Effects of radiation and chemotherapy on adipose stem cells:Implications for use in fat grafting in cancer patients
2021-09-03RebeccaPlatoffMiguelVillalobosAshleighRappHagamanYuanLiuMarthaMatthewsMichaelDiSantoJeffreyCarpenterPingZhang
Rebecca Platoff,Miguel A Villalobos,Ashleigh Rapp Hagaman,Yuan Liu,Martha Matthews,Michael E DiSanto,Jeffrey P Carpenter,Ping Zhang
Rebecca Platoff,Miguel A Villalobos,Ashleigh Rapp Hagaman,Yuan Liu,Martha Matthews,Jeffrey P Carpenter,Ping Zhang,Department of Surgery,Cooper University Health Care,Camden,NJ 08103,United States
Yuan Liu,Martha Matthews,Jeffrey P Carpenter,Ping Zhang,Department of Surgery,Cooper Medical School of Rowan University,Camden,NJ 08103,United States
Michael E DiSanto,Department of Biomedical Sciences,Cooper Medical School of Rowan University,Camden,NJ 08103,United States
Abstract Autologous fat transplantation is a versatile tool in reconstructive surgery.Adipose-derived stem cells(ASCs)increase survival of fat grafts and thus are increasingly used for breast reconstruction in breast cancer patients.However,radiation and/or chemotherapy have been proposed to inhibit soft tissue regeneration in wound healing thus suggesting alteration in stem cell pathways.Therefore,elucidating effects of radiation and chemotherapy on ASCs is critical if one desires to enhance the survival of fat grafts in patients.This review outlines our work evaluating the function and recoverability of ASCs from radiation or chemotherapy patients,focusing specifically on their availability as a source of autologous stem cells for fat grafting and breast reconstruction in cancer patients.Even though evidence suggests radiation and chemotherapy negatively influence ASCs at the cellular level,the efficiency of the isolation and differentiation capacity did not appear influenced in patients after receiving chemotherapy treatment,although fat from radiated patients exhibited significantly altered ASC differentiation into endothelial-like cells.Further,the in vitro growth rates of patient’s ASCs do not differ significantly before or after treatment.Taken together,these studies suggest ASCs as an important new tool for grafting and reconstruction even when radiation and chemotherapy treatment are involved.
Key Words:Fat grafting;Breast reconstruction;Stem cells;Breast cancer;Radiation;Chemotherapy
INTRODUCTION
Autologous fat transplantation is a versatile tool in reconstructive surgery.As a supplement to whole-fat grafts,adipose-derived stem cells(ASCs)can exhibit a positive effect on wound healing and increase survival rates as evidenced by their increasing use in plastic and reconstructive procedures[1-4].ASCs have been used for nearly 20 years in reconstructive surgery to help with soft tissue healing by improving tissue vascularity,elasticity,and healing capacity.At the same time,ASCs help to minimize inflammation,and overall,lead to tissue self-renewal,and differentiation into specialized cell types[5-7].In addition,ASCs,as an abundant source of adult mesenchymal stem cells,have been shown to secrete several growth factors that play a key role in promoting neovascularization which contributes to adipose tissue regeneration[7,8].In addition,ASCs secrete multiple anti-apoptotic growth factors to attenuate adipocyte loss,which ultimately enhances the viability of fat grafts[9].
Recently,clinical studies concluded that autologous fat grafting with a stromal vascular fraction,a rich source of ASCs,improves clinical outcomes in breast augmentation and facial lipoatrophy patients[3,10-12].This is due to ASCs’ versatility to develop into a variety of mature tissues as well as their great capacity for proliferation[13].The ability of ASCs to produce adipose tissue regeneration has been shown to enhance volume and improve cosmesis and symmetry in breast reconstruction[6].The use of ASCs is also thought to improve the survival of fat grafting because it can boost angiogenesisviaASC’s differentiation into endothelial cells(EC)and vascular endothelial growth factor(VEGF)secretion[14].Moreover,ASCs have a longer lifespan in culture than bone marrow stromal cells before becoming senescent which allows greater flexibility in the lab environment[15,16].The beneficial properties of ASCs described above highlight the potential importance of ASCs in the maintenance of transplanted tissue volume,an important consideration in adipose tissue engineering[8].
