Mechanical Properties and Microstructure of Al2O3/SiC Composite Ceramics for Solar Heat Absorber
2021-09-15WUJianfengZHOUYangSUNMengkeXUXiaohongTIANKezhongYUJiaqi
WU Jianfeng , ZHOU Yang, SUN Mengke , XU Xiaohong , TIAN Kezhong , YU Jiaqi
(State Key Laboratory of Silicate Materials for Architectures, Wuhan University of Technology, Wuhan 430070, China)
Abstract: Al2O3/SiC composite ceramics were prepared from α-Al2O3 and SiC by a pressureless sinter method in this study. The effect of SiC contents on the mechanic properties, phase compositions and microstructure is studied. Experimental results show that the vickers hardness ,wear resistance and thermal conductivity of the samples increase with the increase in the SiC content, and the hardness of the sample reaches 16.22 GPa, and thermal conductivity of the sample reaches 25.41 W/(m.K) at room temperature when the SiC content is 20 wt%(B5) and the sintering temperature is at 1 640 ℃. Higher hardness means higher scour resistance, and it indicates that the B5 material is expected to be used for the solar heat absorber of third generation solar thermal generation. The results indicate the mechanism of improving mechanical properties of Al2O3/SiC composite ceramics: SiC plays a role in grain refinement that the grain of SiC inhibits the grain growth of Al2O3, while the addition of SiC changes the fracture mode from the intergranular to the intergranulartransgranular.
Key words: Al2O3/SiC composite ceramics; hardness; thermal conductivity; solar heat absorption material
1 Introduction
With the gradual depletion of traditional fossil energy and a series of environmental pollution problems caused by the large-scale utilization of fossil energy, increasing attention has been paid to the clean energy headed by solar energy. We innovative designed a kind of Al2O3/SiC composite heat-absorbing ceramics which are applied to the part 2 or part 3 of the volumetric solar receiver (Fig.1) of the third generation tower type solar thermal generation which should be less likely damaged by the scour of air and thermal conductivity of the materials at the same time. The most important thing is that the costs must be low. The heat absorbing ceramics will be scoured by the air at 1 100 ℃ with the speed of 5-8 m/s to exchange heat, and the pressure is about 1 MPa[1].
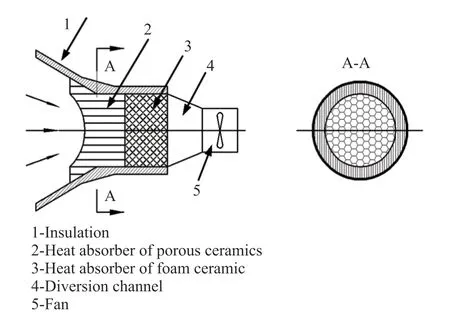
Fig.1 The principle diagram of opening volumetric receiver
Alumina ceramics are currently one of the most widely studied classes of solar heat absorption material, because of theirs excellent properties including mechanical strength, wear resistance,thermal and chemical stability and thermal conductivity. The relatively fragile greatly limits their application, therefore, the strengthening and toughening of Al2O3ceramics have become a core issue in alumina ceramic materials research.
Composite ceramics are one of the main means for improving the mechanical performance of ceramic materials. The toughening and strengthening mechanisms of the second phase in the composite ceramics include microcrack toughening, crack deflection, stress-induced transformation, pinning and bridges. SiC is a widely used engineering ceramic.It is believed that incorporation of SiC into Al2O3matrices will significantly enhance the mechanical properties of monolithic Al2O3[2,3]. Niihara[4]reported that nanocomposite ceramics employing nano-silicon carbide particles as a secondary phase exhibited high mechanical properties. Incorporation of 5 vol%-10 vol% submicron SiC particles into the Al2O3matrix increased the bending strength of the composites from 350 MPa to more than 1 GPa. Ortiz-Merino[5]and Limpichaipanit[6]reported that the addition of SiC could improve the wear resistance of Al2O3/SiC ceramics. Mechanical mixing or precipitation wrapping is a commonly used method for preparing composites using nano SiC and nano (or sub-micron) alumina as starting materials. In addition, high sintering temperature, external pressure and inert atmosphere protection may be required to produce dense Al2O3/SiC composite ceramics[7]. Wang[8]prepared Al2O3/SiC composite powder using a precipitation method. This study showed that the microstructure was changed by the addition of SiC, which increased the mechanical properties of the composites by 40%. Shi[9]and Parchovianský[10]prepared Al2O3/SiC ceramics using a hot pressing method. This improved the hardness and fracture toughness of the product while the bending strength was more than 600 MPa. However, hot pressing is relatively expensive and places restrictions on the product size. Another approach for fabricating Al2O3/SiC composites is pressureless sintering. The most important factors affecting pressureless sintering are the preparation of green body and the atmosphere during sintering[11]. In this instance, sintering aids (usually a small addition of MgO or other metal oxides) are often used to increase the density of the composites[12,13].
In view of the requirements of the third generation solar thermal generation with air as a working medium for the scour resistance of heat absorber materials, we intend to prepare scour resistant Al2O3based composite ceramics, the hardness is used to characterize the scour resistance of the material. To prepare a ceramic composite with better mechanical properties, a pressureless sintering method was employed to prepare Al2O3/SiC ceramics from the raw materials ofα-Al2O3and SiC (with MgO and TiO2as sintering aids). The effects of SiC content and sintering temperature on the final mechanical properties and microstructure of the ceramic composite materials were studied.
