Multiple pathological components in gliosarcoma
2021-10-13NanN.Jiang,RamiroLarrazabal,WaleedAlsunbul等
Dear Editor:
Our recent article "Angiosarcomatous component in Glioblastoma: case report and consideration of diagnostic challenge and hemorrhagic propensity"published inThe Journal of Biomedical Research[1]demonstrated an interesting case of gliosarcoma with alternating gliomatous and angiosarcomatous components, which on imaging, displayed unique intratumorally piliform enhancement pattern and clinically, exhibited an increased tendency for intraoperative hemorrhage. Indeed, gliosarcoma may be composed of various different pathological components and may dictate the radiological features and clinical presentations that make preoperative diagnosis extremely difficult.
We have noted that gliosarcomas may contain not only angiosarcomatous but also other unusual sarcomatous components to varying degrees (in addition of the defining gliomatous component). A primitive neuronal component is occasionally seen in glioblastoma, which is traditionally described as primitive neuroectodermal tumor (PNET)-like components[2–3]; a component of giant cell glioblastoma/variant is not usually found in gliosarcoma[4]. The coexistence of these pathological components within a brain tumor has not been reported in the literature.Here we described a case of gliosarcoma containing multiple pathological components. The patient was a 77-year-old male presented with a 4-month history of progressive right facial palsy, left-sided weakness and memory loss. Brain computed tomography and magnetic resonance (MR) imaging showed a 6.8 cm peripherally-located well circumscribed, mixed solid and cystic mass centered in the right temporal lobe with peritumoral edema (Fig. 1). The mass displayed an irregular thick wall with a large nodular component that has a bubbly appearance. On MR imaging, the mass was hypointense on T1-weighted sequence and intermediate on T2-weighted sequence. The wall and nodular components of this mass demonstrated both heterogeneous enhancement and restricted diffusion.In contrast to the published case of glioblastoma with angiosarcomatous component1, this mass exhibited no piliform enhancement pattern on imaging.
Intraoperatively, the mass is relatively wellcircumscribed and did not display an increased hemorrhagic tendency during the surgery as compared to our previously reported gliosarcoma with angiomatous features. On pathological examination of the resected tissue, the tumor exhibited multiple components including largely alternating gliomatous and sarcomatous features with a focally abundant giant cells intermixed with primitive neuronal features(Fig. 2). The tumor exhibited positive p53 immunostaining in the gliomatous component and negative IDH1(R132H) immunostaining, along with necrosis,microvascular proliferation, and mitotic activity with a high Ki-67 proliferation index (focally more than 90%). These pathological features were diagnostic of a glioblastoma variant consistent with gliosarcoma.
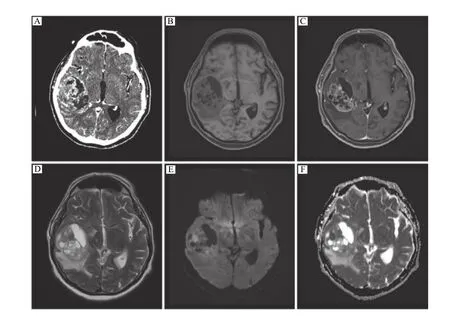
Fig. 1 Preoperative neuroimaging. A: Computed tomography shows a well-circumscribed heterogeneously enhancing, mixed cystic and solid mass centered in the right temporal lobe. B–D: Magnetic resonance imaging reveals hypo- to iso-intensity on T1-weighted image (B)with heterogeneous contrast enhancement of the solid component (C) and hyper-intensity on T2-weighted image (D). E and F: There is restricted diffusion associated with this mass, particularly at the level of the solidly enhancing components.
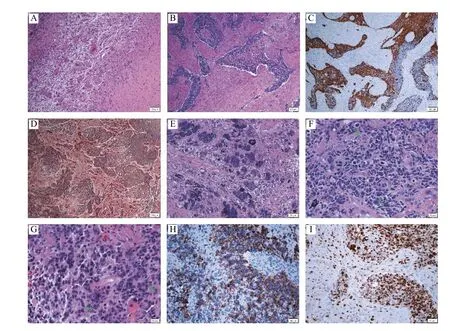
Fig. 2 Photomicrographs of a glioblastoma variant with multiple components. A–H: The tumor is relatively well-circumscribed with a distinct boundary (A), and characterized by largely alternating gliomatous (B, with higher cellularity; C, positive GFAP immunostaining)and sarcomatous (B, with relatively less cellularity; D, positive reticulin staining), along with focally abundant giant cells (E, frequently multinucleated) and foci exhibiting primitive neuronal features including high cellularity with an increased nuclear-to-cytoplasmic ratio, cell wrapping (F, arrows), Homer-Wright rosettes (G, arrows), and focally positive synaptophysin immunostaining (H). I: The Ki-67 proliferation index is markedly high. Original magnification: ×40 (A–D), ×100 (E, H and I), ×200 (F and G). Scale bars: 100 μm (A–D), 50 μm (E, H and I), 20 μm (F and G). GFAP: glial fibrillary acidic protein.
In contrast to our recently published case, this example illustrates the multitude of pathological variations in gliosarcoma with variably complex neuroimaging patterns. Therefore, the key is to be cognizant of the various glioblastoma variants/gliosarcomas and their possible neuroimaging features in order to aid preoperative diagnosis and surgical management.
Yours Sincerely,
Nan N. Jiang1,✉, Ramiro Larrazabal1, Waleed Alsunbul2,Jian-Qiang Lu3
1 Department of Radiology,
2 Department of Surgery/Neurosurgery,
3 Department of Pathology and Molecular
Medicine/Neuropathology,
McMaster University,
Hamilton,
Ontario L8L 2X2,
Canada.
✉Tel: +1-905-527-4322 ext. 46521,
E-mail: nan.jiang@medportal.ca.
杂志排行
THE JOURNAL OF BIOMEDICAL RESEARCH的其它文章
- Differentiation of gestational diabetes mellitus by nuclear magnetic resonance-based metabolic plasma analysis
- Pathogenesis and drug response of iPSC-derived cardiomyocytes from two Brugada syndrome patients with different Nav1.5-subunit mutations
- Dynamic regulation of anti-oxidation following donation repairing after circulatory determined death renal transplantation with prolonged non-heart-beating time
- PUM1 represses CDKN1B translation and contributes to prostate cancer progression
- A nomogram for predicting lymph node metastasis in superficial esophageal squamous cell carcinoma
- Regulatory role of sorting nexin 5 in protein stability and vesicular targeting of vesicular acetylcholine transporter to synaptic vesicle-like vesicles in PC12 cells