In recent years,the use of ASCs within the fat graft is considered particularly useful and has been increasingly employed following breast-conserving cancer surgery.Currently,approximately 93000 breast reconstructions are performed each year in the United States,supporting the clinical significance of this work[12,17,18].Breast reconstruction with fat grafting after surgery is a major area that is enhanced by the use of autologous ASCs following surgery for breast cancer[5,19].However,approximately two-thirds of all cancer patients will undergo radiation and/or chemotherapy as part of their treatment plan.In this context,however,one must consider that radiation and chemotherapy in cancer patients might limit stem cell cellular functions important for soft tissue wound healing.If this possibility is real,then the isolation,banking,and use of autologous ASCs may be critical in order to minimize wound adverse events.Based upon this reasoning,the characterization and usage of ASCs for clinical applications in cancer patients has become a recent focus of research.
Although ASC-assisted autologous fat transfer approaches for breast reconstruction have been shown to enhance graft survival and local angiogenesis,examination of the ability of ASCs to retain their inherent cellular functions and the efficiency of recovering these stem cells in the tumor/cancer treatment arena is lacking.To date,most studies have shown that radiation and chemotherapy have a cytotoxic effect on the stem cell’s proliferative and differentiation potential[20-23].For clinical translation to patients,a better understanding of cancer treatments on ASCs improvement of fat graft survival in actual patients is sorely needed.
This review describes our work defining two critical characteristics of stem cells isolated from human adipose tissue:(1)Availability in cancer patients after receiving radiation or chemotherapy most likely to appear to require a viable source of autologous stem cells;and(2)Ability to retain their great function and recovery capacity post-radiation/chemotherapy.Within this substructure,we summarize and highlight the practical usefulness of these cells in fat grafting and reconstructive procedures in cancer patients undergoing radiation and chemotherapy.
EFFECT OF RADIATION ON ADIPOSE STEM CELLS
Radiation therapy is a routine treatment for patients with cancer,either before or after surgical resection.However,in patients receiving radiation,there is a local injury to the surrounding soft tissues and when the surgical insult is added to the radiated soft tissue,the result is a high rate of wound complications[24,25].An important complication of radiation therapy in breast cancer patients is iatrogenic damage of normal breast tissue that results in chronic,painful,and disfiguring wounds[26,27].Additionally,when tissue damage does occur,the reconstructive surgeon is faced with the challenge of restoring normal tissue health or being left with poor survival[28].To restore the damaged tissue a better understanding is needed concerning whether stem cells contribute to wound healing and the degree to which radiation might hinder the ability of stem cells to participate in tissue recovery.It is well known that ASCs can promote neovascularization and healing of damaged tissues in tissue engineering applications[29,30]yet applicability in the “cancer” population has been questioned as it is likely that cancer treatment and co-morbidity adversely affect many of the cell populations.Thus,it is beneficial to determine the influence of radiation therapy on ASCs isolation and cellular functions that are considered clinically important to improve the radiation-induced wound healing in favor of positive outcomes of tissue reconstruction and repair in patients.Starting from this background,we have studied and evaluated the deficiency that radiated breast tissues have in the number and functional abilities of ASCs with emphasis on endothelial differentiation in breast cancer patients.
Availability and growth rate of ASCs in patients treated with radiation
Many initial studies that sought to evaluate fat as a source of stem cells examined liposuction specimens obtained from young,healthy plastic surgery patients[31-34].However,the effects of radiation on ASC cells remain largely unknown.Several studies have shown that the growth rates of ASCs are decreased and the number of apoptotic cells is remarkably increased after irradiation[19,35-37].However,little is known about the cell viability of ASCs from patients after exposure to radiation therapy.Also,a cell type employed in tissue engineering must be readily and abundantly available in the specific target patient population(e.g.,breast cancer patients)that it is intended for use in[38-40].Therefore,we have studied the availability of ASCs in breast cancer patients undergoing elective radiation surgical procedures(full manuscript in preparation).Each patient that had previously received radiation therapy in one breast,but not in the contralateral breast for cancer treatment,donated approximately 25-50 g of fat from each breast to evaluate any deficiency that the radiated tissues had in ASCs numbers and functions.
