. A: Representative images of hematoxylin and eosin staining on sections of grafts on day 1, 3, and 7 after transplantation in different groups. B: The renal tubular epithelial cell injury was evaluated by ATN score. Data are expressed as mean±SEM. Statistical analyses were performed by two-way ANOVA for comparisons between multiple groups with multiple variables. *P<0.05, **P<0.01, ****P<0.0001; n=5. Scale bars: 100 μm. ATN: acute tubular necrosis;NHBT: non-heart-beating time.

Fig. 3 Prolonged NHBT showed larger parenchymal cell apoptosis in short-term post-transplantation. Immunofluorescence TUNEL staining in graft sections at different time points after transplantation. A: Representative images of TUNEL staining in the graft sections on day 1, 3, and 7 after transplantation. B: The number and proportion of TUNEL positive cells. Data are expressed as mean±SEM. Statistical analyses were performed by two-way ANOVA for comparisons between multiple groups with multiple variables. **P<0.01, ***P<0.001. n=5.Scale bars: 100 μm. NHBT: non-heart-beating time.
Prolonged NHBT delayed the proliferation of tubular cells and microvascular in grafts
By staining the graft sections for PCNA and vWF,we evaluated tubular cell proliferation and microvascular proliferation. Immunohistochemical analysis of the 0-minute NHBT group showed an increased number of PCNA-positive cells on day 1,which was reduced on days 3 and 7 after transplantation (Fig. 4). In the 15-minute NHBT group, abundant PCNA-positive cells could be observed on days 1, 3,and 7. In the DCD groups with prolonged NHBTs,PCNA-positive cells could hardly be detected on day 1, but the number was significantly increased on days 3 and 7. Similar results were observed in vWF staining experiment. The area of vWF-positive cells in the 0-minute NHBT and 15-minute NHBT groups was large on day 1, and then reduced on days 3 and 7(Fig. 5). In the 30-minute NHBT and 45-minute NHBT groups, the vWF-positive area decreased initially but increased afterward. Collectively, these results revealed that the self-repairing of grafts was delayed by prolonged NHBT-induced severe warm ischemia injury.
Prolonged NHBT delayed the expression of antioxidant proteins in grafts
MDA, a product of the reaction between ROS and lipid molecules, reflects the extent of ROS production and oxidative tissue injury[17]. As shown inFig. 6A,MDA content in the DCD grafts were significantly increased in the prolonged NHBT groups (day 1:[8.6±1.1] nmol/mg in the 0-minute NHBT groupvs.[28.2±3.2] nmol/mg, [42.2±7.9] nmol/mg, and[42.6±7.8] nmol/mg in the 15-minute, 30-minute, and 45-minute NHBT groups, respectively;P<0.01; day 3:[1.6±0.4] nmol/mg in the 0-minute NHBT groupvs.[12.6±3.1] nmol/mg, [22.4±5.0] nmol/mg, and[25.6±2.3] nmol/mg in the 15-minute, 30-minute, and 45-minute NHBT groups, respectively;P<0.01; day 7:[0.7±0.2] nmol/mg in the 0-minute NHBT groupvs.[4.0±1.6] nmol/mg, [13.6±2.3] nmol/mg, and[17.8±1.9] nmol/mg in the 15-minute, 30-minute, and 45-minute NHBT groups, respectively;P<0.01).
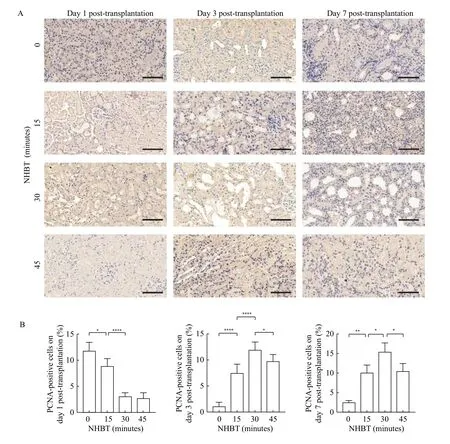
Fig. 4 The graft parenchymal cell proliferation capacity was affected by NHBT. The graft parenchymal cell proliferation capacity was represented by PCNA expression in grafts. A: Immunohistochemical staining of PCNA in graft sections at different time points after transplantation. Representative images of PCNA staining in graft sections on day 1, 3, and 7 after transplantation. Scale bars: 100 μm. B: The number and proportion of PCNA positive cells. Data are expressed as mean±SEM. Statistical analyses were performed by two-way ANOVA for comparisons between multiple groups with multiple variables. *P<0.05, **P<0.01, ****P<0.0001. n=5. NHBT: non-heart-beating time.
