Effect of SiO2 Aerogel-cement Mortar Coating on Strength of Self-Compacting Concrete after Simulated Tunnel Fire
2021-09-15WANGXinjieJIAZhiZHUPinghuaLIUHuiCHENChunhongDONGYanlong
WANG Xinjie, JIA Zhi, ZHU Pinghua*, LIU Hui, CHEN Chunhong, DONG Yanlong
(1. Department of Civil Engineering, Changzhou University, Changzhou 213164, China; 2. Railway 19th Bureau Group Sixth Engineering Co. Ltd, Wuxi 214000, Chinaa)
Abstract: In order to facilitate self-compacting concrete to be better used in tunnel linings that can resist fires, a SiO2 aerogel-cement mortar coating was prepared. Based on the HC curve, a self compacting concrete cube specimens coated and uncoated with SiO2 aerogel-cement mortar (SiO2-ACM) were heated to simulate tunnel fire for 0.5, 1, 1.5, 2, 2.5, 3 and 4 h, respectively.The residual compressive strength was tested after the specimens were cooled to room temperature by natural cooling and water cooling. The results show that, the damages of specimens become more serious as fire time goes on, but the residual strength of specimens coated with SiO2-ACM is always higher than that of uncoated with SiO2-ACM. In addition, the residual strength of specimens cooled by water cooling is lower than that of natural cooling. However, for the specimens coated with SiO2-ACM, the adverse effects of water cooling are lessened. With the increase of fire time, the protective effect of SiO2-ACM is still gradually improved. Finally, a formula was established to predict the residual 150 mm cube compressive strength of specimens protected by SiO2-ACM after a simulated tunnel fire.
Key words: self-compacting concrete; SiO2 aerogel-cement mortar; simulated tunnel fire; residual compressive strength; natural cooling; water cooling
1 Introduction
With the acceleration of urbanization, the demand for tunnel construction in the world has increased significantly. Self-compacting concrete (SCC) is widely used as the tunnel linings because of its higher fluidity and easier construction process[1,2]. However,in the event of a fire, the internal temperature of tunnel structure can rise rapidly to over 1 000 ℃ within 10 minutes[3], which will lead to a sharp increase of internal steam pressure, resulting in the spalling of SCC, and threatening the bearing capacity of tunnel structure or even leading to the collapse of tunnel concrete structure[2,3]. Xargayet al[4]found that the surface of self-compacting high-strength concrete(SCHSC) was distributed with serious and regular cracks at 600 ℃. A conclusion is obtained that the occurrence of explosive spalling of SCC at high temperature is inevitable[5-8]. Therefore, it is vital to improve the fire resistance of SCC for the application of tunnel engineering.
In order to prevent the explosive spalling of SCC in fires, the scholars have conducted many studies[9-11].The precautions have been taken including installing insulation boards, adding polypropylene fibers to concrete, controlling moisture content and so on[12-15].Among them, the application of lightweight fireproof coating on the surface of concrete is an effectively safeguard procedure to protect the tunnel structure from collapsing. Kimet al.[16]developed a bottom ash-based cementitious material for fire protection coatings and found that the thickness between 30 and 40 mm could be considered optimal for fire protection coatings of tunnel linings. Benk and Coban[17]produced lightweight, heat-insulating materials with lightweight aggregates such as pumice and/or expanded perlite.When these bricks were exposed to temperatures up to 825 ℃, they did not lose strength, but rather gained strength. Based on expanded vermiculite, Suvorov and Skurikhin[18]have developed high-temperature insulation materials with physicochemical properties for thermal power plants with the hot-wall temperature not exceeding 1 150 ℃.
SiO2aerogel has attracted people’s attention because of its extremely low thermal conductivity and density. SiO2aerogel is usually prepared by three general steps: gel preparation, aging of the gel and drying of the gel. The dry part can be divided into supercritical drying and ambient pressure drying.In order to reduce costs, the application of ambient pressure drying is more common, and the prepared aerogel is hydrophobic. SiO2aerogel can be used as an ideal thermal insulation material when it combines with other inorganic materials[19,20]. Liuet al[12]successfully prepared the aerogel incorporated mortars (AIM) with a density of 1.2 g/cm3and a thermal conductivity of 0.152 4 W/(m·K) using 60% by volume of SiO2aerogel particles instead of sand in mortar. Nget al[21]used calcined clay and cement as cementitious materials to prepare the SiO2aerogel-cement mortar (SiO2-ACM).The results showed that the thermal conductivity of SiO2-ACM was further reduced. Even though many researches have verified the superior heat insulation performance of SiO2-ACM, there are few studies on the microstructure of SiO2-ACM at ultra-high temperature or the protective effect of SiO2-ACM coating on SCC after tunnel fire environment.
In case of tunnel fire, the fire duration is unforeseeable. Hence, the fire time is a significant factor to affect the performances of concrete at high temperature[22]. However, the study of the effect of tunnel fire time on SCC coated with SiO2-ACM is lacking. In addition, considering that the frequent use of watering cooling at the fire site[3]can lead to the adverse effects on the concrete[23]. The degradation of 150 mm cube compressive strength of SCC coated with or without SiO2-ACM after different simulated tunnel fire time and different cooling methods were investigated in this paper. A law of strength deterioration was revealed, the microstructures of SiO2-ACM after different fire time were observed, and the protective effect of SiO2-ACM on SCC after simulated tunnel fire was analyzed. This paper aims to study the tunnel fire resistance of SiO2-ACM and provide an idea for tunnel lining concrete fireproof coating.