The properties (hardness, thermal conductivity,linear expansion coefficient,etc) that may affect the application of the sample were studied. The two kinds of raw materials(Al2O3and SiC) used in this paper are industrial grade, and the green bodies were pressureless sintered by the graphite-buried sintering method, and the costs are low, which made the study in this paper can be industrialized in the third generation tower type solar thermal generation.
2 Experimental
2.1 Preparation of Al2O3/SiC ceramics
The starting materials used in this work were alumina powder (particle size of 50 µm, Shandong Alumina Industry Group Co., Ltd., China) and SiC powder (particle size of 61 µm, Danjiangkou Hongyuan Silicon Carbide Co., Ltd., China) with MgO(AR, Sinopharm Chemical Reagent Co., Ltd, China)and TiO2(AR, Sinopharm Chemical Reagent Co.,Ltd, China) added as sintering aids. Table 1 shows the chemical compositions of the raw materials. Table 2 shows the formula compositions of six designed samples with different mass ratios of Al2O3and SiC.
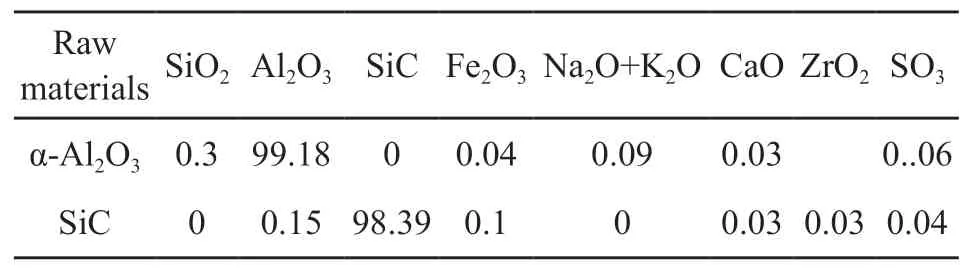
Table1 Chemical compositions of raw materials/wt/%
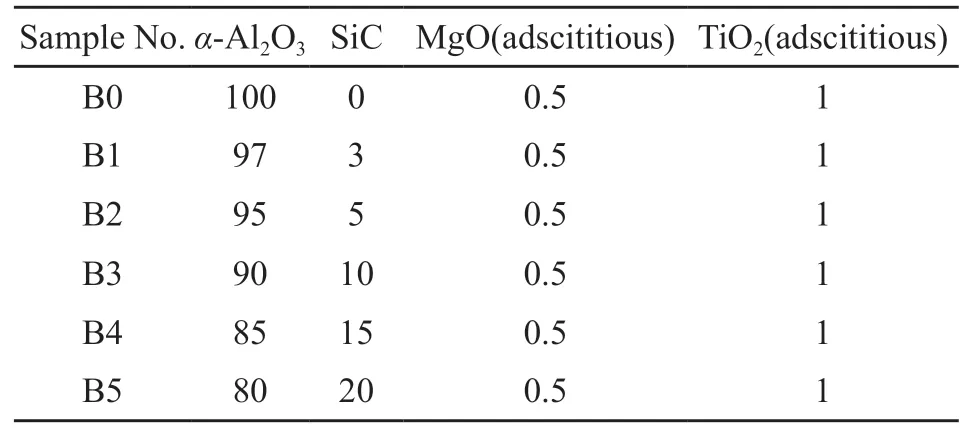
Table2 Batch formulas of samples/(wt/%)
The starting materials were ball-milled in deionized water for 1 h, and then dried. Next, 8 wt%-10 wt% polyvinyl alcohol solution (PVA, 5 wt%)was used as a binder to hold the powders together for pressing. The samples were placed into molds and uniaxially pressed at 60 MPa to prepare the rectangular bars (35 mm×5 mm×5 mm, 3 g) and at 60 MPa to form cylindrical pellets (φ30 mm, 8 g). After being dried at 100 ℃ for 24 h in an electric oven, the green bodies were pressureless sintered by the graphite-buried sintering method (Fig.2) from 1 500 ℃ to 1 640 ℃ in a seggar full of graphite for 2 h. The heating rate of the molybdenum disilicide furnace was 5 ℃/min up to 1 000 ℃ with holding period of 30 min every 200℃, then 3 ℃/min up to the sintering temperature with holding period of 60 min every 100 ℃.
