Differences and similarities between mesenchymal stem cell and endothelial progenitor cell immunoregulatory properties against T cells
2021-09-03MehdiRazazianMaryamKhosraviSheydaBahiraiiGeorgesUzanSaraShamdaniSinaNaserian
Mehdi Razazian,Maryam Khosravi,Sheyda Bahiraii,Georges Uzan,Sara Shamdani,Sina Naserian
Mehdi Razazian,Georges Uzan,Sara Shamdani,Sina Naserian,Institut national de la santé et de la recherche médicale(Inserm)Unité Mixte de Recherche-Inserm-Ministère de la Défense 1197,Hôpital Paul Brousse,Villejuif 94800,France
Maryam Khosravi,Microenvironment &Immunity Unit,Institut Pasteur,Paris 75724,France
Maryam Khosravi,Institut national de la santé et de la recherche médicale(Inserm)Unit 1224,Paris 75724,France
Sheyda Bahiraii,Department of Pharmacognosy,University of Vienna,Vienna 1090,Austria
Georges Uzan,Sara Shamdani,Sina Naserian,Paris-Saclay University,Villejuif 94800,France
Sara Shamdani,Sina Naserian,CellMedEx;Saint Maur Des Fossés 94100,France
Abstract Bone-marrow-derived mesenchymal stem cells and endothelial progenitor cells have some interesting biological properties that make them unique for cell therapy of degenerative and cardiovascular disorders.Although both cell populations have been already studied and used for their regenerative potentials,recently their special immunoregulatory features have brought much more attention.Mesenchymal stem cells and endothelial progenitor cells have both proangiogenic functions and have been shown to suppress the immune response,particularly T cell proliferation,activation,and cytokine production.This makes them suitable choices for allogeneic stem cell transplantation.Nevertheless,these two cells do not have equal immunoregulatory activities.Many elements including their extraction sources,age/passage,expression of different markers,secretion of bioactive mediators,and some others could change the efficiency of their immunosuppressive function.However,to our knowledge,no publication has yet compared mesenchymal stem cells and endothelial progenitor cells for their immunological interaction with T cells.This review aims to specifically compare the immunoregulatory effect of these two populations including their T cell suppression,deactivation,cytokine production,and regulatory T cells induction capacities.Moreover,it evaluates the implications of the tumor necrosis factor alpha-tumor necrosis factor receptor 2 axis as an emerging immune checkpoint signaling pathway controlling most of their immunological properties.
Key Words:Endothelial Progenitor Cells;Mesenchymal Stem Cells;T cells;Immunosuppression;Immunoregulation;TNFα-TNFR2 signaling pathway
INTRODUCTION
Mesenchymal stromal/stem cells
In the year 1968,mesenchymal stromal/stem cells(MSCs)were first isolated from bone marrow(BM)cells[1].These cells represent heterogeneous cell populations that have shown various abilities and potentials such as proliferation and regeneration.According to previous shreds of evidence,they can differentiate into a variety of cell types of several origins like ectodermal,endodermal,and mesodermal such as cardiomyocytes,osteocytes,keratocytes,hepatocytes,and endothelial cells(ECs),neural cells,adipocytes,chondrocytes,and myocytes[2-6].MSCs can express markers such as stem cell antigen 1,CD29,CD51,CD73,CD44,CD146,CD90,and CD105 but are negative for the expression of CD31 endothelial,CD14 monocytes,and CD45 hematopoietic lineage markers[7-10].
Due to their special biological capacities including the ability to differentiate,regulate the immune responses,and produce and release various mediators,MSCs are extensively studied in fundamental research and are one of the best choices for cell therapy and clinical applications[11,12].Based on MSC regenerative and immunomodulatory effect,they have been used for tissue damage repair and anti-inflammatory activities as an effective alternative therapy in over 700 clinical trials such as inflammatory diseases like graftvshost disease[13,14],Crohn’s disease[15],rheumatoid arthritis[16],and lupus nephritis[17],in transplantations like hematopoietic stem cell transplantation[18,19]and kidney transplantation[20,21],cardiovascular diseases[22-24],fibrotic diseases[25,26],spinal cord injury[27,28]and many others.