To determine the influence of radiation on stem cells isolation,ASCs were isolated from normal and radiated breast tissue specimens from the same patient.The major finding of our study was that radiation did not appear to significantly influence the number of stem cells harvested as there was no difference in the number of ASCs obtained between the radiated and non-irradiated breasts.Based on the number of cells obtained one week after harvest,we did not observe any significant change in thein vitrogrowth rate between radiated and non-radiated ASCs in terms of the doubling time.This result suggests that ASCs can be isolated from the patients after radiation exposure and used for fat grafting and reconstruction purposes in patients postradiation treatment(Figure 1A).
Radiation effect on ASCs endothelial differentiation
The role of stem cells in the replacement of senescent or deteriorated cells of the human body is defined by their capacity for self-renewal and multilineage differentiation.Recently,cell-assisted lipotransfer studies concluded that ASCs stimulate angiogenesis and improve both graft revascularization and survival[41-43].Indeed the ability to acquire characteristics of cells resident within neovascularization,as the result of differentiation into EC,represents another important aspect of the ASC’s usefulness in fat grafting and healing of damaged tissues.
ASCs can differentiate into a wide range of cell types,including EC and also ASCs have been shown to express higher levels of the angiogenic factors such as VEGF,hepatocyte growth factor(HGF),and insulin-like growth factor(IGF),which enhance their involvement in angiogenesis that promotes graft retention[27,43,44].Furthermore,a sufficient density of mesenchymal stem cells and proper differentiation is necessary to improve the viability of the fat grafts and promote wound healing.To further understand the use of autologous ASCs in reconstructive procedures following radiation,we have examined the effects of radiation on angiogenic capabilities of ASCs obtained from breast tissue specimens in patients after exposure to the radiation treatment,and especially their ability to differentiate into a functional EC-phenotype which is known to participate in the regeneration of capillary networks.
In these experiments,the functions of ASCs from the radiated breast tissue specimens with an emphasis on endothelial differentiation,including expression of endothelial-specific markers PECAM-1(CD31),von Willebrand factor(vWF),and endothelial nitric oxide synthase(eNOS)were compared to the ASCs from the nonradiated side of the breast.After three weeks of culture in EC-differentiation media,we found there was a very large and significant difference in EC differentiation capacity in the radiated ASCs based on the decreased expression of all three above endothelial markers between radiated and non-radiated breast tissue specimens using real-time PCR analysis(Figure 1B).Taken together,our studies characterized the ASC cell viability and differentiation capacity in response to irradiation and suggest that radiation therapy has deleterious effects on ASC differentiation capacity towards EC which may represent the root cause of chronic wounding and poor fat graft survival in patients,i.e.,radiation appears to damage the ability of ASCs to repopulate the microvasculature with the correct cell types.This finding requires further study with a larger number of patients examining whether ASCs have the function recovery potential of pro-angiogenic and pro-adipogenic phenotypes following radiation treatment after fat transplantation.