2 Experimental
2.1 Constituents and mix proportions
SCC was prepared by P·O 52.5 of ordinary Portland cement (OPC), and its physical and mechanical properties are shown in Table 1. The coarse aggregates (CA) were natural crushed limestone with size of 4.75-16 mm. The fine aggregates were natural river sand (S) with size of 0.35-0.5 mm. The physical properties of normal aggregates are listed in Table 2. The air entraining agent (AEA) and mineral admixtures, such as fly ash (FA), slag and silica fume(SF) were used to meet the strength and workability requirements of SCC. The chemical components of the mineral admixtures are listed in Table 3.
SiO2-ACM was prepared with P·O 42.5 of OPC(Table 1). Aggregates include S and aerogels. The commercial hydrophobic SiO2aerogel was purchased from Guangdong Alison Hi-Tech Co., Ltd. in China,and its main properties are shown in Table 4. The AEA, polycarboxylic acid superplasticizer (PCE)and hydroxypropyl methylcellulose (HPMC) were used to increase the workability of SiO2-ACM, and polypropylene fibers (PP) (length: 12 mm) were added to improve the strength of SiO2-ACM. In order to disperse the aerogel particles uniformly, an inorganic dispersant (VINNAPAS,V) was used. The performance index of PCE is shown in Table 5.
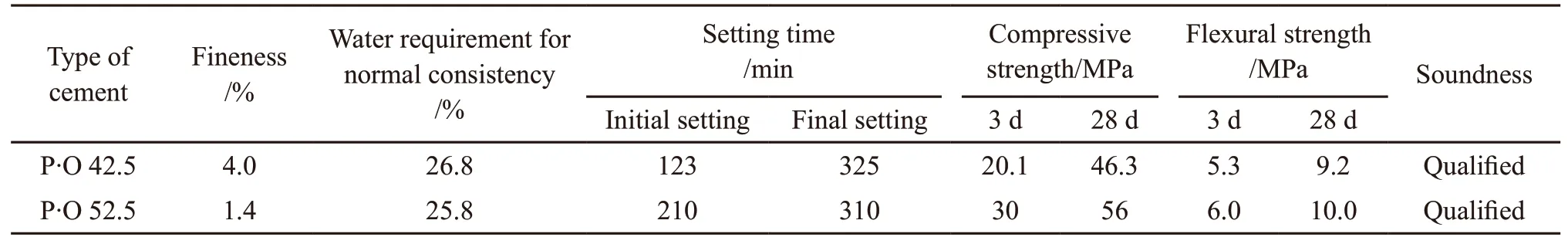
Table 1 Physical and mechanical properties of cement

Table 2 Physical properties of normal aggregates
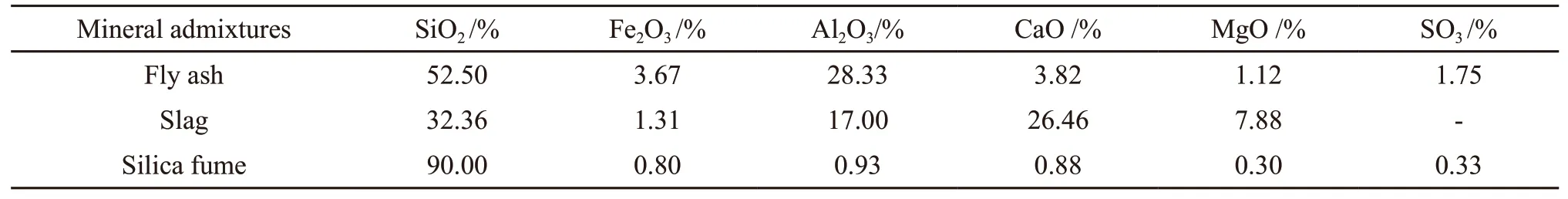
Table 3 Chemical components of the mineral admixtures

Table 4 Main performance index of SiO2 aerogel

Table 5 The performance index of PCE

Table 6 Mix proportion of C60 SCC

Table 7 Mix proportion of SiO2-ACM
The target value of cubic compressive strength is 60 MPa after 28 days of curing. The mix proportion of SCC is listed in Table 6. To prepare the SiO2-ACM,60% of volume of aerogel is used according to the reference reported by Liuet al(2016). As shown in Table 7, the slag and silica fume were taken as 20%and 8% respectively of the mass of binding materials.
2.2 Preparation of specimens
SCC was prepared by the method of sand enveloped with cement, and its slump flow was measured to be around 700 mm, which was in accordance with the selected fluidity index. Molded specimens were cured in a curing chamber at 20 ℃ and relative humidity over 95% until 28 days.
In the preparation of SiO2-ACM, solid components such as aggregates, cementitious materials and additives were mixed firstly, and then water was added while stirring. Stirring time was 180 s, and finally a uniform mortar was obtained. Afterwards, SiO2-ACM was coated on the surface of SCC with a thickness of 6 mm and continued to be cured for another 28 days,which was defined as Group A. While Group B (SCC uncoated with SiO2-ACM) was prepared as the control group.