MSCs can significantly influence their microenvironment.They interact with the other cells,extracellular matrix,bioactive mediators such as cytokines,and therefore they can change the state of the surrounding inflammation[29-34].They can support hematopoietic cells and regulate several types of innate and adaptive immune cells including mast cells,macrophages,myeloid-derived suppressor cells,neutrophils,natural killer cells,dendritic cells,B cells,and especially T cells[8,35-38].
Endothelial progenitor cells
Another population of BM-derived progenitor/stem cells is endothelial progenitor cells(EPCs)that are considered as a circulating reservoir of endothelial progeny and are applied in repairing tissue damages and neovascularization at the damaged endothelial sites[39-41].In vitro,two different cell populations of EPCs have been identified based on their first colony appearance time:early EPCs or colony-forming unit-ECs and late EPCs or endothelial colony-forming cells(ECFCs)[42].Both of these groups can exhibit specific endothelial markers like CD31,CD133,CD144,KDR(vascular endothelial growth factor receptor 2),von Willebrand factor,and CD146 and can engage in angiogenesis function.Nevertheless,colony-forming unit-ECs are unable to form vasculaturein vivobecause they are thought to be hematopoieticderived monocyte/macrophage colonies that primarily exert a paracrine proangiogenic effect.ECFCs,on the other hand,do not express CD45 hematopoietic and CD14 monocyte markers,while it has been recently reported that BMP2,4 and ephrinB2 were exclusively highly expressed[42,43].Furthermore,in comparison to colony-forming unit-ECs,they exhibit a strong clonogenic and proliferative capability[42,44,45].As a result,ECFCs are regarded as true EPC progeny,with all EC characteristics such as endothelial marker expression and vasculogenesis as well as stem/ progenitor cell features such as high clonogenicity and proliferation rate[39,46].ECFCs can be isolated from cord blood(CB)and adult peripheral blood(APB).They have several distinct characteristics that make them particularly appealing for the treatment of cardiovascular,hematological,and degenerative disorders in which angiogenesis regulation is critical(Figure 1).Our team,for example,demonstrated that autologous EPCs can successfully treat right ventricular failure in a piglet model of chronic thromboembolic pulmonary hypertension[47].
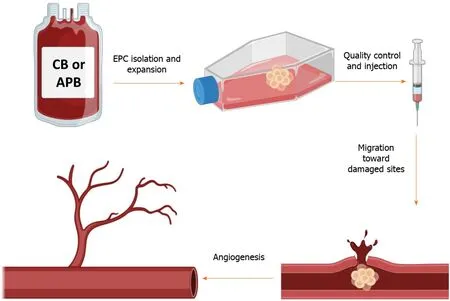
Figure 1 This schematic depicts the simplified procedure from isolation to the application of endothelial progenitor cells.Briefly,endothelial progenitor cells(EPCs)are isolated from the cord blood or adult peripheral blood and are seeded on a culture flask.After the appearance of the first colonies(around 3 wk),cells are passaged and expanded to reach the desired numbers.After several verifications,EPCs could be injected into patients with cardiovascular disorders to take advantage of their regenerative and proangiogenic properties.The graphical images were created with BioRender.com.APB:Adult peripheral blood;CB:Cord blood.
Several other studies during the last decade reported that EPCs bear many therapeutic advantages in clinical therapies for other disorders,notably cardiovascular complications[48-50],thus the clinical capability of EPCs in terms of vascular regeneration is a hotline of trial applications[51,52].Meanwhile,according to Clinical-Trials.gov,numerous disease conditions are investigated according to the regenerative potential of EPCs mostly in patients with ischemic diseases,such as peripheral vascular disease and myocardial infarction.Clinical EPC applications are sorted into three major domains:(1)cellular injections;(2)EPC mobilization therapies;and(3)EPC-capture stents.Heretofore,until April 2021,more than 380 EPCs clinical studies were registered at ClinicalTrials.gov.