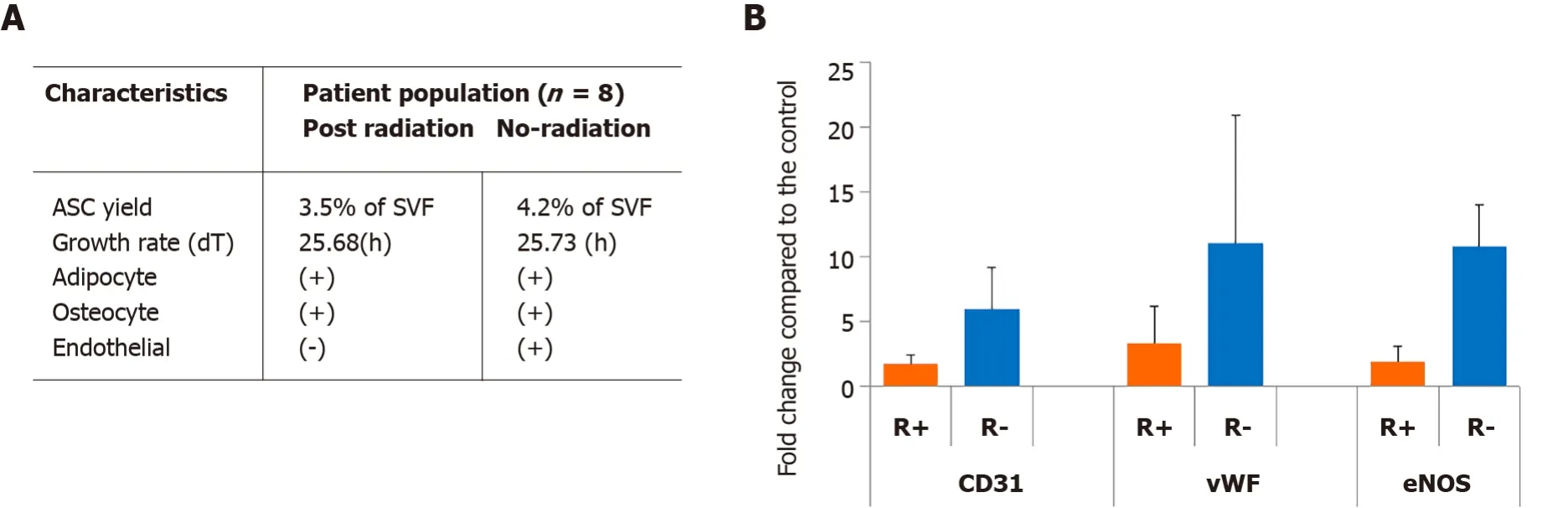
Figure 1 Availability of adipose-derived stem cells in patients after radiation exposure.A:The number of stem cells harvested and growth rates did not appear to be affected by radiation;B:Decreased expression of endothelial markers of CD31,von Willebrand factor,and endothelial nitric oxide synthase between the adipose-derived stem cells from radiated(orange)and non-radiated(blue)breast tissue specimens.vWF:von Willebrand factor;eNOS:Endothelial nitric oxide synthase;ASC:Adipose-derived stem cell.
EFFECT OF CHEMOTHERAPY ON ADIPOSE STEM CELLS
To date,many of the original studies have mainly focused on cytotoxic damage to mesenchymal stem cells resulting from chemotherapy with little attention given to the recovery of the stem cells’ viability and cellular function capability after exposure to chemotherapeutic drug treatment[22,23,45,46].Thein vitrohuman data from our lab and others demonstrate that direct exposure to chemotherapeutic agents decreases the proliferation rate and multi-potency differentiating abilities of ASCs[21,22,24].However,little is known about the effects of chemotherapy on ASCs viability outcomes in patients.It is therefore important and necessary to understand the ASCs damage pattern caused by chemotherapy and to know what preserves or destroys stem cells for best clinical practice.In these studies,we have determined the function recovery potential of ASCs by examining:(1)In vitrohuman ASCs treated with three commonly utilized clinical chemotherapeutic agents:paclitaxel(PTX),5-fluorouracil,and doxorubicin for 3 d followed by a washout period(no drugs)of a week;(2)In vivorats given intravenous PTX injections for 2 wk followed by cessation of drug treatment for an additional 2 wk;and(3)Isolation and evaluation of ASCs functional capacity from patients that received neoadjuvant chemotherapy(NAC)compared with the ASCs from patients not-receiving chemotherapy treatment.
Recovery potential of ASCs after post-chemo-treatment
Recovering cell viability and differentiation capability after treatment with chemotherapeutic drugs represents another important aspect of determining stem cell usefulness in cancer patients for stem cell-based targeted therapy and reconstruction.A few reports have now demonstrated the resistance of ASCs to chemotherapeutic agents and their maintenance of phenotype including their potential of differentiating into multiple cell lineagesin vitroafter treatment[46-48].To further understand the use of autologous ASCs in reconstructive procedures following chemotherapy,we have examined if ASCs can recover their cellular activities post chemotherapy treatment usingin vitroandin vivomodels[23].
