Mesenchymal stem cells for enhancing biological healing after meniscal injuries
2021-09-03HyeChangRhimOkHeeJeonSeungBeomHanJiHoonBaeDongWonSuhKiMoJang
Hye Chang Rhim,Ok Hee Jeon,Seung-Beom Han,Ji Hoon Bae,Dong Won Suh,Ki-Mo Jang
Hye Chang Rhim,T.H.Chan School of Public Health,Harvard University,Boston,MA 02115,United States
Ok Hee Jeon,Department of Biomedical Sciences,Korea University College of Medicine,Seoul 02841,Seoul,South Korea
Seung-Beom Han,Ki-Mo Jang,Department of Orthopaedic Surgery,Anam Hospital,Korea University College of Medicine,Seoul 02841,Seoul,South Korea
Ji Hoon Bae,Department of Orthopaedic Surgery,Guro Hospital,Korea University College of Medicine,Seoul 08308,Seoul,South Korea
Dong Won Suh,Department of Orthopaedic Surgery,Barunsesang Hospital,Seongnam 13497,South Korea
Abstract The meniscus is a semilunar fibrocartilage structure that plays important roles in maintaining normal knee biomechanics and function.The roles of the meniscus,including load distribution,force transmission,shock absorption,joint stability,lubrication,and proprioception,have been well established.Injury to the meniscus can disrupt overall joint stability and cause various symptoms including pain,swelling,giving-way,and locking.Unless treated properly,it can lead to early degeneration of the knee joint.Because meniscal injuries remain a significant challenge due to its low intrinsic healing potential,most notably in avascular and aneural inner two-thirds of the area,more efficient repair methods are needed.Mesenchymal stem cells(MSCs)have been investigated for their therapeutic potential in vitro and in vivo.Thus far,the application of MSCs,including bone marrow-derived,synovium-derived,and adipose-derived MSCs,has shown promising results in preclinical studies in different animal models.These preclinical studies could be categorized into intra-articular injection and tissueengineered construct application according to delivery method.Despite promising results in preclinical studies,there is still a lack of clinical evidence.This review describes the basic knowledge,current treatment,and recent studies regarding the application of MSCs in treating meniscal injuries.Future directions for MSC-based approaches to enhance meniscal healing are suggested.
Key Words:Meniscus;Meniscus healing;Cell-based treatment;Mesenchymal stem cell;Tissue engineering
INTRODUCTION
The meniscus is an essential intra-articular structure of the knee joint.Meniscal injury can negatively affect the overall function of the knee joint,especially in young and physically active populations[1].The incidence rate of meniscal injury ranges from 0.31 to 0.70 per 1000 person-years[2-4].Meniscal disorders include a discoid meniscus in the pediatric population,traumatic injuries in athletes,and degenerative tears in older adults[1].In the past,meniscal injuries were treated with surgical removal because the meniscus was once considered a functionless remnant tissue[5].However,these techniques have been avoided because of the deterioration of articular cartilage and progression to early osteoarthritis(OA)[6].The current meniscal injury management paradigm has shifted toward preserving as much of the native meniscus as possible[7].
Indeed,successful meniscus repair results in better long-term clinical and radiographic outcomes[8].However,after repair,the recovery of the meniscus is inherently affected by its poor vascularity and lack of cellularity[9];therefore,biological augmentation strategies are implemented to overcome such healing limitations[10].Among various methods,mesenchymal stem cells(MSCs)have been extensively explored at the clinical,in vitro,andin vivolevels because of their extensive proliferative ability[11],migration to the site of injury[12],and reparative capacities[13].The purpose of this article is to summarize current treatments for meniscal injury and their limitations and review recent preclinical and clinical studies using MSCs to enhance biological healing after meniscal injuries.
ROLE OF THE MENISCUS:WHY SHOULD WE PRESERVE THE MENISCUS?
The meniscus is a small intra-articular structure;however,it plays an essential role in maintaining knee-joint function.The roles of the meniscus,including load distribution,force transmission,shock absorption,joint stability,lubrication,and proprioception,have been well established[5].Loss of the meniscus predisposes the knee joint to degenerative changes.Biomechanical studies have demonstrated that at least 50% of the compressive load of the knee joint is transmitted through the meniscus during knee extension and that approximately 85% of the compressive load is transmitted at 90° of knee flexion[14].In the meniscectomized status,the contact area is reduced by approximately 75%,and the peak contact stress on the articular cartilage increased by 235%[15].Finally,meniscectomized knees cause damage and degeneration of the cartilage and OA.It has been shown that the degree of OA following meniscectomy is directly proportional to the amount of removed meniscus[16].In an experimental study,after removing 15% to 34% of the meniscus,the contact pressure increased by more than 350%[17].Even in partial meniscectomy,knee biomechanics are remarkably disrupted with increased contact pressure on other soft tissue structures[18].Therefore,the meniscus should be preserved as much as possible.
BASIC ANATOMY OF THE MENISCUS
The menisci are semilunar fibrocartilaginous structures divided into medial and lateral components situated between the tibial plateau and femoral condyle and attached to the tibial plateau by their anterior and posterior roots(Figure 1)[9].Their wedge shape provides better articulation between the flat tibial plateau and the rounded femoral condyle and allows for stability and low contact pressure[19].The menisci are further stabilized by the meniscal ligament complex composed of the medial collateral ligament,transverse meniscal ligament,meniscofemoral ligaments,and coronary ligaments[20].
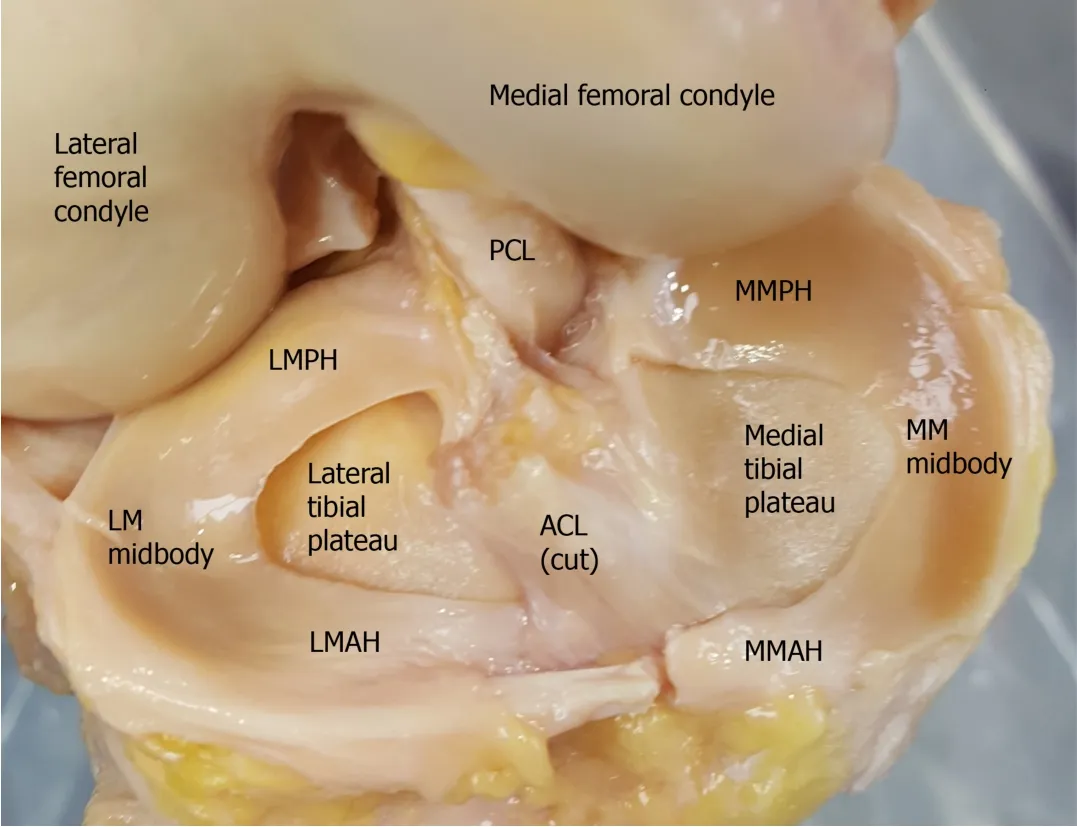
Figure 1 Anatomy of the meniscus in a cadaveric knee joint.ACL:Anterior cruciate ligament;MM:Medial meniscus;MMAH:Medial meniscus anterior horn;MMPH:Medial meniscus posterior horn;LM:Lateral meniscus;LMAH:Lateral meniscus anterior horn;LMPH:Lateral meniscus posterior horn;PCL:Posterior cruciate ligament.
The biochemical composition of the meniscus is 72% water,22% collagen,0.8%glycosaminoglycans(GAGs),and the remaining dry weight is composed of proteins,glycoproteins,and interspersed cells[19,21].The meniscus cells are referred to as fibrochondrocytes because they appear to be a mixture of chondrocytes and fibroblasts[19].The interaction of collagens,GAGs,and proteins contributes to the compressive load resistance,lubrication,and semi-elastic deformation functions of the meniscus[19,22].