Unlike MSCs,there are not many studies to evaluate the immunogenicity and immunoregulatory features of EPCs and their interaction with the immune system.Most of the previous studies have used EPCs to restore blood perfusion notably in hind limb ischemia condition that was performed in immunodeficient mouse models to avoid potential immunological responses[53-55].Nuzzoloet al[56]already demonstrated that EPCs derived from CB have a significantly lower proinflammatory and prothrombotic profile than adult EPCs.Some limitations of this work are that EPCs were not compared to mature ECs,thus one cannot observe whether the reported results are progenitor dependent or not.Furthermore,these evaluations were at the gene expression level,which can be different compared to the protein level.In an allogenic combination,Ladhoffet al[57]demonstrated that rat EPCs are immunotolerated against allogeneic immune responses and particularly humoral-mediated attacksin vitro.Furthermore,when they transplanted these cells as a component of a vascular graft,allogenic EPCs were not rejected[57].However,the interaction of the immune system,notably T cells with EPCs,remains unclear.In an attempt to clarify these missing pieces of information,we have recently reported that EPCs can also regulate the immune response and bear some level of immunoregulation,especially against T cells[40].
This present article aims to review some known similarities and differences between MSCs and EPCs BM-derived progenitor/stem cells that are involved in regeneration and immunoregulatory functions.It describes and compares different mechanisms that they use to suppress conventional T cells(T convs)and/or to induce regulatory T cells(Tregs).Among different mechanisms of action,we emphasize the implication of inflammatory signaling pathways such as the tumor necrosis factor alpha-tumor necrosis factor receptor 2(TNFα-TNFR2)immune checkpoint axis.We try to cover the lack of information by proposing new research directions and their importance for future studies includingin vitroandin vivoapplications in regenerative medicine.
THE INTERACTION OF MSC AND EPC WITH THE IMMUNE SYSTEM
Besides their regenerative and tissue-protective features,many studies have been focusing on the interaction of MSCs and EPCs with the immune system in different biological conditions including inflammation that is caused by tissue damage,transplantation,cancer,etc.The following section describes the impact of these stem/progenitor cells on T cells and specifically compares their immunosuppressive,immunoregulatory,and Treg induction capacity.
MSCs and EPCs demonstrate a dissimilar level of T cell immunosuppression
Plenty of investigations from the past two decades revealed that MSCs have considerable levels of immunosuppressive properties against both innate and adaptive immune responses[35,36].For instance,in the case of natural killer cells that are principal effector members of innate immunity and are believed to play a crucial role in anti-tumor and anti-viral responses,MSCs can inhibit their cytotoxicity,proliferation,and cytokine secretion through prostaglandin E2 and indoleamine 2,3-dioxygenase dependent mechanisms[58].Macrophages are another principal population of innate immunity and regulators of tissue repair such as wound healing that are involved in inflammation by producing different cytokines and growth factors[59].It has been shown that MSCs can alter the polarization of macrophage main subpopulations[60].They change the plasticity of macrophages by polarizing them towards more anti-inflammatory M2 and less proinflammatory M1 subpopulations[37].
In the case of adaptive immunity,T cells are by far the most studied population.Depending on their tissue origin,it has been shown that MSCs can both directly through cell-cell contact and indirectlyviathe production of different mediators such as cytokines and growth factors change the property of these cells.MSC immunoregulatory features are regulated by the secretion of a variety of anti-inflammatory mediators such as IL-10,TGFβ,indoleamine 2,3-dioxygenase,prostaglandin E2,nitric oxide,and many others[7].MSCs can modulate T cell proliferation,expression of different activation markers,and anti- and proinflammatory cytokine production patterns and can regulate the balance of different T cell subpopulations.For example,it has been recently reported that MSCs regulate the Th17/Treg cell balanceviahepatocyte growth factors[61].Moreover,the overexpression of heme oxygenase-1 by MSCs was reported to suppress natural killer cells,decrease the balance of Th1/Th2,and facilitate Th17 into Treg conversionin vitro.During anin vivoassay in a decreased size liver transplant rejection model,understudy animals demonstrated a lower transplant rejection rate and proinflammatory cytokine levels followed by an elevated number of peripheral Treg and greater anti-inflammatory cytokine levels[62].