After the withdrawal of the drugs,the cells were cultured for an additional 9-d.The ASCs showed slow recovery of cell growth capacity in lower doses of the drugs,but not full recovery since ASCs numbers remained significantly below that of controls[23,49].As this time-lapse is considerably after the withdrawal of the drugs for longer than the 9-d,ASCs likely recovered from the inhibitory effects on cell growth with the extra time.Additionally,as the current clinical practice is to provide the patient chemotherapy drugs before and after surgical excision of the tumor,evaluation of the functional recovery of ASCs in the patient should be mapped to the patient’s treatment and recovery time[23].
To complement ourin vitrofindings,we investigatedin vivothe ASCs from the animals treated for 2-wk with PTX followed by washout of drug for an additional 2-wk.We observed that ASCs displayed recovery of adipogenic,osteogenic,and endothelial differentiation potentials in the cells isolated from the 2-wk post cessation PTX injection groups when compared with the active PTX-treated groups[23].However,full recovery of ASC viability was not achieved as the number of ASCs was still significantly lower than for controls[23].This finding requires further confirmation with a comparison of ASCs before,post-drug administration,and after cessation of treatment in the same animal to precisely determine if the chemotherapy is responsible for the alteration in the function and recovery.Taken together,ourin vitrocell andin vivoanimal results support ASCs having the potential to recover differentiation capacity after exposure to chemotherapeutic agents(Figure 2)[23].
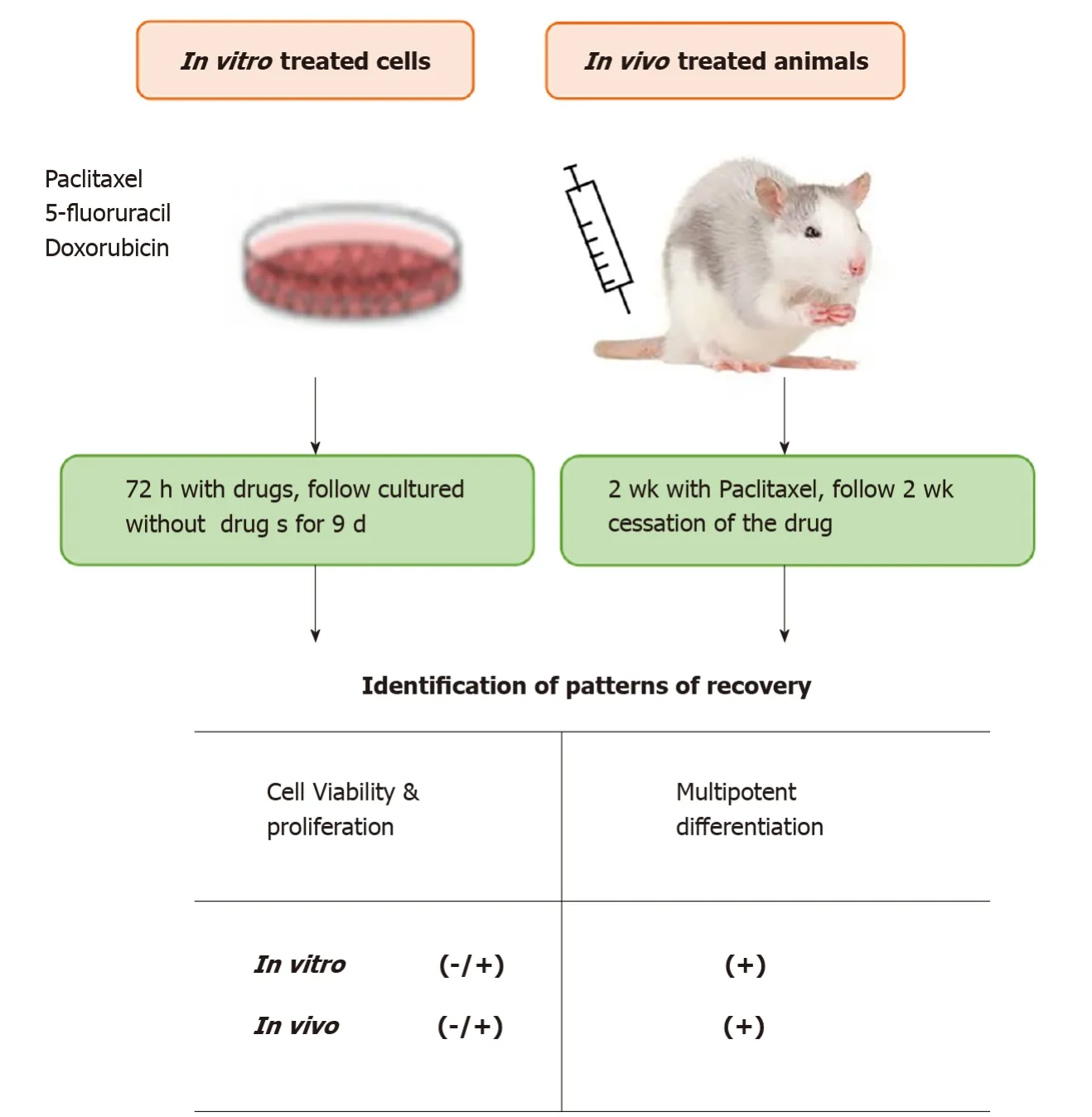
Figure 2 In vitro and in vivo evaluation of recovery potency of adipose-derived stem cells after chemotherapy exposure.In both the in vitro and animal studies,after cessation of drugs,adipose-derived stem cells exhibited partial recovery(-/+)of cell growth and recovery(+)of multipotent differentiation capabilities.
Availability of ASCs in patients receiving chemotherapy