Type I collagen comprises approximately 90% of the collagen expressed throughout the meniscus,while type II collagen is mostly found in the inner portion[9,23].These two types of structural fibers are organized into three distinct layers from the surface to the core:superficial,lamellar,and deep[24].Fibers in the superficial layer show random orientation,and while fibers in the lamellar layer also show no specific orientation,there are radially oriented fibers in the anterior and posterior sections.Circumferential fibers can be found in the outer and inner deep portions and convert the vertical axial load to horizontal tensile forces,dispersing it through the meniscus[25,26].
THE LIMITED HEALING POTENTIAL OF A TORN MENISCUS
The meniscus has limited intrinsic healing capacity,most notably in its avascular and aneural central(inner)two-thirds of the area.The perimeniscal capillary network originating in the capsular and synovial tissues provides a direct blood supply to the outer third of the meniscus[27].The peripheral(outer)rim of the meniscus is described as a red-red vascular zone,while the inner central zone is known as a white-white avascular zone[9].The healing potential of meniscal injury depends on the injury location,in which the red-red zone presents a better prognosis because of a direct blood supply,while the white-white zone has a poor prognosis for repair due to a lack of blood supply[28].The intermediate zone,described as a red-white zone,receives some degree of blood supply and has a fair prognosis if the injury occurs at the border of vascular zone[28,29].
HOW TO EVALUATE THE MENISCUS HEALING
As the importance of meniscal preservation is strongly emphasized,various meniscus repair techniques have evolved and improved.To evaluate healing effects of these new techniques,they are generally first assessed in animal models.In a recent systematic review regarding animal model studies of meniscus repair and regeneration,Bansalet al[30]analyzed a total of 128 full-length peer-reviewed manuscripts.Authors demonstrated that most studies conducted histologic(90%),macroscopic(85%),and healing/integration(83%)analyses in terms of meniscal healing outcome parameters.In addition to these outcome measures,biochemical analysis,magnetic resonance imaging(MRI),assessment of vascularization,gene expression,immunohistochemistry,and arthroscopy were also performed to assess the meniscus healing.In clinical studies,there have been various subjective and objective evaluation methods for assessing the meniscus healing.In terms of subjective evaluation methods,some patients reported outcome measures(PROMs)are currently available to assess the function,symptoms and quality of life of patients with meniscal injuries[31,32].These include the Hughston Clinic Questionnaire[33],International Knee Documentation Committee(IKDC)subjective knee form[34],Knee injury and Outcome Osteoarthritis Score(KOOS)[35],Lysholm Knee Scale[36],Western Ontario McMaster Osteoarthritis Index(WOMAC)[37],EuroQoL-5 dimension(EQ-5D)[38],Twenty-six-item Knee Quality of Life(KQoL-26)[39],Short Form-6 dimensions(SF-6D)[40],Western Ontario Meniscal Evaluation Tool(WOMET)[41],Tegner Activity Scale[42],Cincinnati Knee Score[43],Tapper and Hoover Meniscal Grading Score[44],Knee Outcome Survey-Activities of Daily Living Scale(KOS-ADLS)[45],Short Form-12 Item Health Survey(SF-12)[46].Although there are many PROMs to assess meniscal injuries and healing,evidences for the validity of these PROMs are still insufficient[31].In terms of objective evaluation methods,there are several methods to assess the meniscus healing.Although the most reliable examination tool is arthroscopy[47,48],it is an invasive method and still depends largely on the surgeons’ skills[49].As a noninvasive method,conventional MRI sequences have been widely used to assess meniscal injuries and healing.However,using conventional MRI,assessing meniscal tissue composition changes ahead of surface breakdown is challenging[50].Recently,quantitative MRI techniques,such as T2 mapping,are used to assess the meniscus healing more effectively[50,51].Some authors recommended magnetic resonance arthrography or computed tomography arthrography as an alternative objective evaluation method[52-55].
CURRENT TREATMENT FOR MENISCAL INJURIES
The role of nonoperative treatment for meniscal injuries has been well investigated in patients with degenerative tears.Herrlinet al[56,57]conducted a prospective randomized trial to compare partial meniscectomy with supervised exercise in middleaged patients with non-traumatic medial meniscal tears.The two groups showed similar functional outcomes at six months,maintained throughout the 5-year followup period.Similar results were found in a large,multicenter,randomized controlled trial involving 351 patients over 45 years of age with a meniscal tear and evidence of OA.No significant differences were found between the partial meniscectomy and physical therapy groups in functional and pain improvement up to 1 year.It should be noted that 30% of the patients assigned to the physical therapy group underwent surgery within 6 mo,but their functional outcomes after 1 year were similar to those of patients who underwent surgery initially[58].Another multicenter,randomized controlled trial involving 146 patients aged 35- to 65-years with a degenerative medial meniscus tear and no OA found results consistent with those of previous studies.There were no significant differences in functional outcomes between the partial meniscectomy and sham surgery groups at 1 and 2 years[59,60].These studies suggest that patients with degenerative tears can be treated conservatively,and if this conservative management fails,patients can undergo meniscectomy[29].Unlike the role of non-operative treatment in degenerative tears,its role in acute tears in younger populations remains unclear[5,29].
Meniscectomy is a fast and effective method to relieve pain and joint swelling(Figure 2).In the past,the meniscus was once thought to be an unnecessary tissue that could be sacrificed without further consideration of any sign of injury[5].However,cadaveric studies have decreased the intra-articular contact area and increased the peak contact pressure following total meniscectomy[14,61].These findings were echoed by a clinical study that confirmed that increased pressure leads to radiographic evidence of OA[62].The understanding of devastating biomechanical changes in the knee following total meniscectomy shifted the paradigm to retain as much meniscus as possible.In previous studies,partial meniscectomy,which focuses on removing the only torn piece of the meniscus while preserving as much normal meniscus,resulted in favorable outcomes[63,64]and more satisfactory outcomes than total meniscectomy[65].Nonetheless,long-term studies have found that the amount of degenerative changes after partial meniscectomy is proportional to the amount of removed meniscus and that patients will eventually undergo accelerated degeneration[29,66].Therefore,partial meniscectomy might be beneficial for short-term treatments but may cause long-term damage.
Due to the long-term adverse outcomes after meniscectomy,there has been an increasing tendency toward choosing meniscal repair[29].Indeed,previous studies have shown that compared with partial meniscectomy,healed meniscal repair leads to better long-term outcomes[67,68].Moreover,repair of vertical meniscal tears was protective against the progression of OA compared with partial meniscectomy[69].However,the success of meniscal repair mainly depends on blood supply.Only injuries in the red-red or red-white zone are expected to be repaired,while healing of the inner white-white avascular zone is limited(Figure 3).Therefore,several methods such as fibrin clots and trephination have been used to improve meniscal repair outcomes in avascular zone tears.In a previous case-series study,exogenous fibrin clots were used to repair five cases of a tear involving the posterolateral aspect of the lateral meniscus[70].All patients returned to the previous activity level with healing identified in the second-look arthroscopy.Trephination is the procedure of creating a channel from the vascularized zone to the tear and has shown favorable outcomes[71,72].
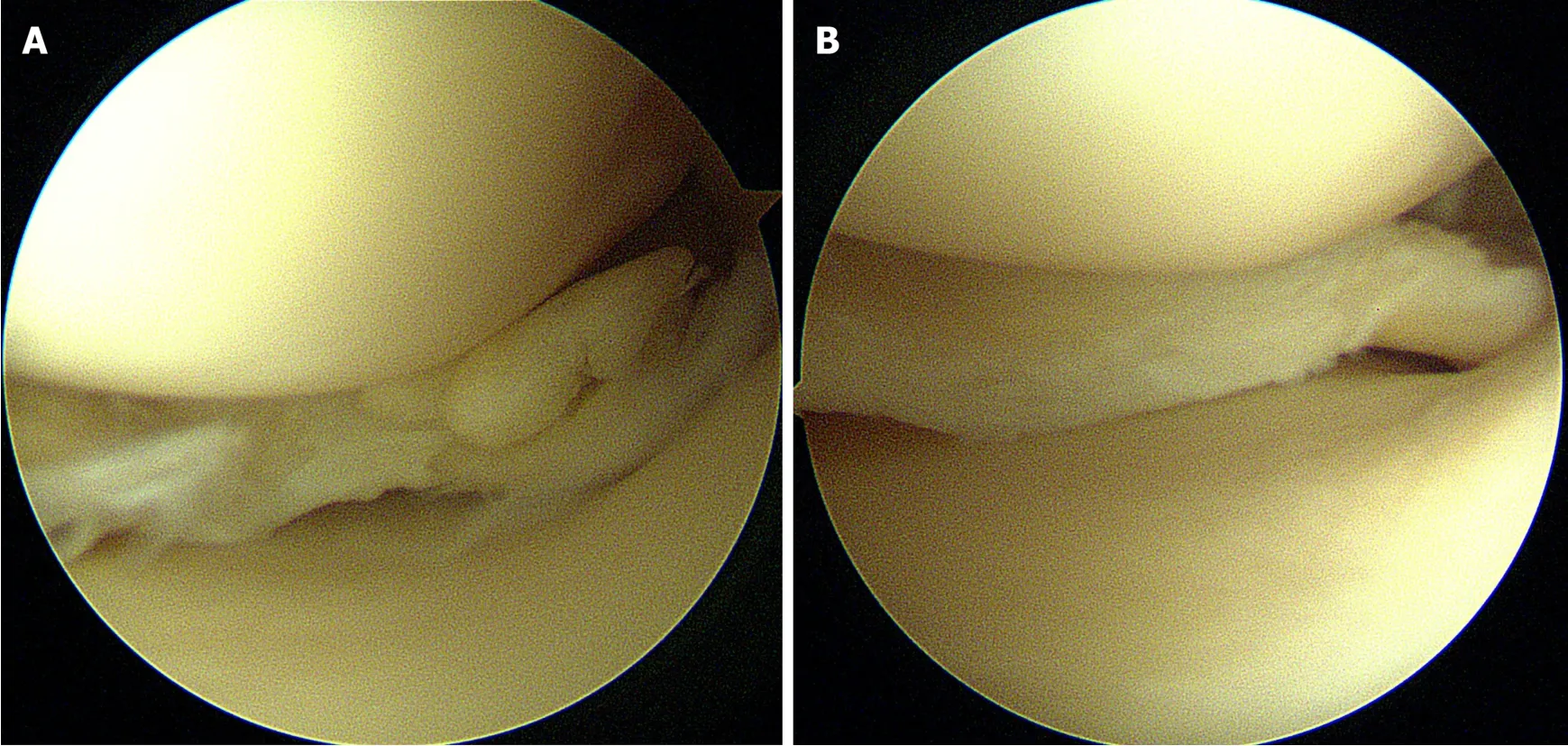
Figure 2 Arthroscopic images of partial meniscectomy.A:Degenerative complex tear of the medial meniscus posterior horn;B:Remnant meniscus after partial meniscectomy.