2.3 Tunnel fire simulation and measurements
The simulated tunnel fire is based on the HC curve, but the heating rate is slightly different as shown in Fig.1(a). Furnace heating is used in all heating methods during the experiment. The five side surfaces of specimens were wrapped with aluminosilicate needle-punched fire blanket to make sure that the single side of the specimen was subjected to the simulated tunnel fire for 0.5, 1, 1.5, 2, 2.5, 3 and 4 h, respectively.Thermocouple continuously recorded the interface temperature of ACM-SCC, as shown in Fig.1(b).For Group A specimens, the surface with SiO2-ACM coating was exposed to the high temperature. After the fire, the same batch specimens were cooled to room temperature by natural cooling (NC) and water cooling(WC), respectively. Then the residual 150 mm cube compressive strength (hereinafter called “compressive strength”) of SCC was tested and recorded.
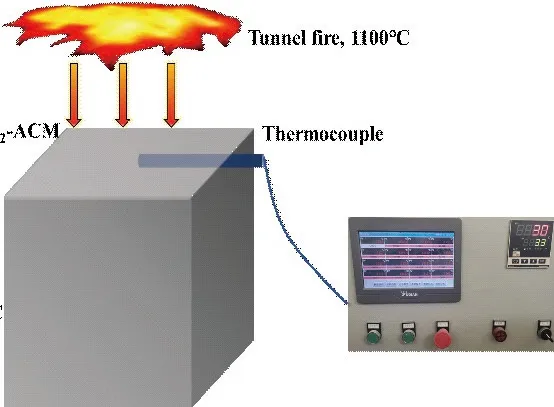
Fig.1 (a) HC and experimental curves of tunnel fire; (b) Schematic of tunnel fire simulation
The measurement of the compressive strength of SCC used a combination of the rebound method and the uniaxial compressive test method, which conforms to the Chinese standards GB/T 50081-2002[25]. The rebound method was performed in different directions and the average value of strength was taken as the result. For the uniaxial compression test, the complete surface was selected for testing to ensure comparability between the results.
Scanning electron microscopy (SEM; SUPRA55,Zeiss) was used to analyze the microstructures of SiO2-ACM before and after simulated tunnel fire.
3 Results and discussion
3.1 Macroscopic characteristics of specimens after simulated tunnel fire
Fig.2 shows the macroscopic characteristics of surface of SiO2-ACM and interface of SiO2-ACM and SCC in Group A after different simulated tunnel fire time.
In the case of the NC, the color of SiO2-ACM coating gradually changes from gray green to yellow brown with the extension of fire time (Fig.2(a)).Correspondently, the cracks on the interface of mortar and SCC gradually increase, and the SCC even is spalled at longer time (more than 2.0 h) as shown in Fig.2(b). When fire time is less than 0.5 h, there are no obvious changes on the mortar, and no cracks on the interface of mortar and SCC. But when fire time reaches to 1.5 h, the surface of mortar become dark red, and cracks appear at the edges. The color of mortar surface starts to turn yellow when fire time is over 2 hours, while the spalling of SCC begins to take place.Until the fire time reaches to 4 h, the mortar protective layer turns yellow brown, the SCC is seriously damaged, and the aggregate is exposed. These results indicate that a 6 mm of SiO2-ACM can protect SCC from spalling for less than 2 h at 1 100 ℃, which is due to the microporous expansion, shrinkage, collapse and complete destruction of silica aerogel particles at high temperatures of 950 to 1 200 ℃[26]. In addition,the decomposition of hydration products of cement is another cause for the color change and insulation failure of SiO2-ACM coating[27,28].