Recently,ASC-based therapies were successfully used for regenerative medicine such as breast cancer and reconstructive surgery[12,44,50].Accordingly,our group examined the availability of ASCs in breast cancer patients.ASCs were isolated from adipose tissue from the cancerous breast and the opposite side noncancerous breast from the same patient post-NAC treatment about 6-8 wk[49].These findings were compared with the patients that did not receive NAC with regard to their ASC cell yield,proliferation rates,and particularly their potential for differentiation into an adipocyte-phenotype[49].
The numbers of ASCs obtained were not altered in patients after receiving the chemo treatment compared with the non-chemotherapy treatment patients.The cellular growth rates of ASCs isolated from the chemo-treatment patients were also not affected upon culturein vitro,and these cells appeared to retain the capacity to acquire adipocyte traits similar to those ASCs isolated from non-chemotherapy patients.Additionally,we found that the number of ASCs yielded and their proliferation rates from the tumor primary breast tissues were decreased compared to ASCs obtained from the normal breast tissue in both NAC and non-NAC treatment groups.However,the adipogenic differentiation capacity of ASCs from the tumor primary breast tissue and the normal breast tissue were similar(Table 1).This finding indicated that in patients who received chemotherapy,the treatment does not significantly change the ASCs phenotype acquired in the tumor environment.In this study,we found that notwithstanding thein vitroevidence of negative effects on ASCs after exposure to chemotherapeutic agents,clinical relevance is questionable as examination of the patient’s ASCs reveals that these cells exhibit functional recovery of adipogenic differentiation after receiving NAC treatment[49].Moreover,the cancerous side of the breast did appear to adversely affect stem cell yield,but not to a point where stem cell harvest would be considered impractical at the post-chemo treatment stage in this patient population.Hence,more studies are needed to evaluate the influence of chemotherapy in breast cellular interactions involving ASCs/or cancer stem cells and tumor cells.Taken together,these results indicate that stem cell availability was not proven inferior for the population receiving chemotherapy or having the cancerous disease.