Oxidative stress is an important pathological mechanism in IRI. Consequently, antioxidative capacity might have a close relationship with graft self-repairing capacity. Therefore we evaluated the antioxidative capacity of grafts[13]. The expression levels of antioxidant proteins, including SOD-1, HO-1, and Nrf2 were assessed (Fig. 6B). The antioxidant capability of the grafts decreased with the prolonged NHBT on day 1 after transplantation. The expression of antioxidant proteins decreased in the 0-minute NHBT and 15-minute NHBT groups over time, which corroborated our results on graft tissue acute injury.The antioxidant proteins increased with the selfrepairing of grafts in the 30-minute NHBT and 45-minute NHBT groups. Immunohistochemical staining revealed that the expression of HO-1 in the 0-minute NHBT and 15-minute NHBT groups peaked on day 1 after transplantation and showed a decreasing trend afterward. HO-1 expression in the long NHBT groups showed an opposite trend: it decreased initially and increased afterward compared with the short NHBT groups (Fig. 7). The expression of antioxidant, which was also dependent on the NHBTs, was parallel with the self-repairing of grafts.

Fig. 5 The graft microvascular proliferation capacity was affected by NHBT. Immunohistochemical staining of vWF in graft sections at different time points after transplantation. A: Representative images of vWF staining in graft sections on day 1, 3, and 7 after transplantation. B: The number and proportion of vWF positive areas. Data are expressed as mean±SEM. Statistical analyses were performed by two-way ANOVA for comparisons between multiple groups with multiple variables. *P<0.05, ***P<0.001, ****P<0.0001. n=5. Scale bars:100 μm. NHBT: non-heart-beating time.
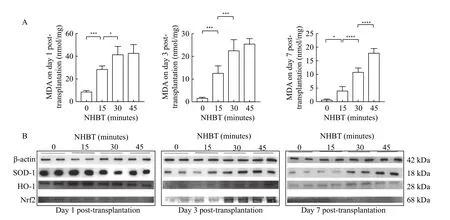
Fig. 6 Prolonged NHBT showed severe oxidant stress injury. Oxidative stress and antioxidant proteins of grafts. A: Content of MDA in graft tissues at different time points. Data are expressed as mean±SEM. Statistical analyses were performed by two-way ANOVA for comparisons between multiple groups with multiple variables. *P<0.05, ***P<0.001, ****P<0.0001. n=5. B: Expression of SOD-1, HO-1 and Nrf2 in graft tissues at different time points after transplantation by Western blotting. MDA: malondialdehyde; NHBT: non-heart-beating time.

Fig. 7 The graft antioxidant capacity was affected by NHBT. Immunohistochemical staining of HO-1 in graft sections at different time points after transplantation. A: Representative images of HO-1 staining in graft sections on day 1, 3, and 7 after transplantation. Scale bars:100 μm. B: The number and proportion of HO-1 positive cells. Data are expressed as mean±SEM. Statistical analyses were performed by two-way ANOVA for comparisons between multiple groups with multiple variables. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001. n=5.NHBT: non-heart-beating time.
Discussion
Bellemaretet alfirst reported the standardized DCD donor rat model[19], which involved cardiac arrest induction by external compression to the heart.Subsequently, several studies were conducted using a similar animal model to study DCD renal transplantation. However, some studies only focused on how the graft quality suffered due to hypoxia and ischemia or organ preservation without the consideration of complete transplantation surgery[2–4,20–22]. A vascular clamping approach is used to induce warm ischemia, which could not fully simulate the pathological environment in DCD renal transplantation[23–25]. Thus, it is necessary to establish an animal DCD renal transplantation model with complete transplantation. In the present study, we have established a stable and reproducible DCD renal transplantation model by completing all process of the surgery to investigate the impact of different NHBTs on the self-repairing and antioxidant capacity of grafts. This animal model could be used to assess the IRI and post-transplantation changes in DCD grafts,which will help to understand the pathological mechanism and future interventions.
Though highly susceptible to hypoxia injury, renal tubular epithelial cells are also capable of rapid regeneration and functional recovery[26]. Self-repairing and antioxidant capacities are associated with graft quality. The gene expression pattern of renal transplantation recipients varies due to different donor graft source (living donation or DCD)[27]. NHBT,which may lead to the severe IRI, is a crucial factor to determine whether the DCD organ should be discarded[28]. Grafts from DCD with a short NHBT would regain function quickly and maintain a high quality because complications such as DGF or PNF could be avoided. In contrast, prolonged NHBT would worsen the graft quality, resulting in a weak or even absent self-repairing capacity. Our study showed initial dysfunction, a weak self-repairing capacity, and delayed functional recovery in grafts with prolonged NHBT compared with those with short NHBT.Moreover, the self-repairing ability of the grafts paralleled the expression of antioxidant proteins in the early post-transplantation period.