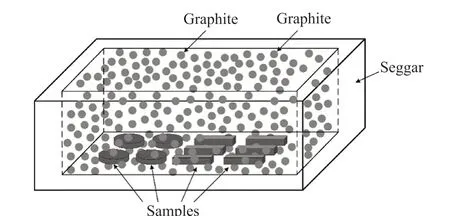
Fig.2 Systematic representation of pressureless graphite-buried sintering
2.2 Characterization
The water absorption (Wa), apparent porosity (Pa)and bulk density (D) of the samples were measured using the rectangular bars. The test approach was that using an AUY120 electronic analytical balance(Japanese Shimazu) through a static weighing method based on the Archimedes principle, and determination of the grain size distributions was accomplished using Nanomeasure software. The bending strength of the samples was determined by the three-point bending test with a span of 28 mm and at a loading rate of 1 mm/min using a computer-controlled electronic universal testing machine (Model RGM-4100, Shenzhen Reger Instrument Co., Ltd., China). The hardness of the samples was determined using a digital microsclerometer (Model HVS-1000, Shanghai Taiming Optical Instrument Co., Ltd., China) with a load of 4.9 N for 10 s. The wear testing was carried out on friction and wear tester (Model HT-600, Lanzhou Zhongke Kaihua Science and Development Co., Ltd., China)under the following condition: 2 mm diameter GCr15 steel grinding ball (hardness of 58 HRC) rotated against the ceramic sample, rotation speed of 1 000 rpm with a radius of 5 mm, constant load of 1 141 g. The tests were conducted at room temperature for 60 min. And the weight loss during the test (Δm) was determined by weighing the samples before and after the test. The wear rate (WR) (mm3/(N•m) ) was defined as:

where D (g/cm3) is the density of the sample,F(N)is the applied load andL(m) is the length of the wear track. The phase compositions of the samples were analyzed by X-Ray diffraction (XRD) (Model D/max-RA, Rigaku Industrial Co., Japan) with the following conditions: Cu Kα radiation, scanning speed of 0.02 °/s,operating voltage of 40 kV, operating current of 30 mA and the scan angle from 5° to 80°. The morphologies of the fractured surface of the samples were observed by scanning electron microscope (SEM) (Model JSMIT300, JEOL Ltd., Japan) operating at 20 kV. The morphologies of the worn surface of the samples were observed by field emission scanning electron microscopy (FE-SEM) (Model Quanta 250 FEG, FEI,USA).
3 Results and discussion
3.1 Physical properties of as-prepared composites
Fig.3 shows the water absorption (Wa), apparent porosity (Pa) and bulk density (D) of the samples sintered at different temperatures. It is seen that the water absorption and porosity are decreased, while the bulk density increases as the sintering temperature is increased. The bulk density of the samples increase significantly in the temperature range from 1 540 to 1 580 ℃, indicating rapid densification of the samples over this temperature range. The water absorption of all the formulae is below 0.5% except for formula B5.The bulk densities of samples B4 and B5 are lower than the monolithic Al2O3(sample B0) and when SiC content is ≥10% (sample B3-B5), the bulk densities of the samples decrease with the increase of SiC content.These results are mainly because the incorporated SiC particles block the movement of the Al2O3grain boundaries and suppress the densification process resulting in an increase in the porosity of the samples.Another reason for the decreased bulk density is the added SiC of low density. The higher densities of samples B1 and B2 are attributed to the small amount of added SiC particles that fill the gaps between Al2O3matrix particles, promoting densification. On the other hand, the SiC particles inhibit Al2O3grain growth,which promotes the grain refinement to form a fine uniform structure resulting in an increase in the density of the composite samples[14,15]. Near full densities in the samples can be achieved when they are sintered at higher temperature or under higher pressure.
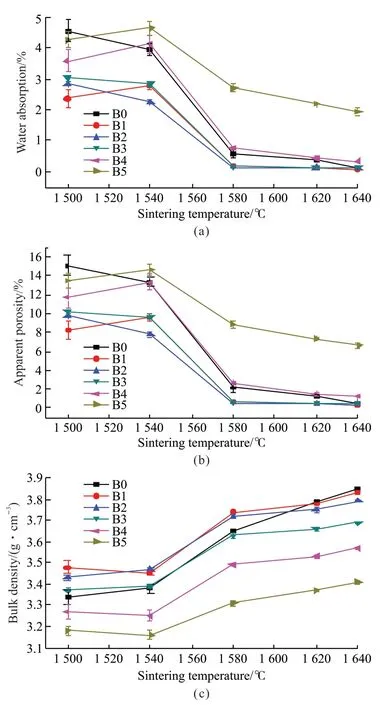
Fig.3 Relationship between Wa(a), Pa(b) and D(c) and sintering temperature of the samples
3.2 Mechanical properties
The best sintering temperature of different formulations is different. According to the test results ofWa(%),Pa(%),D(g/cm3) andσ(MPA), we choose the samples sintered at the optimum temperature for comparison. In this work, the bending strength is used to characterize the mechanical properties, while surface hardness and wear rate of the samples are used to characterize the scour resistance of various solar heat absorption material. The relationships between hardness, bending strength and SiC content are shown in Fig.4.
As shown in Fig.4, the Vickers hardness of the samples increase with the increase in SiC content,the Vickers hardness of sample B5 (containing 20 wt% SiC) is 16.22 GPa, whereas the hardness of the monolithic Al2O3is only 13.61 GPa, which is consistent with the results reported by many researchers[9,10,16].The improvement of hardness is attributed to the incorporation of a hard secondary phase (SiC) and the refinement of the grains in the composites.