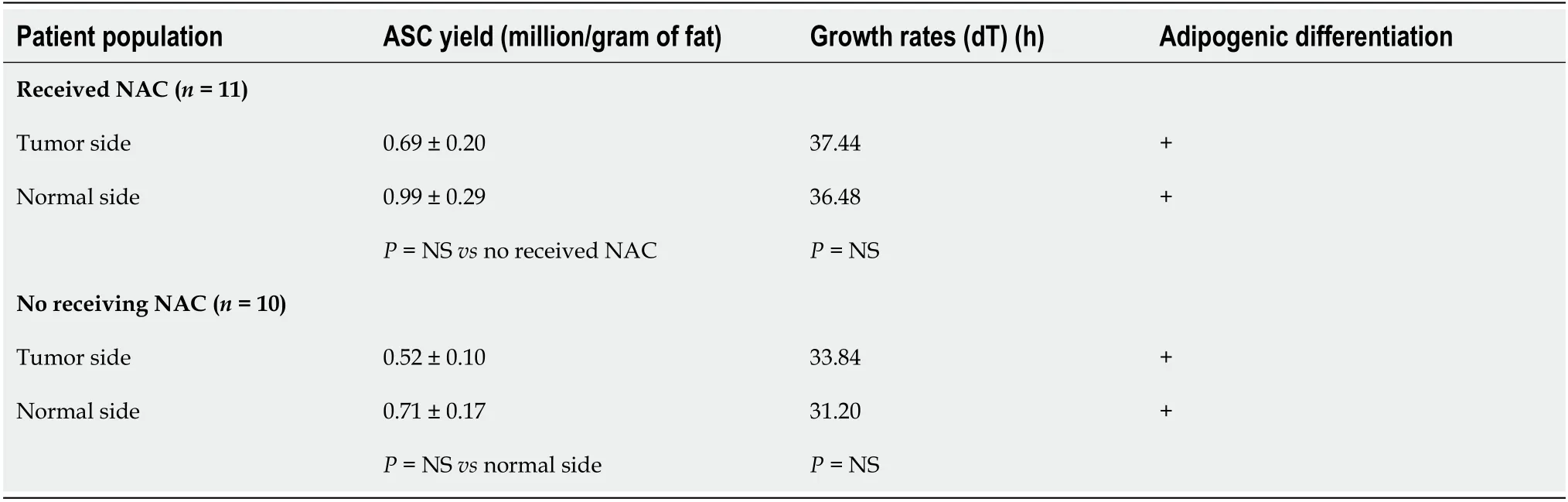
Table 1 Availability of adipose-derived stem cells in patients receiving chemotherapy
ADDITIONAL STUDIES WITH ASCS
An important aspect of designing a fat graft is to enhance the viability and survival rate of transplanted fat tissues.However,one of the main challenges in enhancing the viability of fat grafting is to provide a sufficient and functional vasculature[43].The preliminary data above suggests that radiation therapy has deleterious effects on ASC differentiation capacity towards EC,i.e.,appears to damage the ability of ASCs to repopulate the microvasculature.Therefore,successful use of these stem cells will likely require isolation and banking before radiation treatment.Fat grafting used in breast reconstruction following surgery and radiation for breast cancer is accompanied by a relatively high failure rate due to absorption of the grafted fat,and it is suspected that this absorption results from the lack of vascular support to the grafted tissue[17,51].Therefore,it remains a challenge for researchers to seek an effective solution to improve angiogenesis that would result in boosting the efficiency of survival in fattransplantation[42,43,52].
In our study,we focused on investigating the potential of ASCs differentiation toward an endothelial phenotype requirement into the fat transplantation,thereby improving both survival and neovascularization for autologous fat transplantation.Hereby,taking advantage of the ASCs responsiveness to endothelial differentiation,we have examined the effect of fat grafting assisted with ASCs,or ASCs differentiated to a functional EC-phenotype,on the degree of survival and neovascularization in the animal model.Some studies suggested that fat graft survival is mainly dependent on successful vascularization so that adipogenesis is associated with capillary angiogenesis allowing adipocyte differentiation within clusters of endothelial and stromal cells[53,54].Importantly,ASCs induced to an EC-like phenotype with lipotransfer increased the graft volume retention and revascularization,which represent potential mechanisms for adipose transplantation(Figure 3)[43].