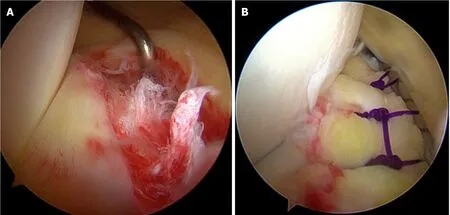
Figure 3 Arthroscopic images of meniscus repair.A:Longitudinal tear in red-red zone of the medial meniscus posterior horn;B:Meniscus repair using allinside suture technique.
When patients have irreparable meniscal tears or have already undergone total or partial meniscectomy,meniscal allograft transplantation(MAT)may be considered as an option to restore knee function[73,74].Indications for MAT included patients younger than 50 years of age with a history of meniscectomy without articular cartilage damage,uncorrectable joint malalignment,or knee instability.Previous studies have reported survivorship as high as 89% at 10 years and functional improvements[74].However,complications associated with MAT include graft failure,infection,extrusion,and the need for revision.Furthermore,it is challenging to resolve resources,shape matching,and ethical issues[75].
A CELL-BASED APPROACH FOR ENHANCED MENISCUS HEALING
The meniscus has a limited healing capacity,especially in the inner two-thirds avascular zone.To overcome the poor intrinsic healing capacity,cell-based and tissue engineering approaches have emerged as an alternative means for the regeneration of the injured meniscus[76,77].Several preclinical studies have recently shown that cellbased regenerative treatment could be a good option[78-82].
Different types of cell sources have been investigated to enhance the biological healing of the meniscus.The meniscus contains distinct cell populations,such as fibrochondrocytes,fibroblast-like cells,and superficial zone cells.These meniscal cells can be isolated from the meniscus tissue and reinserted into the lesions.Although these cells have been studiedin vitro[83-85],there is a lack ofin vivostudies[77].In recent years,the use of MSCs has dramatically increased in the regenerative treatment of musculoskeletal disorders.MSCs are pluripotent cells found in numerous human tissues,including bone marrow and adipose tissue,and have self-renewal capacity and can differentiate into multiple lineages[86].In addition,owing to their specific immunological properties,MSCs can be applied even in a non-autologous manner[87].MSCs have received increasing interest as a potential biological approach for treating meniscal injuries.In a recent animal study comparing autologous MSCs and meniscus cells,Zellneret al[77]demonstrated that both MSCs and meniscal cells improved meniscal healing.However,donor-site morbidity,reduced availability,and reduced chondrogenic differentiation of meniscal cells favor MSCs for clinical use in cell-based meniscus regeneration[77].
APPLICATION OF MSCS IN BIOLOGICAL MENISCUS HEALING
The use of MSCs seems to be an attractive approach for meniscal healing,as these cells have high proliferative and chondrogenic potential.The precise roles and mechanisms of MSCs for enhanced meniscus healing are still unknown.Although there are some controversies,it is recently accepted that the therapeutic effects derived from MSCs in various musculoskeletal therapies are mainly due to their paracrine effects[88,89].Various growth factors can be secreted by the paracrine functions of MSCs,which might lead to enhanced angiogenesis,cell differentiation and migration,and various additional regenerative effects[88,90,91].Preclinical studies using MSCs have shown promising results in regenerating meniscus tissue[77,81,82,92,93].In addition to these direct MSC application studies,Driscollet al[94]demonstrated that marrow stimulation by drilling on the femoral intercondylar notch apex led to modest improvements in the quality and quantity of reparative tissue bridging a meniscal defect in a rabbit model study.The authors reported an increased intra-articular population of pluripotent MSCs might stimulate meniscal healing and regeneration.However,clinical data regarding MSC application for meniscal lesions are still limited[95-99].
MSCs can be harvested and isolated from various sources.Among these MSC sources,bone marrow-derived MSCs(BM-MSCs)[77,96,100-102],synovium-derived MSCs(S-MSCs)[81,92,93,98,103],and adipose-derived MSCs(A-MSCs)[99,100,104-106]have been used to demonstrate promising results in meniscal healing.Currently,there is no consensus on which source is the best,because each has its own advantages and disadvantages.
BM-MSCs have been investigated and most commonly used for various mesenchymal tissue healing processes.BM-MSCs were first discovered by Friedensteinet al[107-109]in 1968 and have been widely used and investigated since then.Although it is clear that BM-MSCs are a very attractive cell source with high osteogenic,chondrogenic,and adipogenic potential,the procedure for harvesting BMMSCs can be invasive and painful,yielding only a small amount as they only make up 0.0017% to 0.0201% of bone marrow cells.Furthermore,these cells have a propensity for hypertrophic differentiation,which may be detrimental to tissue regeneration[110-113].
S-MSCs are a relatively new source of MSCs compared with BM-MSCs.The number of S-MSCs is known to increase after meniscus injuries,and it has been suggested that this cell population might be crucial in meniscal repair[77,114,115].S-MSCs are colonyforming cells isolated from the synovium of the knee during arthroscopy[116,117].Some studies have indicated that S-MSCs might be better than BM-MSCs in terms of chondrogenic,osteogenic,and adipogenic capacities[118-121].Nonetheless,the synovium contains only a few multipotent cells that form colonies[117,122].In addition,in the long term,a high osteogenic capacity may induce joint diseases such as OA from stem cell-induced mineralization and calcification[6].Therefore,long-term studies are needed to establish the efficacy of S-MSCs in meniscal healing.
A-MSCs are also a promising cell source for a biological meniscus healing strategy.A-MSCs are easier to producevialiposuction or adipose tissue biopsy and can be collected in larger quantities through less invasive methods than BM-MSCs[123-127].A-MSCs have been shown to have low risk of rejection and to be more genetically stable[128-131].The most accessible source for A-MSCs is subcutaneous fat tissue,but recently,several studies found supra- and infra-patellar fat pads to be suitable sources for A-MSC harvest[132-134].Although these cells are derived from adipose tissue,they possess chondrogenic capacity.Nonetheless,A-MSCs have limited chondrogenic potentials compared with BM-SMCs[111,135]and inferior chondrogenic and osteogenic differentiation capacities compared with S-MSCs[118].Furthermore,one study suggested that repetitive induction of A-MSCs might confer an elevated hypertrophic state with concurrent decreased chondrogenic potential which may be detrimental to meniscus repair[136].
In addition to BM-MSCs,S-MSCs,and A-MSCs,meniscal fibrochondrocytes(MFCs)and meniscus-derived MSCs(M-MSCs)have recently been used for biologic meniscus healing.MFCs were first described by Webberet al[137].These cells have the unique properties of both fibroblasts and chondrocytes[138,139].Previous studies suggested that the periphery of the meniscus,responsible for shock absorption,behaves more like fibroblasts,while the inner rim of the meniscus,acting as a direct contact point for the femoral condyle,resembles chondrocytes[139-141].Therefore,MFCs and M-MSCs display different morphologies and biochemical properties depending on whether they are located at the periphery or inner zone[85,142,143].MFCs and M-MSCs have been shown to exhibit multilineage differentiation and express common markers of MSCs[85].However,there are several problems associated with applying autologous MFCs and M-MSCs for meniscal healing.First,these cell populations are scarce in the meniscus[85,144,145],and the procedure to harvest these cells is invasive and lengthy.Even worse,this procedure may inevitably injure a healthy meniscus[61,85,146].
INTRA-ARTICUALR INJECTION OF MSCS FOR MENISCUS HEALING
Intra-articular injection is a simple treatment for intra-articular pathologies that improves structure and function.Recently,there have been ongoing preclinical and clinical trials regarding intra-articular or direct intralesional injection using different sources of MSCs(Table 1).