To investigate the self-repairing and antioxidant capacity of DCD grafts with different NHBTs, we harvested tissues at different time points after transplantation. We found that the increased incidence of initial dysfunction in grafts with a prolonged NHBT was caused by severe IRI. No difference was observed in renal histology and renal function among DCD groups on day 1 after transplantation. Grafts in the 0-minute NHBT and 15-minute NHBT groups exhibited quick recovery in the early posttransplantation period. However, grafts with long NHBT showed an increased risk of DGF, which could result in a lower survival rate. The renal histology of the grafts in 30-minute NHBT group was better than that in the 45-minute NHBT group. Grafts in both 30-minute NHBT and 45-minute NHBT groups had an abnormal structure even on day 7 after transplantation.Results of the TUNEL assay also demonstrated that cardiac arrest appeared to sensitize the grafts to cell death and decrease cell regeneration of in the grafts due to IRI.
The self-repairing capacity of grafts was analyzed by staining for PCNA and vWF, which reflected the renal tubular epithelial cell proliferation and microvascular proliferation, respectively[29–30]. Our results showed that PCNA-positive cells and vWFpositive areas could hardly be detected in grafts of the 30-minute NHBT and 45-minute NHBT groups because of the extensive damage on day 1 after transplantation. On days 3 and 7 after transplantation,the numbers of PCNA-positive cells in grafts with prolonged NHBTs were significantly increased,whereas they were decreased in the 0-minute NHBT and 15-minute DCD groups. The vWF-positive areas followed a similar trend with PCNA. Grafts with 30 minutes NHBT had a higher regenerative potential than those with 45 minutes NHBT. This suggests that the self-repairing capacity may be finite and related to NHBT. Our results indicate that an NHBT longer than 45 minutes is not suitable for establishing rat DCD renal transplantation model because the grafts may suffer severe IRI, and thus could not easily regain renal function and structure.
Pathological mechanisms contributing to IRI involve oxidative stress, calcium overload, apoptosis,and inflammation, and oxidative stress is pivotal to initiate IRI[31–32]. During reperfusion, excessive ROS are produced due to the abrupt regaining of oxygen,which can cause serious damage to cellular proteins,lipids, and DNA, and then lead to parenchymal cell apoptosis and necrosis in the grafts[32–33]. The endogenous antioxidant system of grafts produces antioxidants to eliminate or neutralize ROS,diminishes graft injury, and facilitates self-repairing and functional recovery of grafts[15]. As a part of the antioxidant system, Nrf2 is a central transcriptional regulatory factor that could regulate the expression of antioxidant enzymes (such as HO-1) to protect cells from IRI-triggered oxidative damage[34–37]. HO-1 is a rate-limiting enzyme that catalyzes the degradation of heme into the antioxidant biliverdin, the antiinflammatory agent carbon monoxide, and ferrous iron[38–40].
We found that MDA content significantly increased in the grafts after reperfusion, and the increase depended on NHBT. In addition, a dynamic decrease in the levels of SOD-1, HO-1, and Nrf2 was observed at different time points following transplantation in the 0-minute NHBT and 15-minute NHBT groups.The levels of these antioxidant enzymes on day 1 after transplantation were lower in the 30-minute NHBT and 45-minute NHBT groups than in other groups and then kept rising with the post-transplantation time.The expression of antioxidant proteins showed changes correlated well with histological and immunohistochemical analyses. Thus, the antioxidant system participates in the endogenous protection of grafts to promote early recovery following non-heartbeating and transplantation.
In summary, the up-regulation of antioxidant capacity, which is dependent on NHBTs, reduces grafts oxidative stress and promotes self-repairing of grafts. Prolonged NHBTs could delay the expression of antioxidant proteins. These findings support that enhancing the antioxidant capacity of grafts can reduce post-transplantation IRI and protect graft function following DCD renal transplantation.
Acknowledgments
This work was supported by funds from the National Natural Science Foundation of China (Grant No. 81570613 and No. 81370853), Jiangsu Provincial Social Development Project (Grant No. BE2017615),and 2016 Jiangsu Provincial Medical Innovation Team (Grant No. 0536).
杂志排行
THE JOURNAL OF BIOMEDICAL RESEARCH的其它文章
- Differentiation of gestational diabetes mellitus by nuclear magnetic resonance-based metabolic plasma analysis
- Multiple pathological components in gliosarcoma
- Pathogenesis and drug response of iPSC-derived cardiomyocytes from two Brugada syndrome patients with different Nav1.5-subunit mutations
- PUM1 represses CDKN1B translation and contributes to prostate cancer progression
- A nomogram for predicting lymph node metastasis in superficial esophageal squamous cell carcinoma
- Regulatory role of sorting nexin 5 in protein stability and vesicular targeting of vesicular acetylcholine transporter to synaptic vesicle-like vesicles in PC12 cells