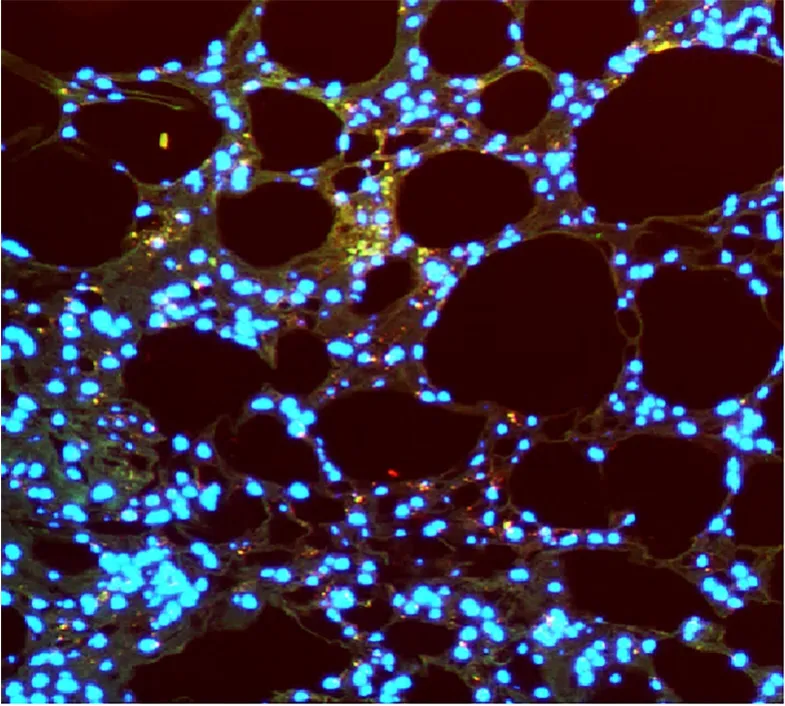
Figure 3 Adipose-derived stem cell-assisted transplanted fat lipoaspirate improved fat graft angiogenesis.Immunofluorescence micrograph(200 ×;CD31 and human nuclear stain)of human adipose-derived stem cells(ASCs)with fat lipoaspirate injected into the rat for 8 wk.Fat lipoaspirate was mixed with human endothelial differentiated ASCs and then subcutaneously injected into the adult male Sprague-Dawley rat’s dorsum.Immunofluorescence staining analysis of the transplants was performed with an anti-human nuclear antibody(red)to detect if the human ASCs proliferated in transplanted tissues.The CD31 staining(green)was used to detect capillary endothelial cells.The merging of the red fluorescence of anti-human nuclear with the green fluorescence of CD31 revealed 3 yellow endothelial cells,indicating that the delivery of human ASCs promoted neovascularization.
CONCLUSION
Adult autologous adipose derived-stem cells represent an important source of cells for fat grafting and breast reconstruction.Their usefulness is directly related to the availability of the cells in patients that undergo cancer treatment as well as their maintenance of cell yield,growth,and multipotent differentiation potential postradiation or chemo-treatment.Studies from our group and others suggest that stem cells derived from cancer patient adipose tissue appear to maintain their important characteristics;ASCs provide a potential to retain their capacity as a source of autologous stem cells for fat grafting and reconstruction in cancer patients.Going forward,it will be necessary to determine whether fat grafting assisted with ASCs differentiated to a functional EC phenotype can be achieved following post-radiation therapy.This will likely depend upon the ASCs’ ability to neovascularize secondary to improved graft survival and healing of damaged tissues.To determine if ASCs altered function and recovery components may be due to the chemotherapy,it is important to perform more preclinical and clinical studies to elucidate the effects of chemotherapy before,during,and after the cessation period in the same patient.Furthermore,there has been a keen interest in optimizing the microenvironmental cues important to modulate the local environment and thus stimulate angiogenesis of the ASCs for improving grafted fat survival in a previous radiotherapy-treated field and this line of research should be aggressively pursued.
杂志排行
World Journal of Stem Cells的其它文章
- Differences and similarities between mesenchymal stem cell and endothelial progenitor cell immunoregulatory properties against T cells
- Inter-regulatory role of microRNAs in interaction between viruses and stem cells
- Mesenchymal stem cells for enhancing biological healing after meniscal injuries
- Modulating poststroke inflammatory mechanisms:Novel aspects of mesenchymal stem cells,extracellular vesicles and microglia
- Antler stem cells and their potential in wound healing and bone regeneration
- Therapeutic prospects of mesenchymal stem/stromal cells in COVID-19 associated pulmonary diseases:From bench to bedside