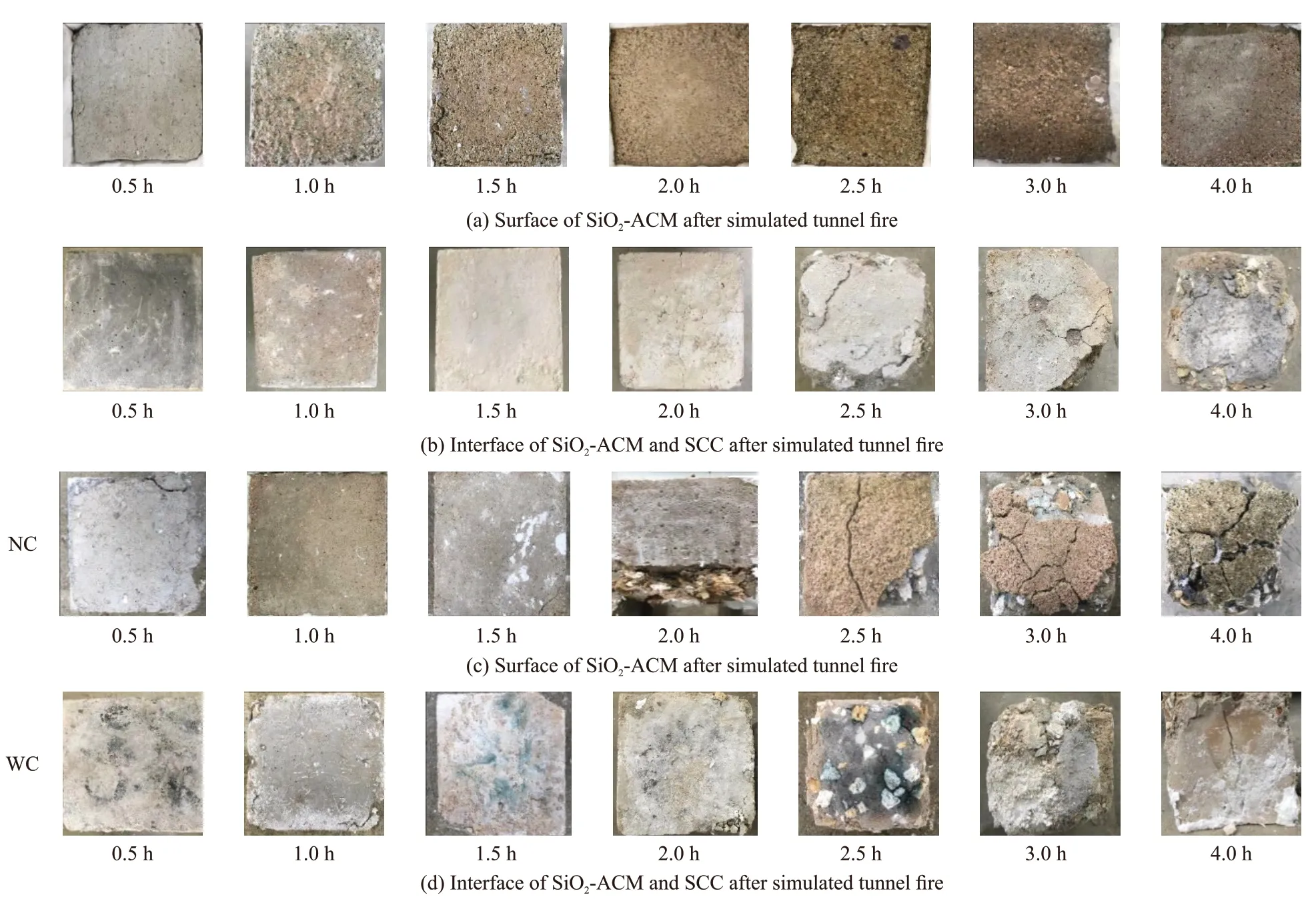
Fig.2 Macroscopic characteristics of Group A specimens after simulated tunnel fire for different time
However, in the case of WC, both of the SiO2-ACM and SCC in Group A are destroyed more seriously than that in the case of NC, when fire time is more than 1.5 h (Figs.2(c)-(d)). The holes created by the melting of the polypropylene fibers[29,30]and the loose structure of cement mortar after 2 h led to the direct contact of cooling water with the SCC.Hence, the specimen is suddenly cooled after the high temperature. The temperature gradient stress between inside and outside specimen results in a large number of cracks inside the mortar and concrete[31]. This is the reason that gives rise to the spalling of SCC after simulated tunnel fire by WC method.
Predictably, the specimens without SiO2-ACM in Group B are damaged more seriously after simulated tunnel fire, especially in the case of WC (Fig.3). After 2 h of fire time, it can be found that the high temperature damage of the specimens is extremely serious and collapse occurs in the case of NC. However, under the condition of WC, the time threshold is only 1.5 h.The failure time of SCC is earlier than that of group A,which indicates that SiO2-ACM has a certain protective effect on concrete specimens to prevent spalling. But the thickness of protective layer needs to be confirmed in further research.

Fig.3 Macroscopic characteristics of Group B specimens after simulated tunnel fire for different time
Chung[32]proposed a simple classification of fire damage based on the visual classification of concrete components in the United Kingdom as shown in Table 8. The damage level is mainly determined based on hazard information and subjective judgment. According to the results mentioned above, in order to meet the concrete damage level of no more than 2, the effective protection time of the SiO2-ACM in the case of NC can be considered as 2 h, while in the case of WC, it is 1.5 h. When the surface of SiO2-ACM is yellow and loose,and the graininess is obvious, and the mortar can be considered invalid.
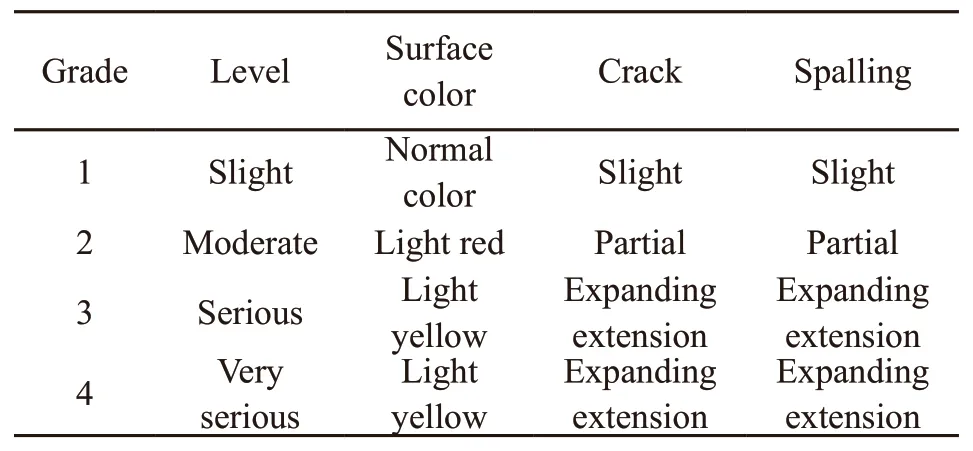
Table 8 Classification of fire damage of concrete components
3.2 Influence of fire time on the residual compressive strength
The residual compressive strength ratio is defined as the ratio of the residual compressive strength of the specimens after high temperature to the compressive strength before high temperature. The effects of fire time on the residual compressive strength ratio of specimens in Group A and B are shown in Fig.4. It can be seen that the residual compressive strength of specimens decreases with fire time under different conditions (cooling methods, and whether or not the SiO2-ACM were coated). But the residual compressive strength ratio of specimens in Group A is much higher than that of Group B specimens. What’s more, the presence of SiO2-ACM slows down the decline rate of residual compressive strength of SCC. These results suggest that SiO2-ACM can be used as an effective fireproof material for tunnel structure.