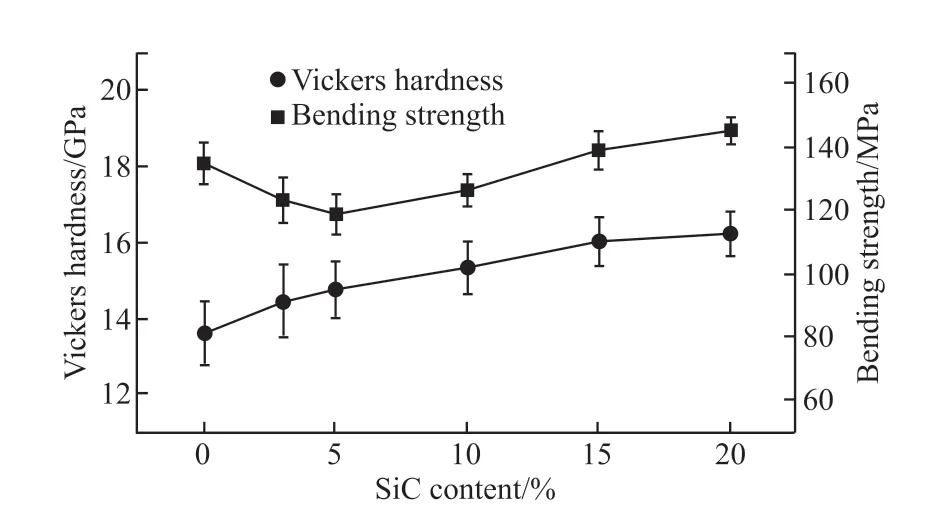
Fig.4 Vickers hardness and bending strength of the samples as functions of SiC content
According to the Griffith theory, there are two factors responsible for improving the bending strength of ceramic materials: the reduction in the size of inherent flaws or micro-cracks in the ceramic material can increase the energy barrier to crack propagation[17].The size of micro-cracks in ceramic is usually related to the grain size of the ceramic material. In the case of the composite ceramics in this work, with the use of SiC, the Al2O3matrix grains are refined and the size of micro-cracks is reduced, leading to an improvement in the overall strength of the material. However, as the change of the fracture mode and the refinement of the grains are not obvious when the content of SiC is low,at the same time, the difference of thermal expansion coefficients of Al2O3(8.8×10-6℃-1) and SiC (4.7×10-6℃-1) is large, the samples added SiC(B1-B5)are more prone to defects during the sintering and cooling process than the samples without SiC (B0),therefore, when the content of SiC is low (B1, B2),the bending strength of the samples decreases with the increase of SiC content. The bending strength of the samples with more SiC content (B3 - B5) increases with the increase of SiC content because the change of the fracture mode and the refinement of the grains caused by SiC are more obvious.
Crack deflection of the SiC particles is also one of the reasons for strength improvement in the samples.When a propagating crack meet a SiC particle in its path, the crack is deflected because the SiC particles pin the grain boundary causing deflection and crack bridging. This process consumes some of the fracture energy. When the cracks extend around the SiC particles and interact with the micro-cracks around the SiC particles, further crack propagation and breaking are limited, which improves the macro crack resistance of the material.
Generally, a material with high hardness can be worn at a lower rate, and when it is used in the solar heat absorber of third generation solar thermal generation,it is less likely to be damaged by the scour of air. The wear mechanism of ceramic material is dependent on operating parameters of the wear test, material intrinsic properties and microstructure or defects[18]. The main factors affecting the wear resistance of ceramics are hardness and fracture toughness microstructure of the samples. Fig.5 shows the relationship between the wear rate and SiC content of the samples. The addition of SiC results in a reduction in wear rate compared with the monolithic sample, and the wear rate of the samples decreases with the increase in SiC content, and the composite sample with the highest hardness also has the lowest wear rate which is 1.83×10-5mm3/N•m.
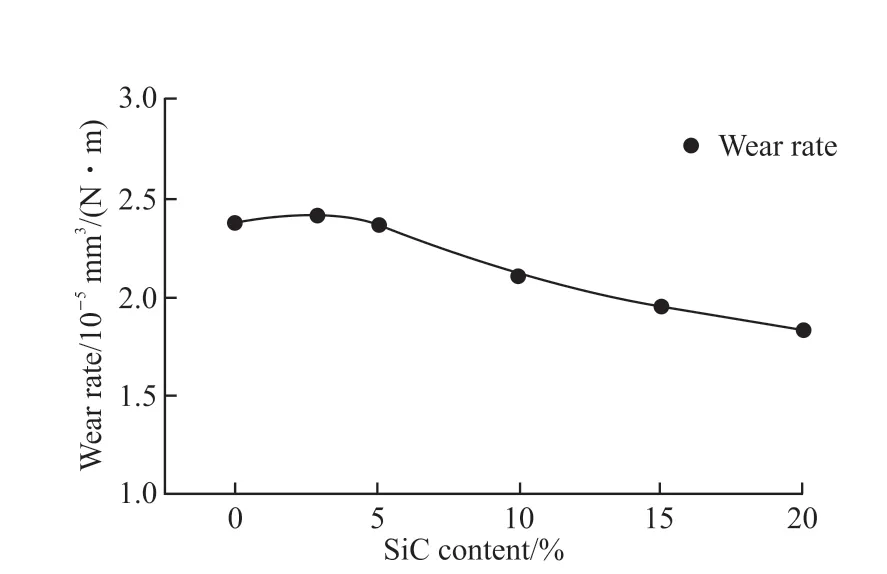
Fig.5 Relationship between the wear rate and SiC content of the samples
The main wear mechanism for the monolithic alumina is intergranular fracture and grain pull-out,as observed in Fig.6. When adds SiC particles, the addition of SiC changes the fracture mode from the intergranular to the intergranular-transgranular (shown in Fig.8) and it has a great impact on the improvement of the wear resistance because of the geometrical consequence that the grain pull-out through brittle fracture will be smaller in size[5,6]. Moreover, the thermal residual stress in the composites is due to the mismatch in the thermal expansion coefficients of Al2O3(8.8×10-6℃-1) and SiC (4.7×10-6℃-1)[19]will reduce grain pull-out during wear process[2].