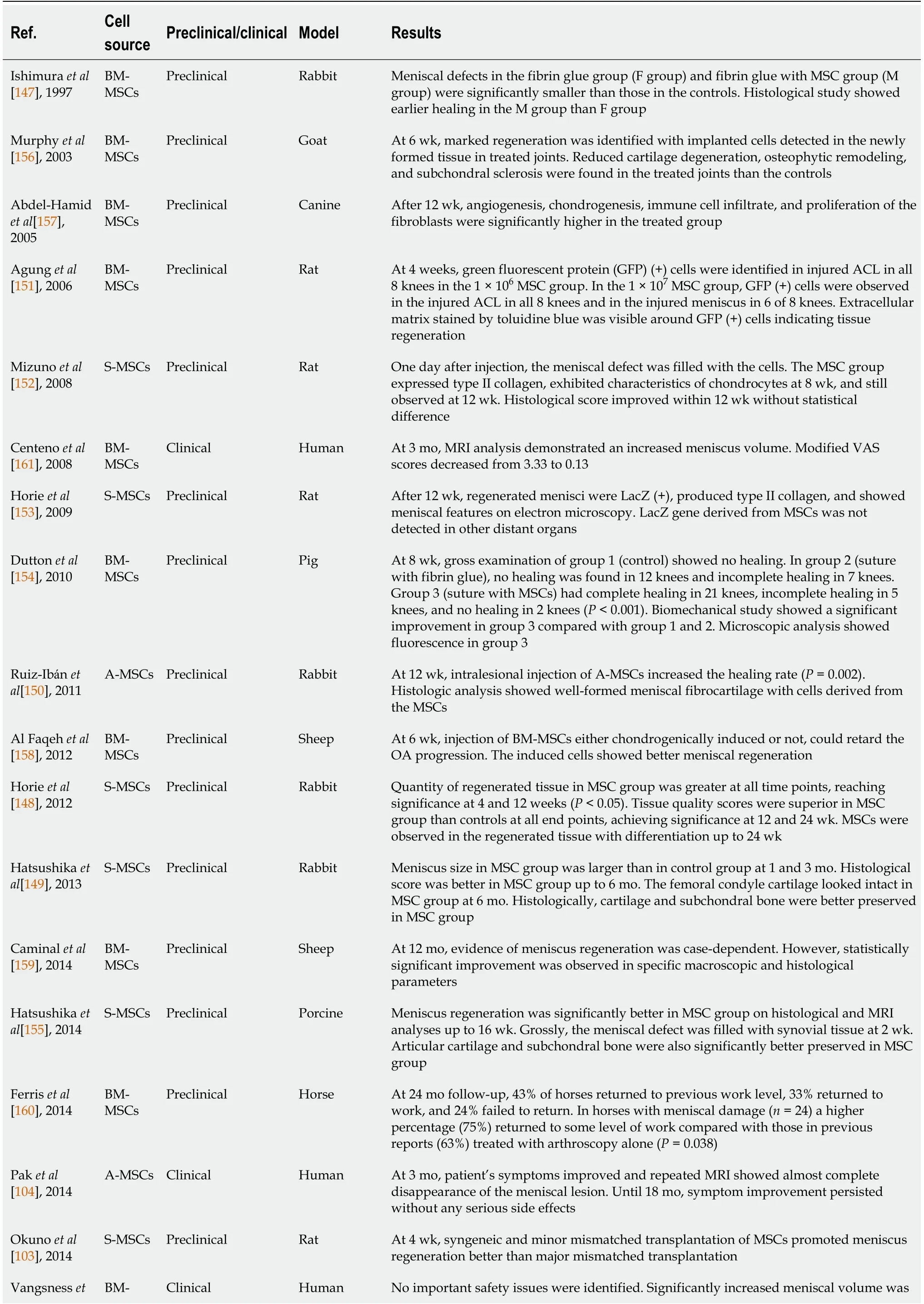
Table 1 Summary of recent preclinical and clinical studies regarding intra-articular mesenchymal stem cell injection for meniscus healing
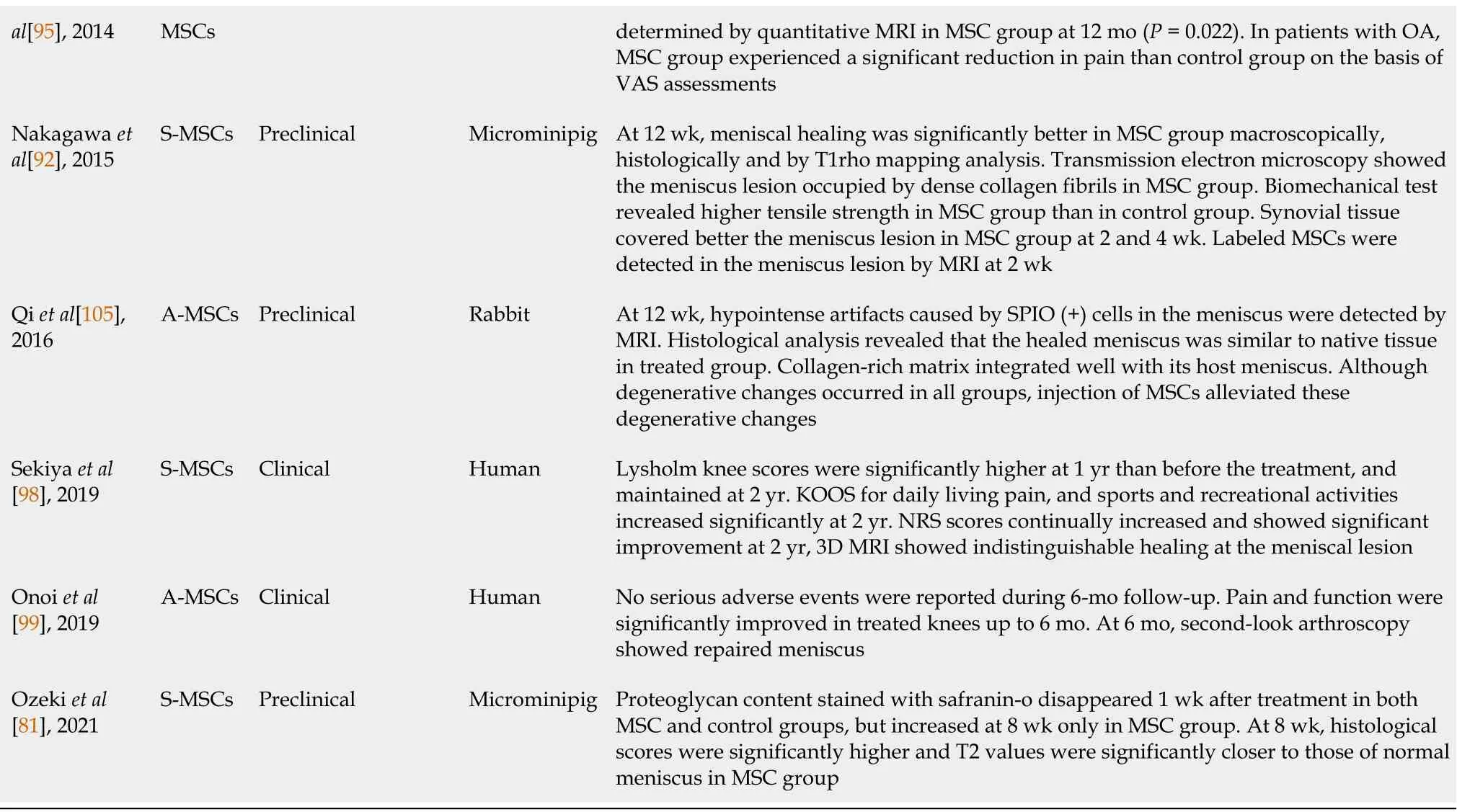
BM-MSCs:Bone marrow-derived mesenchymal stem cells;MSC:Mesenchymal stem cell;GFP:Green fluorescent protein;ACL:Anterior cruciate ligament;S-MSCs:Synovium-derived mesenchymal stem cells;MRI:Magnetic resonance imaging;VAS:Visual analog scale;A-MSCs:Adipose-derived mesenchymal stem cells;OA:Osteoarthritis;SPIO:Superparamagnetic iron oxide;KOOS:Knee injury and outcome osteoarthritis score;NRS:Numerical rating scale.