For Group B specimens as shown in Fig.4(a),when fire time is less than 1.0 h, the decline rate of residual strength is sluggish. Because in the short fire time, the internal temperature of specimen is not high, and the heat transferred to the inside forming an autoclave environment promotes the hydration of excess cement clinker, which reduces the adverse effect of high temperature on the residual strength of specimen[20]. However, as time goes on, the residual compressive strength sharply decreases. In this stage(1.0-3.0 h), the water inside SCC specimen vaporizes to produce vapor pressure leading to the cracks, thus generating the spalling of SCC. With the increase of time, the temperature inside and outside of specimen gradually becomes uniform. While at high temperature,Ca(OH)2and C-S-H inside the specimen begin to decompose[22,33-34], the aggregates are thermally expanded, and the skeleton effect is weakened. A variety of factors lead to the residual compressive strength of the specimen decreasing sharply at this stage. After that, the decrease rate of residual compressive strength tends to slow down. The possible reason is that the strength is maintained only by the friction between the aggregates at this stage[35], and the influence of high temperature is no longer obvious.
For the Group A specimens as shown in Fig.4(a),the residual compressive strength is always higher than that of Group B specimens due to the low thermal conductivity of SiO2-ACM. As Pietraket al[36]and Liet al[37]pointed out, silica aerogel composites could provide excellent resistance to heat transfer. Even though the SiO2-ACM shows its fireproof performance,the curves of Group A specimens also show linear descent functions regardless of the cooling methods.This is because that the aerogel properties are changed with the fire time increasing. In this process, the volume of aerogel is reduced, the pores collapse, and the bonding ability with the cement paste is weakened[26].As a result, SiO2-ACM gradually loses its original high thermal insulation performance. At the same time, the mortar matrix is also cracked at high temperature for longer fire time.
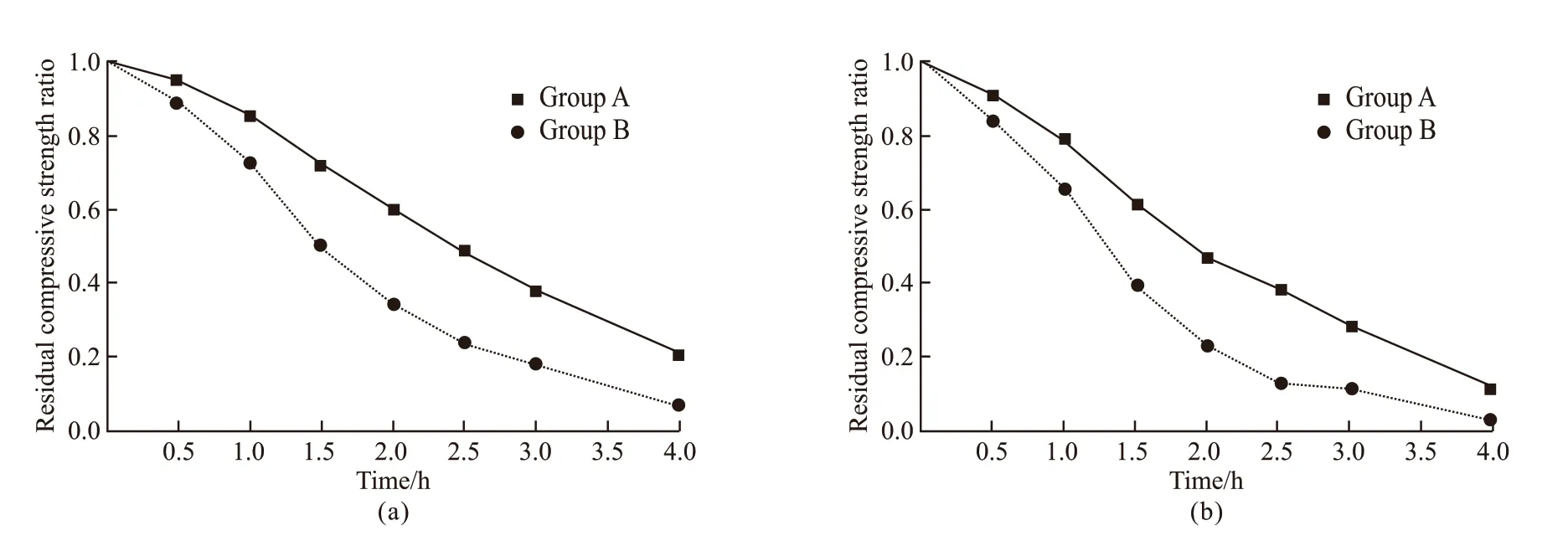
Fig.4 Relationship curves between residual compressive strength ratio and fire time for Group A and B specimens under different cooling methods: (a) NC; (b) WC
3.3 Influence of cooling methods on the residual compressive strength
In Fig.5, it can be seen that the residual compressive strength in the case of WC is lower than that in the case of NC, regardless of the Group A or Group B specimens. In the case of NC, the changes of temperature between the inside and outside of specimens are small, resulting in smaller temperature stress. Compared with NC, WC method can cause the rapid cooling on the surface of SCC specimen,but its internal temperature is still high. Hence, larger temperature stress is generated during this process,which accelerates the development of cracks, resulting in greater strength loss[38].