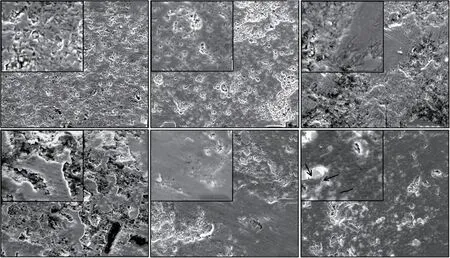
Fig.6 SEM micrographs of worn surface of samples sintered at different temperatures: (a)B0(1 640 ℃); (b)B1(1 640 ℃); (c)B2(1 620 ℃);(d)B3(1 580 ℃); (e)B4(1 640 ℃); (f)B5(1 640 ℃)
3.3 Phase constituents and microstructure analysis
The phase constituents of the samples from each formulation that exhibited the highest bending strength were examined by XRD analysis, as shown in Fig.7. As these data shows, the phases of samples B2-B5 consist corundum and silicon carbide with small amounts of graphite. SiC is not detected in sample B1, because the SiC content in this sample is lower than the detection limit of XRD instrument. As shown in Fig.8, the grain size of the Al2O3decreases as the SiC content increases.This proves that the Al2O3grain refinement helps to enhance the strength of the ceramic composite.
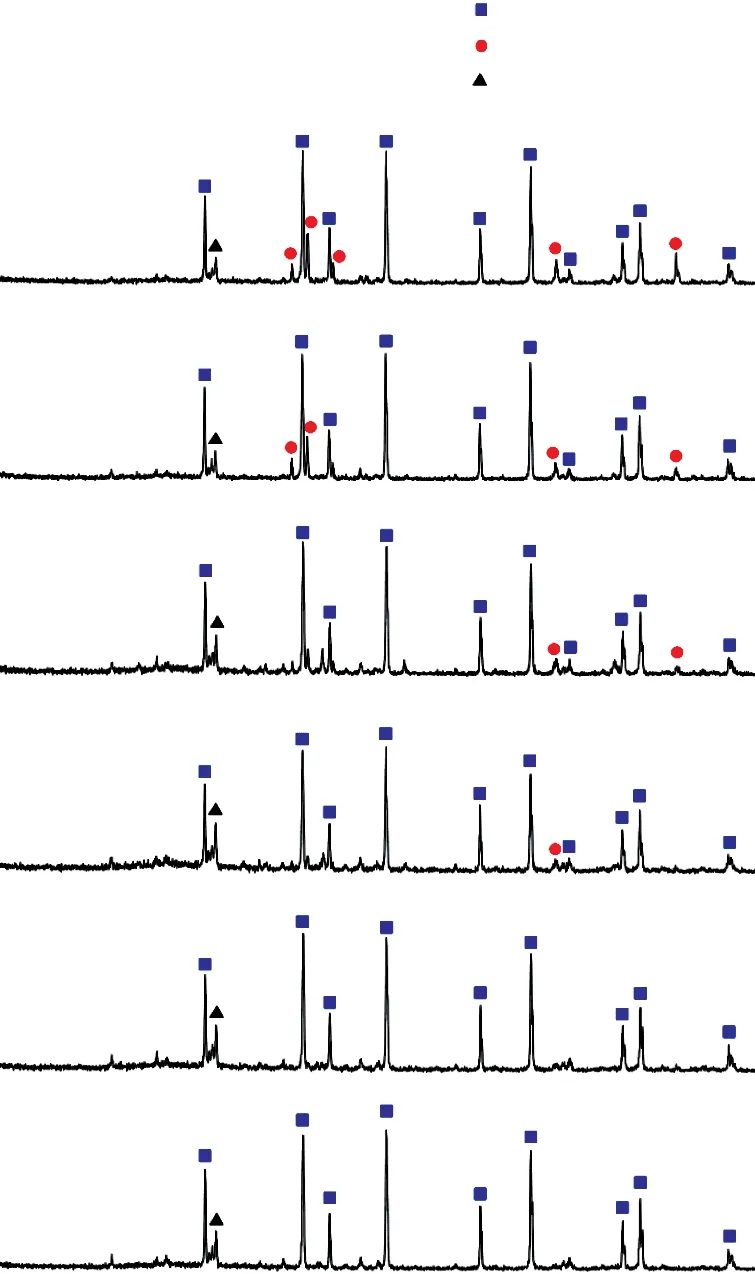
Fig.7 XRD patterns of samples sintered at different temperature
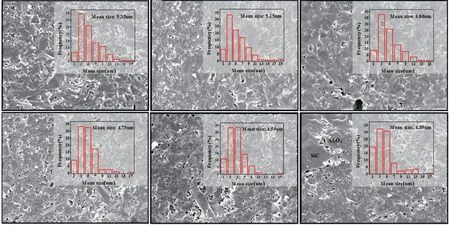
Fig.8 SEM micrographs of fractured surface of samples:(a)B0(1 640 ℃); (b)B1(1 640 ℃); (c)B2(1 620 ℃); (d)B3(1 580 ℃); (e)B4(1 640℃); (f)B5(1 640 ℃)
To better understand the relationship between the ceramic microstructure and mechanical properties of the samples, SEM analysis was performed to define their microstructure together with elemental mapping.The microstructure of the fractured surfaces of the samples sintered at various temperatures is shown in Fig.9. As shown, the samples appear to have a compact structure with the Al2O3and SiC grains closely bonded.In these micrographs, the plate-shaped grains are SiC,while the square-shaped grains areα-Al2O3. Abnormal grains growth of ceramic matrix is observed in sample B0 (Fig.9(a)), while the ceramic composite grains tend to be uniform and refined. The mean grain size of the Al2O3decreases with increasing SiC content,which is an indication of the inhibitory effect of SiC on the growth of Al2O3grains. The grain refinement strengthening is one of the reasons for the improved strength of the material.