MSCs are isolated from different neonatal and adult sources that could affect their regenerative and immunological properties.MSCs harvested from different sources have different characteristics,advantages,and drawbacks,such as their differentiation,colony-forming unit,and proliferation capacities that influence their potential in clinics[63].We have recently revealed that MSCs derived from fetal sources such as fetal liver(FL)are more immunosuppressive than BM-derived MSCs[9].In this setting,we have reported that FL-MSCs were more efficient to suppress the proliferation of both CD4+CD25-and CD8+CD25-T conv populations in comparison to commonly used BM-MSCs.Furthermore,FL-MSCs were more functional in decreasing the expression of T cell activation markers such as CD25,GITR,ICOS,and TNFR2 in both populations[9].
As already mentioned EPCs are harvested from CB and APB sources.CB-EPCs are better cell therapy possibilities for the treatment of cardiovascular diseases,according to previous evidence comparing these two accessible sources[64,65].Nevertheless,because CB-EPCs are obtained from allogeneic sources,it is critical to understand how the host immune system reacts when they are administered.Before their extensive clinical implementation,two major difficulties must be addressed:(1)Do allogenic EPCs have immunogenic qualities,and as a result,may they elicit an immune response? and(2)Is it possible for the host immune system to recognize EPCs as pathogen targets leading to their eventual rejection?
To find answers to these concerns,we evaluated the EPC immunogenicity by investigating if human leukocyte antigen(HLA)-mismatched total peripheral mononuclear cells(PBMCs)can recognize them as allogenic stimulating cells.Allogenic CB-EPCs were unable to stimulate peripheral blood mononuclear cell proliferation as compared to the allogenic HLA-II+ lymphoblastoid cell line,which resulted in increased proliferation of peripheral blood mononuclear cells[66].Co-culturing them with peripheral blood mononuclear cells,on the other hand,resulted in dose-dependent immunosuppression,which was verified in third-party donors.Furthermore,Proustet al[66]administered human CB-EPCs into xenogeneic immunocompetent ischemic mice and showed that these cells were tolerated by the murine immune system for at least 14 d and could correctly integrate into the ischemic site and perform their proangiogenic function.
In addition,recently published articles report that EPCs have also some level of immunoregulatory functions.For instance,Naserianet al[40]implanted EPCs derived from CB into a bio-artificial vessel model and reported that in complete contrast to mature ECs like human aortic ECs,which are already differentiated cells,CB-EPCs could suppress CD4 and CD8 T cells in a dose-dependent manner[40].Further attempts to clarify the underlying mechanism for their immunosuppressive effect revealed that similar to MSCs,EPCs can also produce anti-inflammatory molecules such as IL-10,TGFβ,and HLA-G[66-68].The comparative pieces of information from our team reveal a significantly higher immunosuppressive function of MSCs in comparison to EPCs.Our findings demonstrate while CB-EPCs are more immunosuppressive than APB-EPCs,both FL and BM-MSCs are remarkably more suppressive than EPCs(Figure 2).
Furthermore,we have evaluated the ability of EPCs from different sources to decrease the activation profile of the T cells.Interestingly,we have noticed that both CB-EPCs and APB-EPCs were capable to reduce the activity of CD4 and CD8 T cells with a higher immunoregulatory effect observed with CB-EPCs[68].Comparing MSCs and EPCs let us conclude that once more MSCs are stronger regulators of T cell activation markers such as CD25,GITR,ICOS,and TNFR2(Figure 2).Consequently,after immunosuppression and deactivation,the ability of T cells to produce different cytokines is altered.Our recent results made it clear that similar to MSCs,EPCs can also decrease the secretion of T cell proinflammatory cytokines such as TNFα,IFNγ,IL-17,and IL-2,but unlike MSCs they do not elevate the production of anti-inflammatory cytokines such as TGFβ and IL-10(Figure 2)[7,8,68].