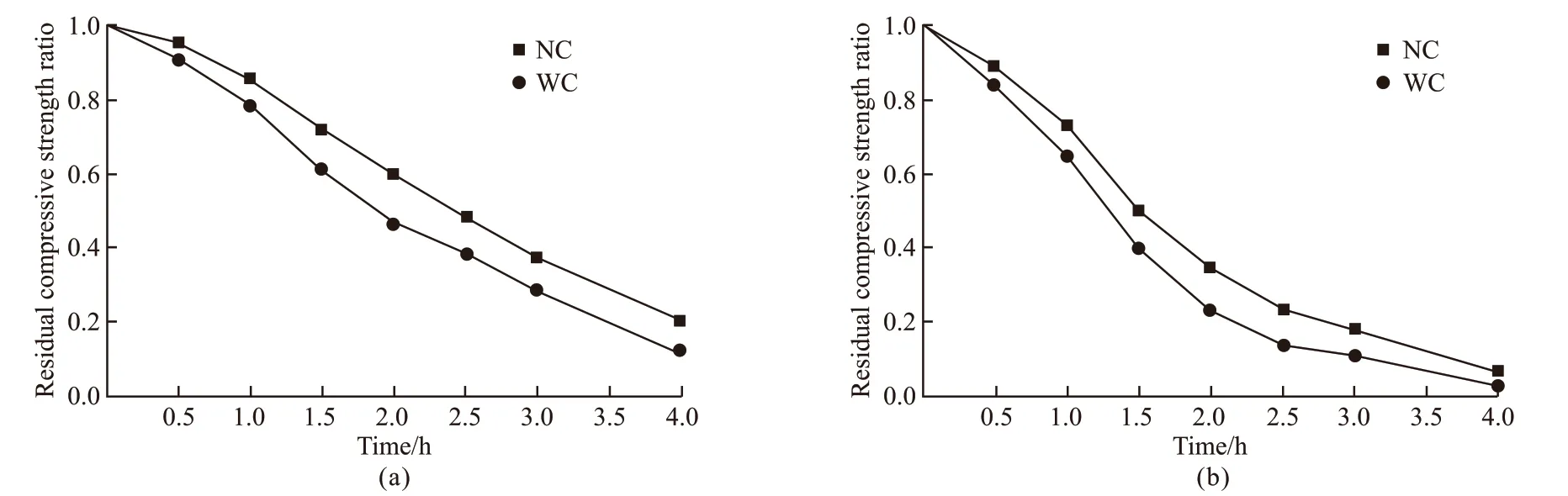
Fig.5 Relationship curves between residual compressive strength ratio and cooling methods for Group A (a) and B (b) specimens
Since the SiO2-ACM is a cement-based material that is hard to withstand the effects of cooling water,WC method weakens the protective effect of SiO2-ACM. However, due to the hydrophobicity of SiO2aerogel, SiO2-ACM can prevent the water from contacting with SCC to some extent. Therefore, the strength of the Group A specimens is higher than that of the Group B specimens in the case of WC.
3.4 The protective effect of SiO2-ACM
The residual compressive strength ratio of Group A specimens cooled by NC method is around 0.6 at 2 hours. However, the residual compressive strength ratio of group B is only 0.501 at 1.5 hours. In the condition of WC, the residual compressive strength ratio of Group A specimens is 0.611 at 1.5 hours, while that of the Group B specimens is only about 0.4 at 1.5 hours.In order to quantitatively study the protective effect of SiO2-ACM, the residual compressive strength ratios of two groups of specimens after simulated tunnel fire are discussed.