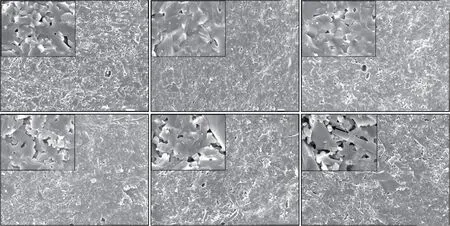
Fig.9 SEM micrographs of fractured surface of samples sintered at different temperatures: (a)B0(1 640 ℃); (b)B1(1 640 ℃); (c)B2(1 620℃); (d)B3(1 580 ℃); (e)B4(1 640 ℃); (f)B5(1 640 ℃)
Fig.10 shows the elements distribution of the fractured surface of sample B5 sintered at 1 640℃. The elemental mapping results show that the microstructure of the sample is comprised of Al2O3and SiC as indicate by the Al-rich and Si-rich areas in the images of the Al and Si signals. There is also a small amount of Al and O found at the edge of the SiC particle as indicated by the arrows in the images of Al, Si and O signals. This is an indication that the SiCparticle is slightly oxidized and the SiO2generated by this oxidization reacts with the Al2O3to form a small amount of mullite. This effect causes tight bonding between the individual particles, which also improves the strength of the sample. Additionally, the image for the Mg signal shows a Mg-rich area in the sample,together with Al and O in the same area where the Si is not contained. This result indicates that the Mgrich area is magnesia alumina spinel (MgAl2O4), since only 0.5 wt% MgO is added, the amount of MgAl2O4formed is too low to be detected by XRD. EDS (energy dispersive spectroscopy) analysis results of the three selected spots in Fig.10 (Fig.11 and Table 3) confirm this rationale. With the MgAl2O4pinned at the grain boundaries of the Al2O3, the grain boundary movement is inhibited, which promotes grain refinement in the Al2O3matrix, yet another reason for improvement in strength of the composite ceramic.
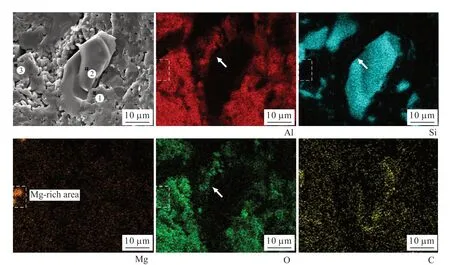
Fig.10 Secondary electron image and elements distribution of the fractured surface of sample B5 sintered at 1 640 ℃
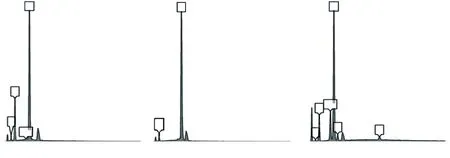
Fig.11 EDS patterns of the three selected spots in Fig.10
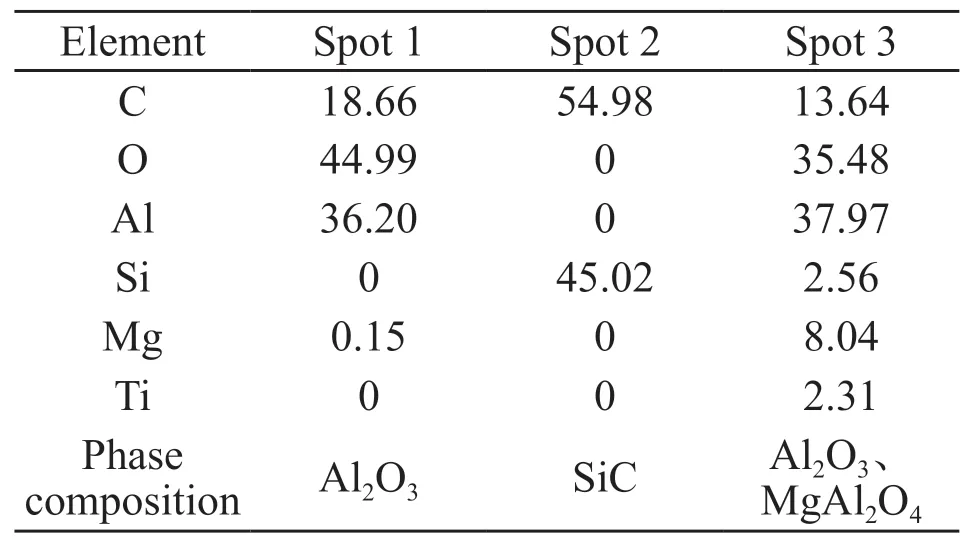
Table3 The element composition analysis at the given point in Fig.10 /wt%
With respect to the fracture modes of the sample,the grain boundary of the Al2O3grains can be seen clearly in the fracture surface morphology of sample B0, which is a clear indication of a typical intergranular fracture. Since the thermal expansion coefficient of the Al2O3grains is anisotropic, residual stress is formed at the interface during cooling process, so that when the cracks grow, the interface is subjected to residual tensile stress and the fracture mode is primarily intergranular fracture. When SiC particles are added to the alumina, transgranular fracture occurs in the samples together with intergranular fracture. This so called intergranular-transgranular mixed fracture, is conducive to improving the mechanical properties of the material. The intergranular fracture can be explained by grain boundary strengthening and matrix weakening