Animal studies
Rabbit model:Among various MSC sources,BM-MSCs have been the most frequently investigated for meniscal healing in various animal models.The first preclinical study using BM-MSCs was published in 1997[147].Ishimuraet al[147]evaluated the healing properties of a full-thickness defect in the avascular portion of the rabbit meniscus in the control group(C group),fibrin glue only group(F group),and fibrin gluecontaining BM-MSCs group(M group).The authors demonstrated that the remaining defects in F and M groups were significantly less than those in the C group,and the histological study showed earlier mature healing of the meniscal defects in the M group than in the F group.Horieet al[148]used allogeneic S-MSCs in a rabbit model.The authors made a 1.5-mm diameter full-thickness cylindrical defect in the inner twothirds of the anterior horn of the medial meniscus.Then,2 × 106S-MSCs in 50 mL of phosphate-buffered saline(PBS)solution were injected directly into the defect using a 27-gauge needle.The quantity of regenerated tissue in the treated group was greater at all time points,reaching statistical significance at 4 and 12 wk(P< 0.05).Tissue quality scores were also higher in the treated group at all endpoints,achieving statistical significance at 12 and 24 wk(P= 0.008 and 0.021,respectively).Injected S-MSCs were identified in the regenerated tissue with differentiation into type I and II collagenexpressing cells for up to 24 wk.Hatsushikaet al[149]reported relatively long-term results of intra-articular injection of S-MSCs in a rabbit massive meniscal defect model.After creating a large defect in the anterior half of the medial meniscus,1 × 107S-MSCs in 100 mL PBS solution were injected into the knee joint.The S-MSCs and control groups were compared macroscopically and histologically for up to 6 mo.The meniscus size in the treated group was larger than that in the control group at 1 and 3 mo.However,the difference in the size between the two groups was indistinct at 4 and 6 mo.The histological score was better in the treated group at 1,3,4,and 6 mo.Grossly,although the surface of the medial femoral condyle was fibrillated in the control group,it appeared nearly intact in the treated group at 6 mo.Histologically,articular cartilage and subchondral bone were better preserved in the treated group.Ruiz-Ibánet al[150]performed a preclinical study to determine whether A-MSCs affect the healing rate of meniscal lesions in the avascular zone in a rabbit model.In the treated group,the authors injected 1 × 105allogeneic A-MSCs marked with bromodeoxyuridine in the lesion before suturing.After 12 wk,in group A(short lesion,acute repair),six of 12 A-MSC-treated menisci and none of 12 controls showed some healing(P= 0.014).In group B(short lesion,delayed repair),two of eight A-MSC-treated menisci and one of eight controls had some healing(P=0.5).In group C(long lesion,acute repair),six of 12 A-MSC-treated menisci and none of 12 controls showed some healing(p= 0.014).In group D(long lesion,delayed repair),four of eight A-MSCtreated menisci and none of eight controls showed some healing(P= 0.07).Generally,the intralesional injection of A-MSCs enhanced the healing rate[odds ratio,32(range,3.69 to 277);P= 0.002].Histological analysis of the healed areas showed well-formed meniscal fibrocartilage with cells derived from A-MSCs.Qiet al[105]assessed the effect of targeted intra-articular injection of superparamagnetic iron oxide(SPIO)-labeled A-MSCs in a rabbit model of a massive meniscal defect.At 12 wk,clear hypointense artifacts caused by SPIO(+)cells in the meniscus were detected using MRI.Histological analysis revealed that the anterior portion of the medial meniscus was similar to that of the native tissue,with typical fibrochondrocytes surrounded by richer extracellular matrix in the SPIO(+)A-MSCs group.The collagen-rich matrix bridging the interface and the neo-meniscus integrated well with the host meniscus.Although degenerative changes occurred in all groups,intra-articular injection of AMSCs alleviated these degenerative changes.Prussian blue staining showed that the injected A-MSCs were directly associated with tissue regeneration.
Rat model:Agunget al[151]investigated the mobilization of BM-MSCs into injured intra-articular tissues and their regeneration in a rat model study.BM-MSCs were obtained from green fluorescent protein(GFP)transgenic Sprague-Dawley(SD)rats and injected into normal SD rats with injured anterior cruciate ligament(ACL),medial meniscus,and femoral condyle cartilage.Four weeks after intra-articular injection,GFP(+)cells were identified only in the injured ACL in all eight knees in the 1 × 106BM-MSC injection group.In the 1 × 107BM-MSC injection group,GFP(+)cells were observed in the injured ACL of all eight knees and in the injured medial meniscus and articular cartilage in six of eight knees.In addition,extracellular matrix stained by toluidine blue was found around GFP(+)cells in the injured femoral condyle cartilageand medial meniscus,indicating tissue regeneration.The authors concluded that injected BM-MSCs could mobilize into injured intra-articular tissues,and might contribute to tissue regeneration.In 2008,Mizunoet al[152]first reported intraarticular injection of S-MSCs in an animal model.Authors injected 105,106,or 107GFP(+)S-MSCs into the knee joint after creating a defect in the anterior horn of the medial meniscus in a rat model.One day after injection,green fluorescence was not detected in the 105MSC group.In the 106MSC-injected group,only very faint fluorescence was visible.However,GFP fluorescence was visible in the 107MSC-injected group.Therefore,the authors analyzed the data obtained from 107MSC-injected rats.At 8 wk,the meniscal defect was filled with cartilage matrix in both the treated and control groups.Injected GFP(+)cells still existed in the meniscal defect,producing type II collagen,although its expression was lower than that in the native meniscus.Electron microscopy showed several short processes with lacunae,characteristic of chondrocytes.At 12 wk,the chondrocytes matured morphologically in both groups.The authors concluded that the injected S-MSCs adhered to the meniscal defect and survived to differentiate into cartilage cells.Horieet al[153]investigated whether intraarticular injection of S-MSCs enhanced meniscal regeneration in a massive meniscal defect in a rat model.After creating a meniscal defect,5 × 106Luc/LacZ(+)S-MSCs were injected into the knee joint.Two to eight weeks after Luc/LacZ(+)S-MSC injection,macroscopic meniscal regeneration was much better in the treated group than in the control group.After 12 wk,the regenerated menisci were LacZ(+),producing type II collagen,and the characteristic meniscal features were identified by transmission electron microscopy.The LacZ gene derived from S-MSCs could not be detected in other distant organs by real-time PCR.Okunoet al[103]compared the effect of syngeneic and allogeneic transplantation of S-MSCs for meniscal regeneration in a rat model to identify the influence of allogeneic transplantation of S-MSCs.After removal of the anterior half of the medial meniscus in the knees of F344 rats,5 × 106SMSCs derived from F344(syngeneic transplantation),Lewis(minor mismatched transplantation),and ACI(major mismatched transplantation)were injected into the knee joints of F344 rats.Four weeks after intra-articular injection of S-MSCs,the regeneration area was significantly larger in the F344 group than in the ACI group.At 8 wk,histological assessment using the modified Pauli’s score showed significantly better scores in the F344 and Lewis groups than in the ACI group.DiI-labeled cells were observed in the F344 group;however,they were hardly identified in the ACI
group after 1 wk.The immunological analysis showed that the number of ED1(+)macrophages and CD8 T cells in the synovium around the meniscal defect was significantly lower in the F344 group than in the ACI group at 1 wk.Therefore,the authors concluded that syngeneic and minor mismatched transplantation of S-MSCs induced better regeneration of the meniscus defect than major mismatched transplantation.
Porcine model:Duttonet al[154]applied BM-MSCs together with a conventional suturing technique to assess improved healing in meniscal tear site in a porcine model.In their study using 28 adult pigs(56 knees),group 1(nine knees)had a radial meniscal tear left untreated.In group 2(19 knees),the tear was repaired with sutures and fibrin glue,and in group 3(experimental group,28 knees),the tear was treated by suturing with an injection of fibrin glue containing at least 2 × 106autologous BMMSCs.At 8 wk,macroscopic examination showed no healing in any of the specimens in group 1.In group 2,no healing was found in 12 knees,and incomplete healing was observed in seven knees.Group 3 showed complete healing in 21 knees,incomplete healing in five knees,and no healing in two knees(P< 0.001).A biomechanical study using the mean modulus of elasticity showed that there was a significant improvement in Group 3 compared with Group 1(P< 0.001)and Group 2(P< 0.002).In the microscopic analysis,although fluorescence was absent from group 2,fluorescence was observed in group 3.The authors concluded that avascular meniscal tears treated with autologous BM-MSCs could achieve good macroscopic healing with the formation of fibrocartilage-like tissue and improved biomechanical properties.Following a rabbit model study[149],Hatsushikaet al[155]attempted repetitive allogeneic S-MSC injection in a porcine model.After creating a large defect in the anterior half of the medial meniscus,5 × 107allogeneic S-MSCs were injected into the knee joint at 0,2,and 4 wk.Then,the authors evaluated the regenerated meniscus,adjacent articular cartilage,and subchondral bone using sequential MRI at 2,4,8,12,and 16 wk.They were also assessed histologically and macroscopically at 16 wk.Based on histological and MRI analyses,meniscus regeneration was significantly better in the treated group than in the control group.The modified Pauli’s histological score was significantly better in the treated group.Sequential MRI analysis showed that the resected area appeared less disorganized in the MSC group than in the control group at 8,12,and 16 wk.In addition,quantification analyses demonstrated that the T2 value in the regenerative meniscus was significantly lower in the treated group for up to 16 wk.Grossly,the meniscal defect appeared to be filled with synovial tissue at 2 wk.Articular cartilage and subchondral bone at the medial femoral condyle were also significantly better preserved in the treated group based on macroscopic,histological,and MRI analyses.The intra-articular S-MSC injection effects were also investigated in a microminipig model by Nakagawaet al[92]and Ozekiet al[81].Nakagawaet al[92]examined whether transplantation of S-MSCs promoted healing after meniscal repair in a pig model.After making a full-thickness longitudinal tear in the junction between the internal third and middle third of the meniscus in the avascular area,2 × 107SMSCs were injected after meniscal repair in the treated group.At 12 wk,the meniscal healing was significantly better in the treated group than in the control group macroscopically,histologically,and by T1rho mapping analysis.Transmission electron microscopic analysis showed that dense collagen fibrils occupied the meniscus lesion only in the treated group.The tensile strength to failure was higher in the treated group than in the control group.At 2 and 4 wk,the synovial tissue covered the superficial layer from the outer zone into the meniscal lesion in the treated group.SMSCs labeled with ferucarbotran were detected in the meniscus lesion and surrounding synovium by MRI at 2 wk.Recently,Ozekiet al[81]injected allogeneic SMSCs at the injured site of the medial meniscus posterior horn after puncturing 200 times with a 23G needle.Proteoglycan content stained with safranin-O disappeared 1 wk after treatment in both the treated and control groups,but increased at 8 wk in the treated group.At 8 wk,histological scores were significantly higher in the treated group,and the T2 values were significantly closer to those of the healthy meniscus.