The residual compressive strength ratio of Group A specimens is expressed byfA, while the residual compressive strength ratio of Group B specimens is expressed byfB. Fig.6(a) shows the residual compressive strength ratios of two groups of specimens cooled by NC method after different fire time. The data was correlated by using a linear regression method, and a regression equation with a coefficient of determination (R2) of 0.99 was obtained:
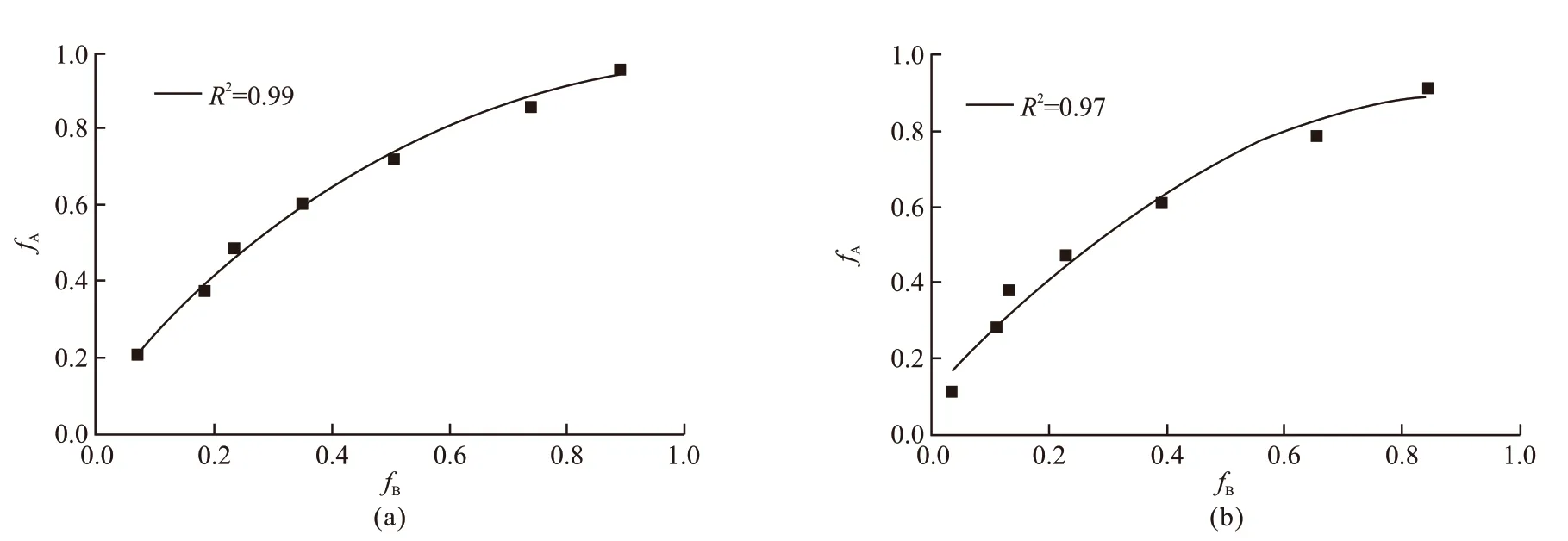
Fig.6 Relationship between residual compressive strength ratio of Group A and B specimens: (a) NC; (b) WC

Similarly, Fig.6(b) shows the relationship in the condition of WC. Eq.(2) is the regression equation with a coefficient of determination (R2) of 0.97:
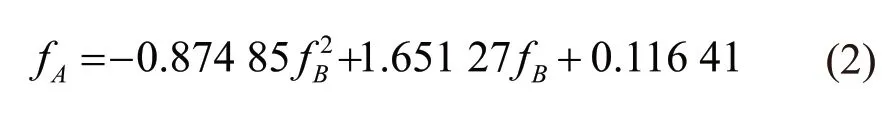
By comparing Eq.(1) with Eq.(2), it is found that the difference between the two regression equations is not significant, that is to say, the effect of the cooling methods on the protective effect of SiO2-ACM is not significant. According to the relationship, this paper establishes a general relationship formula between the residual compressive strength ratios of two groups of specimens under different cooling methods as shown in Fig.7. The resulting regression equation with a coefficient of determination of 0.98 is given as follows:
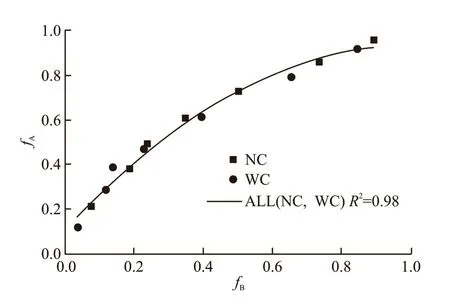
Fig.7 General relationship between residual compressive strength ratio of Group A and Group B specimens under different cooling methods
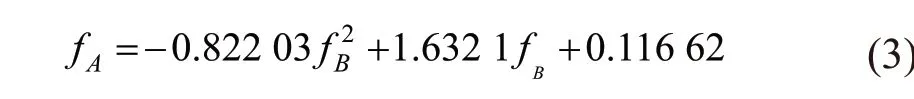
Eq.(3) can be used to predict the residual compressive strength of specimens protected by SiO2-ACM after a tunnel fire. The slope can indicate the protective effect of SiO2-ACM. The larger the slope,the better the protective effect. AsfBincreases, the slope decreases gradually, that is, as the fire time increases, the protective effect of SiO2-ACM is better.Considering that the strength of the specimen is gradually reduced, and when the strength is too low,the protective effect of SiO2-ACM is meaningless.According to Yan’s evaluation method[39], in order to ensure that the residual compressive strength ratio is 0.5 or higher, it is considered that SiO2-ACM fails after 2 hours.
3.5 Interfacial temperature of composite of SiO2-ACM and SCC
The temperature at the interface between SiO2-ACM and the concrete was recorded in the test. In the case of NC, the effective protection time of SiO2-ACM was 2 h, so the time interval of the interfacial temperature curve was set to 0-2 h, and the specific interfacial temperature curve is shown in Fig.8. It can be found that the interfacial temperature gradually increases as the fire time increases. Between 0 and 0.5 h, the temperature curve rises slowly. This time period is the heating process of the simulated tunnel fire test, and the maximum interfacial temperature is only about 100 ℃. After 0.5 h of fire time, the rise rate of interfacial temperature becomes faster, which is caused by the loose surface of SiO2-ACM and the decrease in fire resistance. When the fire time reaches 2 h, the interfacial temperature reaches about 520 ℃. At this time, micro cracks appear on the concrete surface, and the strength is only 60% of the original strength, which is consistent with the research results of Uysal and Tanyildizi[40].