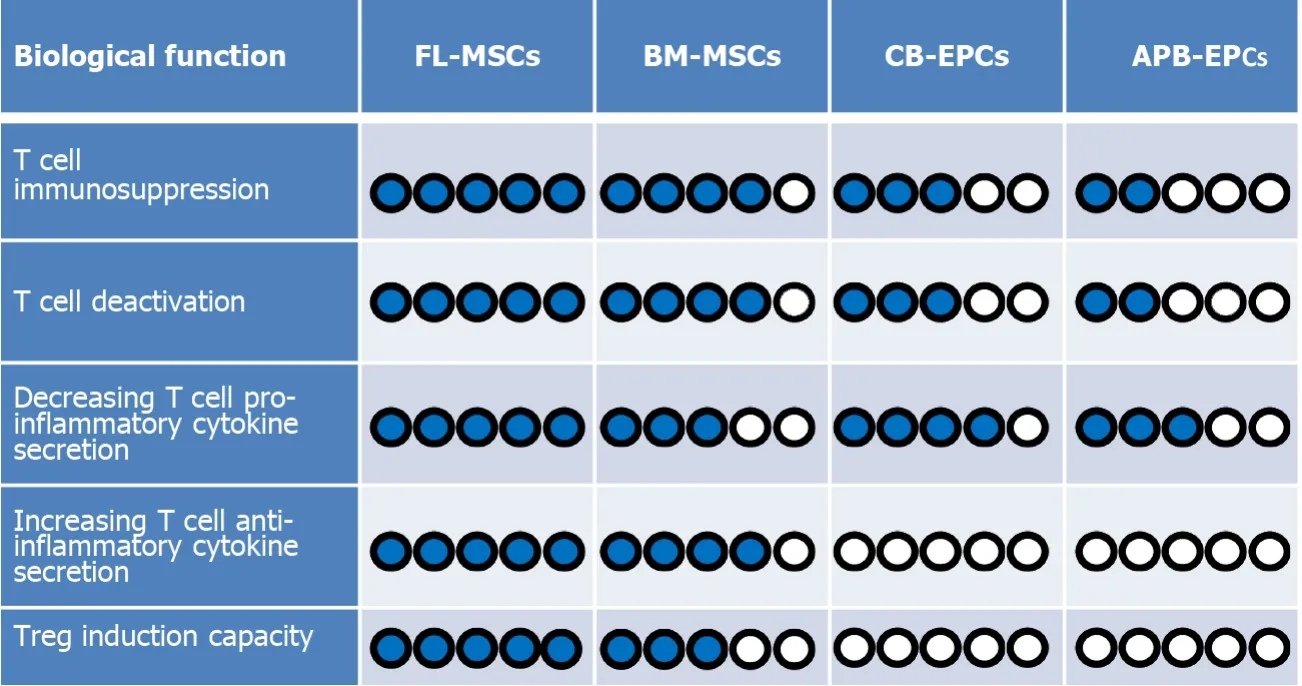
Figure 2 A comparison between immunoregulatory functions of mesenchymal stem cells and endothelial progenitor cells.According to our evaluations,among the four cell types[fetal liver(FL)derived mesenchymal stem cells(MSCs),bone marrow-derived(BM)-MSCs,cord blood-derived(CB)-endothelial progenitor cells(EPCs),and adult peripheral blood-derived(APB)-EPCs].Fetal liver-derived(FL)-MSCs are the most immunomodulatory cells because they:(1)suppress T cell proliferation;(2)decrease T cell activation phenotype;and(3)decrease T cell secretion of proinflammatory cytokines.On the other hand,they could more efficiently increase:(1)the secretion of anti-inflammatory cytokine;and(2)the induction of regulatory T cells(Tregs)more than the other cells.Therefore,we have considered FL-MSCs as the reference(5/5 blue points)regarding the measured criteria and compared the capacity of the other cells with them.In the case of CB-EPCs and APB-EPCs,because we did not notice any Treg induction or elevation of anti-inflammatory cytokine secretion we attributed a 0/5 score.Blue circles represent elevated levels while white circles represent the absence of the effect.