Goat model:Murphyet al[156]explored the role of BM-MSCs in tissue repair or regeneration of the OA-induced knee in a goat model.The authors injected 10 × 106autologous BM-MSCs suspended in a dilute solution of sodium hyaluronan directly into the injured knee.At 6 wk following intra-articular MSC injection,marked regeneration of the meniscus was identified with implanted cells detected in the newly formed tissue of the treated group.Reduced cartilage degeneration,osteophytic remodeling,and subchondral sclerosis were found in the treated group(sodium hyaluronan only group).The authors concluded that intra-articular injection of BMMSCs stimulated regeneration of the meniscal tissue and retarded the progressive destruction of the OA joint.
Canine model:Abdel-Hamidet al[157]attempted autologous BM-MSCs in a canine model study.The authors evaluated the healing process both clinically and histologically.At 12 wk,the MSC-treated menisci showed significantly enhanced angiogenesis,chondrogenesis,immune cell infiltration,and proliferation of fibroblasts with marked deposition of collagen fibers compared with the non-treated group.
Sheep model:Al Faqehet al[158]performed a sheep model study to determine whether intra-articular injection of autologous chondrogenic-induced BM-MSCs could retard the progressive destruction of surgically induced OA knees.At 6 wk after intraarticular injection,although undifferentiated BM-MSCs and control groups showed damage and absence of a meniscus,the chondrogenic-induced BM-MSC group showed the appearance of meniscal-like tissue formation at the injured meniscus.In a study by Caminalet al[159],a meniscal tear was created arthroscopically in the anterior horn of the medial meniscus.The animals were monitored by MRI and ultrasound,and macroscopic and histological analyses were conducted at 6 and 12 mo.Twelve months after injection,four out of the eight medial menisci treated with BMMSCs presented healthy tissue features.However,the rest of the menisci showed some areas of erosion on the inner edge of the femoral side.
Horse model:Ferriset al[160]reported clinical outcomes after intra-articular injection of BM-MSCs in a horse model.At a mean 24 mo follow-up after intra-articular administration of autologous BM-MSCs,43% of the enrolled horses returned to the previous level of work,33% returned to work,and 24% failed to return to work.In horses with meniscal injury,a higher percentage(75%)returned to some level of work than those in previous reports(60%-63%)that were treated with arthroscopy alone(P= 0.038).
Human studies
BM-MSCs:As mentioned above,there have been promising preclinical results in intra-articular injection using various sources of MSCs over the past 20 years.Based on these successful preclinical results,though a limited number of studies,there have been clinical trials regarding the effects of intra-articular injection of MSCs for meniscal healing[95,98,99,104,161].In 2008,Centenoet al[161]first reported intraarticular injection of BM-MSCs in a 36-year-old man.After 3 mo,MRI showed increased meniscus volume.The modified visual analog scale(VAS)scores decreased from 3.33 to 0.13.In 2014,Vangsnesset al[95]reported a randomized,double-blind,controlled study.The authors examined the effect of a single intra-articular injection of BM-MSCs following partial meniscectomy.After partial meniscectomy,a single intraarticular injection of allogeneic BM-MSCs was administered.Patients were randomized into one of the three groups.In group A,patients received an injection of 50 × 106allogeneic BM-MSCs.In group B,15 × 107allogeneic BM-MSCs were administered.Group C was the control group with only sodium hyaluronate(hyaluronic acid,hyaluronan).After treatment,no ectopic tissue formation was observed in the blinded MRI evaluation.After 1 year,a significant meniscal volume increase(defined as a 15% increase in meniscal volume)was determined by quantitative MRI in 24% of patients in group A and 6% in group B(P= 0.022),and the overall group comparison was significant at 2 years(P= 0.029).Based on VAS assessment,among patients with OA at the time of surgery,a significant improvement was observed in the BM-MSC groups compared with the control group up to 2 years.
S-MSCs:Sekiyaet al[98]examined the effects of intra-articular S-MSC injection after repairing the degenerative tears of the medial meniscus in five patients.No major adverse events leading to the study termination were identified.Lysholm scores were significantly higher at 1 year than before MSC treatment and were maintained at 2 years.KOOS scores for “daily living,” “pain”,and “sports and recreational activities”improved significantly at 2 years.The numerical rating scale(NRS)scores continually increased and showed significant improvement at 2 years.Three-dimensional MRI showed indistinguishable healing at the meniscal lesion at 2 years.
A-MSCs:In 2014,Paket al[104]reported a case report of intra-articular injection of autologous A-MSCs in a 32-year-old woman.Three months after injection,the patient’s symptoms improved,and repeated MRI showed almost complete disappearance of the meniscus lesion.After 18 mo,her symptom improvement persisted without any serious side effects.Onoiet al[99]reported second-look arthroscopic findings in two cases of knee OA treated using an intra-articular injection of AMSCs.The authors performed a second-look arthroscopy 6 mo after a single intraarticular injection of A-MSCs.During the 6-month follow-up,no serious adverse events were reported.Pain and function assessed by VAS and KOOS were significantly improved from baseline in the treated knees at 1,3,and 6 mo.The second-look arthroscopy showed that almost all the cartilage defect areas were covered with regenerated cartilage,and the degenerative meniscal tear areas were repaired.
TISSUE ENGINEERING USING MSCS FOR MENISCUS HEALING
Tissue engineering is a promising approach for the treatment of various tissue defects.MSCs can be used as a source of repair cells or essential growth factors for tissue engineering.In the past 20 years,there have been several preclinical and clinical trials using various MSCs for meniscal healing(Table 2).Although one study did not show any significant differences between the control and MSC-treated groups[162],other studies have generally shown promising results.