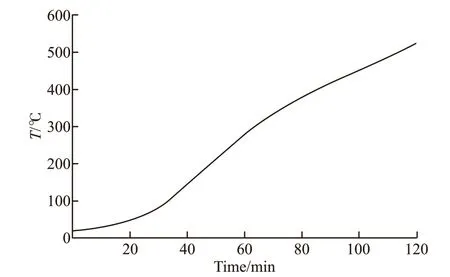
Fig.8 Interfacial temperature curve of composite SiO2-ACM and SCC
3.6 Microscopic analysis of SiO2-ACM
The microstructure of SiO2-ACM samples at the composite interface for different fire times in the case of NC were observed, which is showed in Fig.9.
The SEM image of cement paste in a nonthermally treated SiO2-ACM is showed in Fig.9(a). The surface of mortar paste is rough and there are many pores caused by the addition of AEA, which reduces the influence of heat conduction[41-43], thereby reducing the thermal conductivity. As shown in Fig.9(a2), the aerogel particles are embedded in the cement paste.But due to the hydrophobicity of aerogel, there is a gap between it and the hydrophilic cement paste, which is consist with the reference[42].
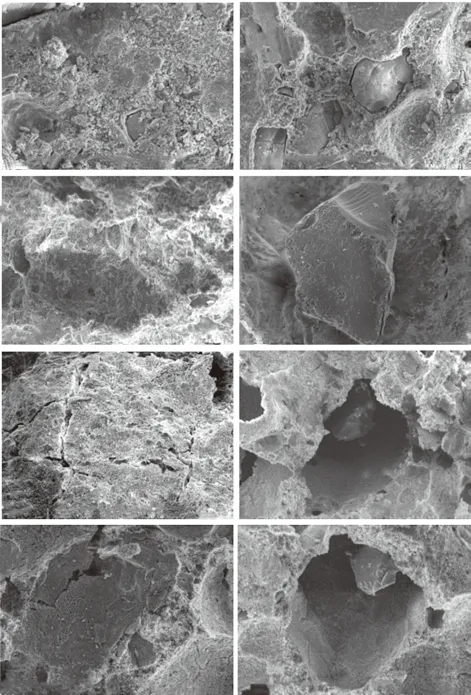
Fig.9 SEM images of cement paste (left) and SiO2 aerogel (right)under different fire time: (a) 0 h; (b) 0.5 h; (c) 2 h; (d) 2.5 h
Fig.9(b) displays the microstructure of SiO2-ACM after a simulated tunnel fire of 0.5 h. The structure of ACM is intact and there are no obvious cracks in ACM,which is consist with the results in Fig.2(a). While SiO2aerogel begins to slightly atrophy. With the time increasing, the aerogel atrophies seriously (Figs. 9(c)-(d)).
In addition, the mortar is partially distributed with wide cracks after 2 h of fire time. The bond between the aerogel particles and paste is weakened. This implies the failure of SiO2-ACM as fireproof coating, which is corresponding to the results mentioned above.
The SEM images analysis in this work shows that the internal structure of the non-heat treated SiO2-ACM specimen is complete, and the aerogel particles are well connected with the cement matrix. As the fire time increases, the aerogel particles shrink and the cement paste cracks, and the link between the two is weakened.This is the reason for the decline in the protective effect of SiO2-ACM.
4 Conclusions
a)As the fire time increases, the damages of mortar and SCC become more and more serious. But the SiO2-ACM shows an effectively protective effect on the anti-spalling of SCC.In order to meet the concrete damage level not exceeding 2, the effective protection time of SiO2-ACM in the case of NC can reach 2 hours,and in the case of WC, it is up to 1.5 hours. At this time, the surface of the mortar turns yellow, cracked and porous. From a microscopic point of view, the aerogel particles shrink and the cement paste cracks,and the link between the two is weakened. This is the reason for the decline in the protective effect of SiO2-ACM.
b)Due to the low thermal conductivity of SiO2-ACM, the residual compressive strength of Group A specimens is always higher than that of Group B specimens no matter what cooling method is used. And the residual compressive strength of SCC cooled by WC is lower than that of NC, regardless of the Group A or Group B specimens. As fire time increases, the adverse effects of WC are more significant. However,for the Group A specimens, the adverse effects of cooling water are lessened.
c)The effect of the cooling methods on the protective effect of SiO2-ACM is not significant. However,with the increase of fire time, the protective effect of SiO2-ACM is still gradually improved.
杂志排行
Journal of Wuhan University of Technology(Materials Science Edition)的其它文章
- Mechanical Properties and Microstructure of Al2O3/SiC Composite Ceramics for Solar Heat Absorber
- Effect of Friction Stir Welding on Bulk Metallic Glasses
- Effects of Lay-up Types of Out-of-autoclave Prepregs on Preparation Quality of L-shape Composite Laminates
- Hypereutectic Al-Si Matrix Composites Prepared by In Situ Fe2O3/Al System
- Preparation of Heavyweight Ultra-high Performance Concrete Using Barite Sand and Titanium-rich Heavy Slag Sand
- Effects of Shale and CaO Incorporation on Mechanical Properties and Autogenous Deformation of Early-age Concrete