due to the thermal residual stress during the cooling process[4,19,20]. Since the thermal expansion coefficient of Al2O3is significantly larger than that of SiC, radial compressive forces and tangential tensile forces will develop around the SiC particles. When the Al2O3grain boundary is perpendicular to the radial direction,the grain boundary is strengthened by the presence of compressive stress. But when the grain boundary is parallel to the radial direction, the grain boundary will be weakened by the presence of tensile stress. The radial compressive forces increase with the increase of SiC content, which leads to more transgranular fracture in the samples[9,21]. In addition, the change of fracture mode also reduces the wear rate of the sample.In pure alumina ceramics, the fracture mode is mainly intergranular, most of the cracks will propagate along the grain boundary, but the propagation range may not be limited to one grain, so the size of grain spalling is large, and the wear rate of the sample is high (as shown in Fig.12). With the adding of SiC, the fracture mode of composite ceramics changes from intergranular fracture to transgranular fracture. Compared with pure alumina ceramics, there are more cracks propagating into the crystal. The crack ends at the wear surface, which reduces the crack propagation range. The grain spalling size is smaller, and the wear rate of samples is lower.
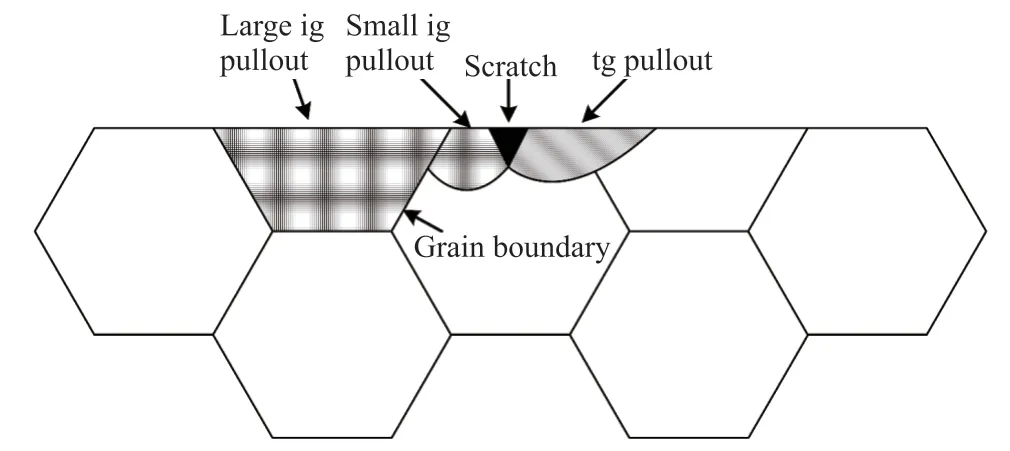
Fig.12 The schematic of effect of SiC on the change of fracture mode
The thermal conductivity of B0(1 640 ℃), B1(1 640 ℃), B2(1 620℃), B3(1 580 ℃) , B4(1 640 ℃)and B5(1 640 ℃) was tested at room temperature. The results are shown in Fig.13. Addition of SiC could significantly improve the thermal conductivity of the samples. The thermal conductivity of B5 is 25.41 W/(m·K) at room temperature, while that of B0 is only 18.12 W/(m.K). As shown in Fig.13, the increase rate of thermal conductivity of the samples decrease with increasing SiC content. Besides the thermal conductivity of each component, the density of samples also has a significant effect on the thermal conductivity.Barea[22]prepared composite ceramics with different content of SiC by hot pressing. The relative density of the composite ceramics is over 97%. The thermal conductivity is 32-40 W/(m·K) at room temperature,and the thermal conductivity of pure Al2O3is 28-30 W/(m·K). In this study, the apparent porosity of B1-B5 are 0.35%, 0.48%, 0.59%, 1.19% and 6.61%. At the same time, there are some closed pores in the samples, which will impede the improvement of thermal conductivity of the samples. Therefore, with the increase of SiC content, the increase rate of thermal conductivity of the sample decreases gradually. The thermal conductivity of the sample reaches the maximum when the SiC content is 20%.