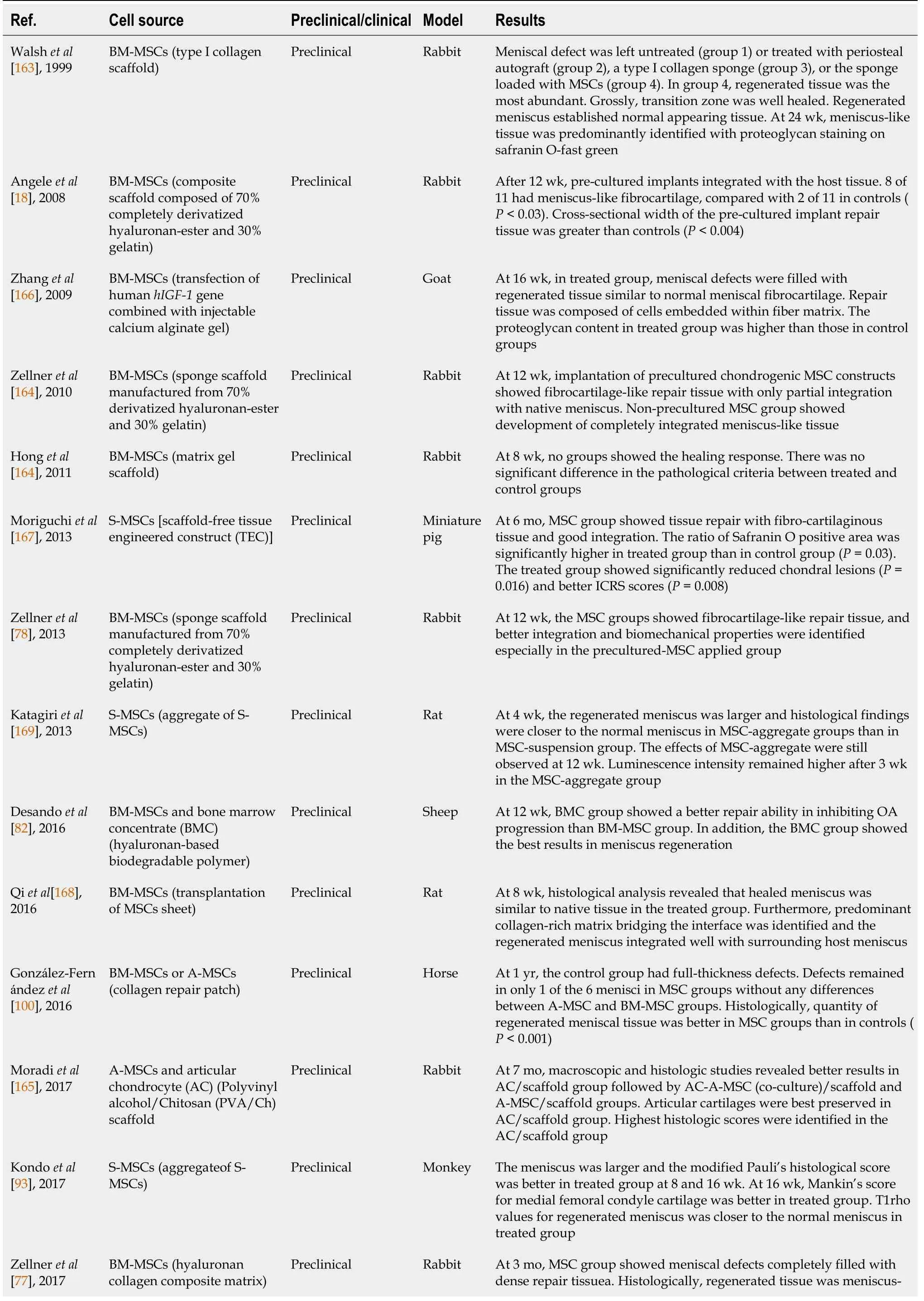
Table 2 Summary of recent preclinical and clinical studies regarding tissue engineering using mesenchymal stem cells for meniscus healing
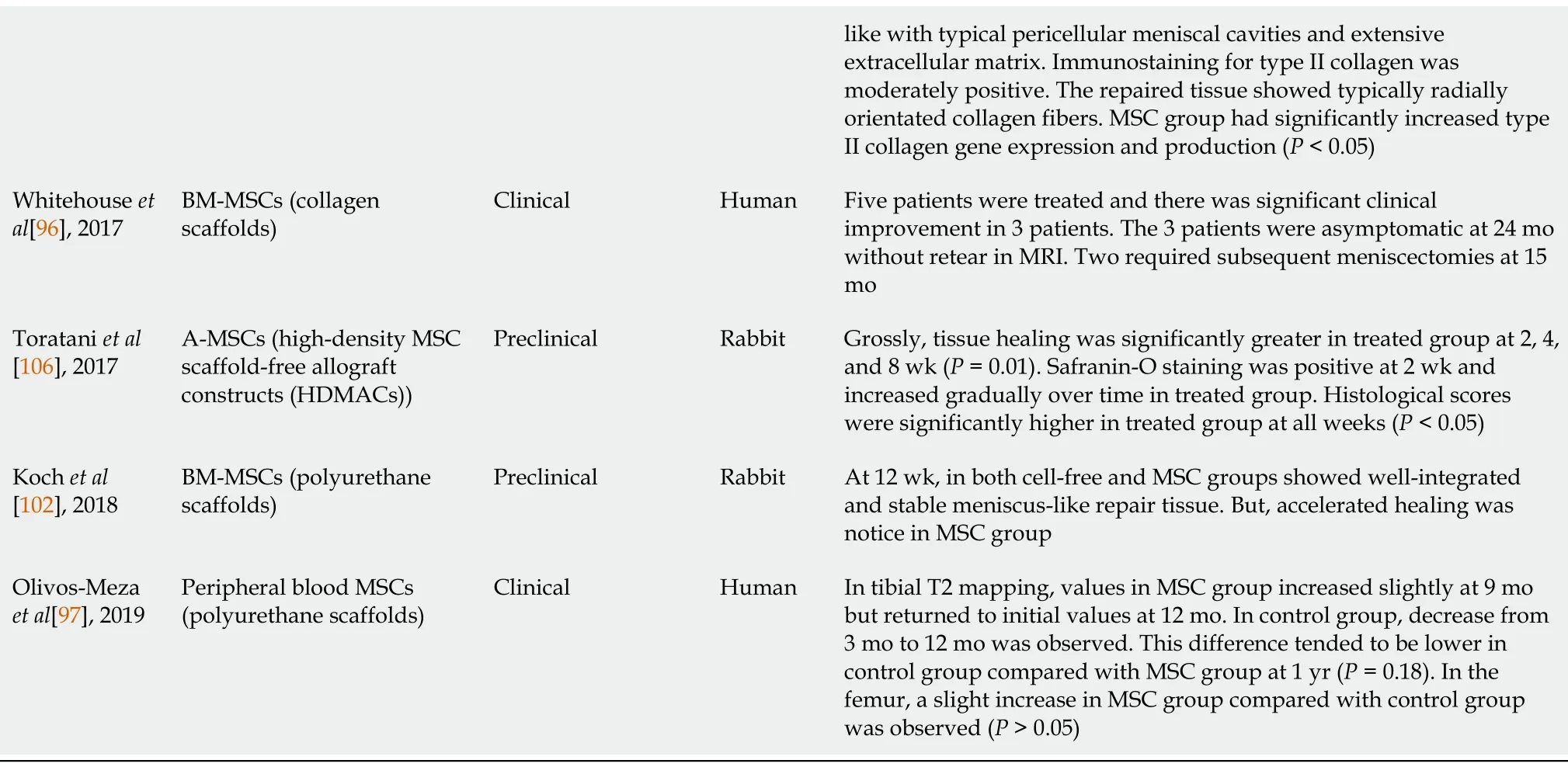
BM-MSCs:Bone marrow-derived mesenchymal stem cells;MSC:Mesenchymal stem cell;S-MSCs:Synovium-derived mesenchymal stem cells;ICRS:International Cartilage Repair Society;A-MSCs:Adipose-derived mesenchymal stem cells;OA:Osteoarthritis;MRI:Magnetic resonance imaging.
Animal studies
Rabbit model:BM-MSCs are the most frequently used in preclinical studies.In 1999,Walshet al[163]reported a preclinical study on applying a type I collagen scaffold containing BM-MSCs for meniscal defects in a rabbit model.After partial meniscectomy,the meniscal defect was left untreated(group 1)or treated with one of the following:a periosteal autograft(group 2),a type I collagen sponge(group 3),or the same sponge loaded with autologous BM-MSCs(group 4).In group 4,the regenerated tissue was more abundant at each time point than the other groups.Grossly,the transition zone was smooth and healed well.The regenerated meniscus established normal-appearing tissue connecting to the peripheral capsular attachments.At 24 wk,a predominant meniscus-like tissue with fibrochondrocytes was identified with mature collagen bundles and proteoglycan staining with safranin O-fast green.However,OA changes were observed in Group 4.Angeleet al[18]tested a BM-MSC-loaded composite scaffold composed of 70% completely derivatized hyaluronan-ester and 30% gelatin for meniscal defects in a rabbit model.At 12 wk,the untreated defects showed a muted fibrous healing.Defects treated with cell-free scaffolds also showed predominantly fibrous tissue.The cross-sectional width of the repair tissue after treatment with cell-free scaffolds was significantly greater than that of the controls(P< 0.05).BM-MSC-loaded scaffolds integrated well with the host tissue,and eight of 11 contained meniscus-like fibrocartilage,compared with two of 11 in controls(P< 0.03).The mean cross-sectional width of the BM-MSC-loaded scaffolds group was greater than that of the control group(P< 0.004).Zellneret al[164]applied BM-MSCs in sponge scaffolds manufactured from 70% derivatized hyaluronan-ester and 30% gelatin for circular meniscus punch defects(2 mm)in a rabbit model.After 12 wk,untreated defects and defects treated with cell-free scaffolds exhibited muted fibrous healing responses.The implantation of precultured chondrogenic MSC constructs showed fibrocartilage-like repair tissue,which was only partially integrated with the native meniscus.Non-precultured MSCs in hyaluronan-collagen composite scaffolds showed the development of completely integrated meniscus-like repair tissue with significant differences compared with cell-free scaffolds(P= 0.005).Honget al[162]investigated the biological healing responses after application of human BMMSCs in a matrix gel scaffold for complete radial tear of the anterior horn of the medial meniscus in a rabbit model.However,after 8 wk,enhanced healing was not observed in the treatment group.There was no significant difference in the pathological criteria between the treated and control groups.Zellneret al[78]applied BM-MSCs in sponge scaffolds manufactured from 70% derivatized hyaluronan-ester and 30% gelatin for 4-mm longitudinal meniscal tears in the avascular zone of the lateral menisci in a rabbit model.At 12 wk,untreated defects,defects treated with suturing alone,with cell-free,or with platelet-rich plasma seeded scaffolds showed a muted fibrous healing.The MSC groups showed fibrocartilage-like repair tissue,and better integration and biomechanical properties were identified,especially in the precultured MSC-treated group.Zellneret al[77]compared the regeneration capacity of BM-MSCs seeded in a hyaluronan collagen composite matrix with autologous meniscal cells in a rabbit model.At 3 mo,the MSC group showed meniscal defectscompletely filled with dense repair tissue with stable integration to the native meniscus.Histologically,the regenerated tissue was meniscus-like with a low cell density but typical pericellular meniscal cavities and an extensive extracellular matrix.Immunostaining for type II collagen was moderately positive.The architecture of the repaired meniscus typically showed radially oriented collagen fibers.The meniscal scoring system showed no statistically significant difference between the meniscal cell and MSC groups at 6 wk or 3 mo.However,the MSC group had significantly increased type II collagen gene expression and production compared with the meniscal cells group(P< 0.05).Furthermore,macroscopic joint assessment demonstrated donor-site morbidity during meniscal cell treatment.Kochet al[102]applied a polyurethane scaffold loaded with BM-MSCs to repair critical meniscus defects(7-mm broad lesions)in the avascular zone in a rabbit model.At 12 wk,both the cell-free and MSC-loaded groups showed well-integrated and stable meniscus-like repair tissue.However,accelerated healing was observed in the MSC group.Both groups showed dense vascularization throughout the repaired tissue.Moradiet al[165]evaluated the effects of polyvinyl alcohol/chitosan(PVA/Ch)scaffolds seeded with A-MSCs and articular chondrocytes(AC)on meniscus regeneration.At 7 mo,macroscopic,histologic,and immunofluorescent studies revealed better results in the AC/scaffold group followed by the AC-A-MSC(co-culture)/scaffold and AMSCs/scaffold groups.In the control(cell-free scaffold)group,no apparent meniscal regeneration was observed.Articular cartilage was also best preserved in the AC/scaffold group.Modifying Pauli's scoring system showed the highest score(15 points)in the AC/scaffold group.Torataniet al[106]implanted scaffold-free 3-dimensional allogeneic A-MSC constructs for meniscal defects in a rabbit model.Macroscopically,the height of the healing tissue was significantly greater in the treated group than in the control group at 2 wk(P= 0.01),4 wk(P= 0.01),and 8 wk(P= 0.02).Histologically,safranin-O staining was positive at 2 wk and increased gradually over time in the treated group.Histological tissue quality scores were significantly higher in the treated group than in the control group at all weeks(P<0.05).
Goat model:Zhanget al[166]investigated whether BM-MSCs transfected with the human insulin-like growth factor 1(hIGF-1)gene combined with injectable calcium alginate gel improved the repair of full-thickness meniscal defects in a goat model.At 16 wk,the meniscal defects in the treated group were filled with regenerated tissue,similar to that in normal meniscal fibrocartilage.The repaired tissue was composed of cells embedded within the fiber matrix.The proteoglycan content in the treated group was higher than that in the control group,and less than that in the normal meniscus.
Sheep model:Desandoet al[82]investigated the regenerative potential of bone marrow concentrate(BMC)and BM-MSCs engineered with a hyaluronan-based biodegradable polymer(Hyaff-11)for OA knees in a sheep model.At 12 wk,the BMC group showed a better repair ability in inhibiting OA progression than the BM-MSCs group,with a reduction in inflammation in the cartilage,meniscus,and synovium.In addition,the BMC group showed the best results for meniscal regeneration.
Porcine model:Although limited,S-MSCs have also been used preclinically in tissue engineering for meniscus healing.Moriguchiet al[167]performed a preclinical study to test the feasibility of a scaffold-free tissue-engineered construct(TEC)derived from SMSCs for meniscal defects(4-mm cylindrical defect)in a miniature pig model.At 6 mo,the treated group showed tissue repair with fibrocartilaginous tissue and good integration to the host meniscal tissue.However,the control group showed either partial or unrepaired defects.The ratio of Safranin O-positive area was significantly higher in the treated group than in the control group(P= 0.03).Moreover,the treated group showed significantly reduced size and severity of post-traumatic chondral lesions on the tibial plateau(P= 0.0163)and better International Cartilage Repair Society(ICRS)scores(P= 0.008).
Rat model:Qiet al[168]investigated whether the use of BM-MSC sheets could effectively regenerate the meniscus in a rat model.At 8 wk,histological analysis revealed that the healed anterior portion of the meniscus was similar to that of the native tissue with typical fibrochondrocytes surrounded by richer extracellular matrix in the treated group.The histological scores in the treated group were 5.5 ± 0.8 and 3.8± 0.6 at 4 and 8 wk,respectively.These scores were significantly lower than those in the control group,9.8 ± 1.5 and 16.3 ± 2.4 at 4 and 8 wk(P< 0.05).Furthermore,a predominant collagenrich matrix bridging the interface was identified,and the regenerated meniscus integrated well with the surrounding host meniscus.Katagiriet al[169]investigated whether S-MSC aggregates regenerated a meniscus more effectively than S-MSC suspension when the same number(25000)of the cells was used in a rat model.At 4 wk,the regenerated meniscus area was more extensive and the histological findings were closer to that of the normal meniscus in the S-MSC aggregate groups than in the S-MSC suspension group.The effects of S-MSC aggregate transplantation remained at 12 wk.Luminescence intensity remained higher at 3 wk and thereafter in the aggregate S-MSC group than in the suspension S-MSC group.
Monkey model:Based on the results of Katagiriet al[169],Kondoet al[93]tried aggregates of S-MSCs for meniscus regeneration in a monkey model.After the anterior halves of the medial menisci were removed,the authors transplanted an average of 14 aggregates consisting of 250000 S-MSCs for the meniscus defect.The meniscus was larger and the modified Pauli’s histological score was better in the treated group than in the control group at 8 and 16 wk.At 16 wk,Mankin’s score for the medial femoral condyle cartilage was better in the treated group than in the control group in all primates.T1rho values for both the regenerated meniscus and medial femoral condyle cartilage were closer to the normal meniscus in the treated group than in the control group at 16 wk.
Horse model:González-Fernándezet al[100]examined the ability of meniscus regeneration using a collagen repair patch(scaffold)seeded with BM-MSCs or AMSCs in a horse model.At 1 year,macroscopically,the control group had fullthickness defects in all samples.Defects remained in only one of the six menisci in the MSC groups.There were no macroscopic differences between the A-MSC and BMMSC groups.Histologically,the quantity of regenerated meniscal tissue(determined by staining with Safranin O fast green)was better in meniscal defects treated with MSCs than in control defects(P< 0.001).
Human studies
BM-MSCs:Tissue engineering techniques using engineered scaffolds and MSCs have achieved promising results in preclinical studies.Some authors have attempted tissue engineering using MSCs in clinical studies based on these preclinical studies.However,these clinical studies are limited in number.In 2017,Whitehouseet al[96]reported the clinical outcomes of BM-MSC seeded collagen scaffold application in five patients.The BM-MSCs/collagen-scaffold implant was applied to the meniscal tear prior to repairing vertical mattress sutures.The follow-up period was two years.Of the five treated patients,the implant survived without further treatment in three cases.However,the remaining two patients subsequently underwent meniscectomy due to recurrent symptoms after approximately 15 mo.The three successfully treated patients showed improvements in all clinical scores over the first year,and these changes were maintained for up to 2 years.After 1 year,MRIs of the three patients showed no evidence of displacement or re-tearing.
Peripheral blood MSCs:Olivos-Mezaet al[97]attempted the first clinical application of polyurethane scaffolds with peripheral blood MSCs in 11 patients with meniscal defects.Patients enrolled in the treated group received a daily subcutaneous injection of 300 μg of G-CSF(Filgrastim)for 3 consecutive days to increase the pool of MSCs in the peripheral blood stream.The author assessed the articular cartilage changes with T2 mapping in the femur and tibia.The tibia values in the treated group increased slightly after 9 mo but returned to their initial values after 12 mo(P> 0.05).In the control group,a marked decrease from 3 mo to 12 mo was observed(P> 0.05).This difference was significantly lower in the control group than in the treated group(P=0.18).In the femur,a slight increase in the treated group(47.8 ± 3.4)compared with the control group(45.3 ± 4.9)was observed(P> 0.05).The authors concluded that the addition of MSCs did not show any advantage in protecting articular cartilage over acellular scaffolds.
CONCLUSION
Meniscus injuries are common and can lead to rapid degeneration of the knee joint.Due to limited intrinsic healing capacity,current reparative treatments fail to produce long-term improvements,and thus,alternative regenerative strategies,including MSCs and tissue engineering are being tried.Recent preclinical and clinical studies have suggested that MSCs appear to be safe and effective for enhanced meniscal healing.However,the number of studies was limited.Therefore,despite the positive and promising results,there is still no consensus on the ideal cell sources and scaffold manufacturing techniques for meniscus regeneration.Most of the current studies have tried BM-MSCs,S-MSCs,and A-MSCs.To identify proper cell sources and optimize their clinical application,further investigations of multiple cell source types,including meniscal cells and MSCs derived from other tissues,are necessary.In terms of tissue engineering,the application of various scaffolds should be validated based on their long-term efficacy and safety.Considering the complexity of the structure and function of the meniscus,further anatomical and biomechanical investigations are necessary to manufacture successful scaffolds.Although many preclinical studies have shown positive and promising results,only a few clinical studies have been conducted with a limited number of patients and a short follow-up period.Further advancements in future clinical studies with many patients,long-term follow-up,various cell sources,and proper control groups could validate the safety and efficacy of MSC application in clinical settings to enhance meniscal healing and slow or delay the progression of knee OA.
杂志排行
World Journal of Stem Cells的其它文章
- Differences and similarities between mesenchymal stem cell and endothelial progenitor cell immunoregulatory properties against T cells
- Inter-regulatory role of microRNAs in interaction between viruses and stem cells
- Modulating poststroke inflammatory mechanisms:Novel aspects of mesenchymal stem cells,extracellular vesicles and microglia
- Antler stem cells and their potential in wound healing and bone regeneration
- Therapeutic prospects of mesenchymal stem/stromal cells in COVID-19 associated pulmonary diseases:From bench to bedside
- Mesenchymal stem cells as a potential therapeutic tool to cure cognitive impairment caused by neuroinflammation