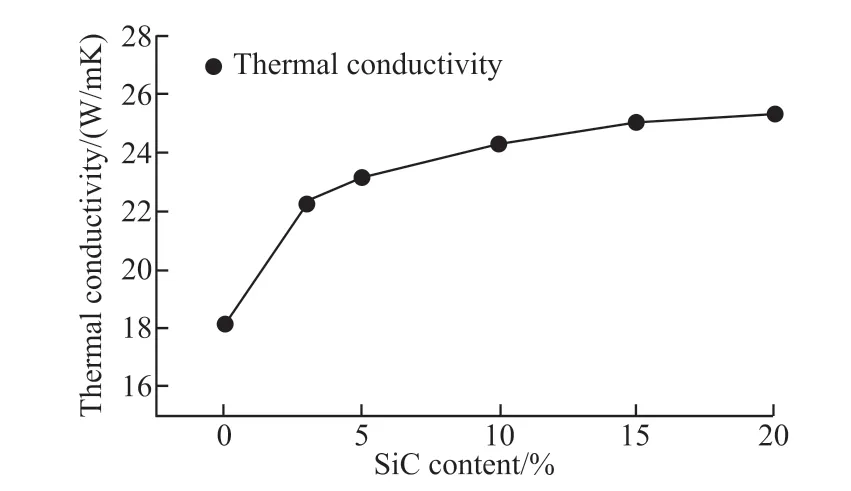
Fig.13 Relationship between the thermal conductivity and SiC content of the samples
The linear expansion coefficient of multiphase ceramics will affect the size design when the sample is applied to the volumetric solar receiver of the third generation tower type solar thermal generation. The coefficient of linear expansion of SiC is smaller than that of Al2O3, and the coefficient of linear expansion of the sample satisfy additivity principle, so adding SiC will reduce the coefficient of linear expansion of the sample. B5 sintered at 1 640 ℃ exhibits the best mechanical properties and the linear expansion coefficient is 7.52×10-6/K at 1 000 ℃. The results are shown in Fig.14.
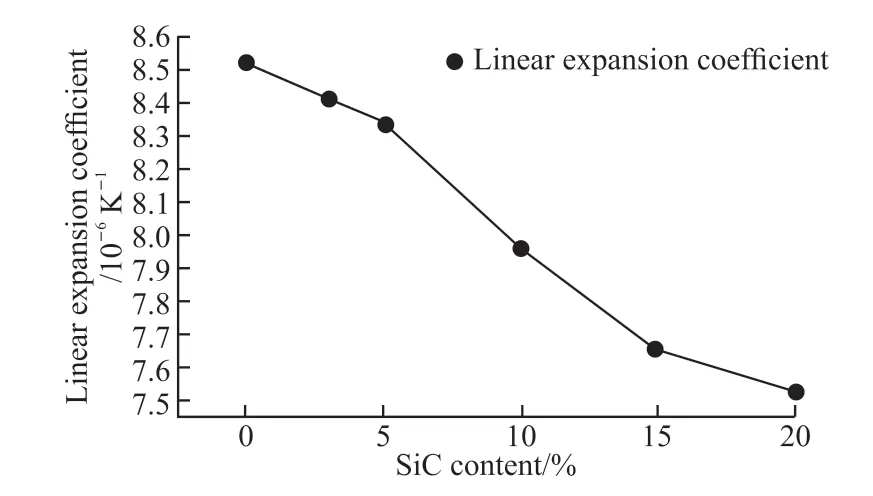
Fig.14 Relationship between linear expansion coefficient and SiC content of the samples
4 Conclusions
Al2O3/SiC composite ceramics were prepared using a pressureless sintering process employingα-Al2O3powders and SiC powders as starting materials. The Al2O3/SiC composite containing 20 wt% SiC(B5) sintered at 1 640 ℃ exhibits the best mechanical properties. The test results are as follows:the Vickers hardness is 16.22 GPa, and the thermal conductivity is 25.41 W/(m.K) at room temperature,and the linear expansion coefficient is 7.52×10-6/K at 1 000 ℃.The sample of B5 is significantly improved over that of the monolithic Al2O3, and it will meet the high requirement of solar heat absorber of third generation solar thermal generation for air scour resistance, therefore, B5 formula can be used for Al2O3/SiC solar heat absorption material. Adding SiC particles is found to be an effective way of improving the strength and hardness of alumina ceramics. The resulting improvement in mechanical properties of the composite ceramic is attributed primarily to matrix grain refinement, crack deflection of the second phase particles and the intergranular-transgranular mixed fracture mode that result from the addition of SiC.
杂志排行
Journal of Wuhan University of Technology(Materials Science Edition)的其它文章
- Effect of Friction Stir Welding on Bulk Metallic Glasses
- Effects of Lay-up Types of Out-of-autoclave Prepregs on Preparation Quality of L-shape Composite Laminates
- Hypereutectic Al-Si Matrix Composites Prepared by In Situ Fe2O3/Al System
- Preparation of Heavyweight Ultra-high Performance Concrete Using Barite Sand and Titanium-rich Heavy Slag Sand
- Effects of Shale and CaO Incorporation on Mechanical Properties and Autogenous Deformation of Early-age Concrete
- Diffusion and Regeneration Mechanism of Waste Composite Oils Rejuvenator in Aged Asphalt