MSCs and EPCs have different Treg induction capacities
One of the main mechanisms of immunosuppression by MSCs(regardless of their isolation source)is through induction of the expression of the forkhead box P3(Foxp3)molecule in T convs[69-73].Foxp3 is a transcription factor that is accepted as the master of Treg development and function[74-76].Tregs are a rare subpopulation of T cells,discovered by Sakaguchiet al[77],that are specialized in immune suppression and maintenance of immunological tolerance[77,78].It is now clear that any disruption in the development or functionality of Tregs will lead to autoimmune and inflammatory diseases[79-81].Interestingly,it is revealed that through a variety of mechanisms,MSCs can convert T convs to Foxp3 expressing Tregs.Signaling pathways involving TCR,costimulatory molecules,TGF receptor,IL-2R,programmed deathligand 1,and Notch upregulate Foxp3 expression[82-84].Induced Tregs exert their immunosuppressive activity mainly by producing IL-10,TGFβ,and IL-35[85,86].
Indeed,we and others have demonstrated several mechanisms behind this complex biological process.For instance,the modulation of ubiquitination factors[71],runtrelated transcription factor complex[72],Treg-specificdemethylatedregions demethylation[71],micro RNAs such as miR126a[73],and mitochondrial and extracellular vesicle transfer from MSCs to T cells[87,88]are among some of the principal mechanisms.Besides,we have recently demonstrated that Treg induction by MSCs is a reciprocal phenomenon that requires both MSC and T cell interaction.In this setting,the expression of TNFR2 by T cells was shown to be crucial for their conversion towards functional Foxp3+and Foxp3-Tregs[89].Our data demonstrated that MSCs were incapable of converting T cells harvested from TNFR2 knockout(KO)mice.Moreover,in comparison to wild-type T cells,MSCs-exposed TNFR2 KO T cells secreted decreased amounts of IL-10 and TGFβ[89].Furthermore,the production of anti-inflammatory mediators such as IL-10 and TGFβ by MSCs has been shown to be essential in Treg induction[90,91].
Concerning EPCs,recent findings based on various techniques including enzymelinked immunosorbent assay,flow cytometry,and immunofluorescence staining have revealed that these progenitor cells are also able to produce significant amounts of IL-10,TGFβ,and HLA-G anti-inflammatory cytokines[66,68].Therefore,based on their immunosuppressive effect and secretion of immunoregulatory mediators,our first assumption was that probably similar to their MSC counterparts,EPCs could also induce the expression of the Foxp3 molecule in CD4 and CD8 T cell subpopulation.Surprisingly,our result revealed that the EPC immunosuppressive effect was Treg independent as they did not increase the expression of Foxp3 in T convs(Figure 2)[68].This is in accordance with the absence of IL-10 and TGFβ production by T cells after co-culturing with CB-EPCs or APB-EPCs[68].These results contradict other studies showing the capacity of mature ECs such as liver sinusoidal ECs,human umbilical vein ECs,and dermal microvascular ECs to induce CD4+CD25+Foxp3+Tregs mostly through a TGFβ dependent mechanism[92-94].Whether,unlike mature ECs,their progenitors(EPCs)are incapable of Treg induction or this deficit was due to the xenogenic context of our experimentation are two questions that need to be further investigated.
The implication of the TNFα-TNFR2 signaling pathway in MSC and EPC immunoregulatory function
Stem cells are very sensitive to an inflammatory environment and their biological function could significantly alter in the presence of surrounding proinflammatory mediators including IFNγ,IL-17,IL-1,and especially TNFα[31,95-97].For example,pretreating MSCs with TNFα is shown to have a boosting impact on the production of anti-inflammatory cytokines like TGFβ and IL-10 that consequently participate in immunosuppression and the Treg induction[98,99].Equally,Nouri Barkestaniet al[100]recently reported that priming EPCs with a proper dose of TNFα could efficiently upregulate the expression of the TNFR2 molecule and increase their immunosuppressive and immunoregulatory effect in HLA mismatched combinations.
TNFα recognizes two transmembrane receptors(TNFR1 and TNFR2)with two completely distinct biological functions[100-102].While the interaction of TNFα with TNFR1 leads to proapoptotic and deleterious outcomes,its interaction with TNFR2 generally causes cell activation,proliferation,and survival[101-103].TNFR1 and TNFR2 are different subgroups of the TNF receptor superfamily[104].TNFR1 is a death receptor because it bears a death domain in its cytoplasmic compartment and its activation ends in caspase-8 function and cell death[105-107].TNFR2,on the other hand,recruits TRAF2 with its associated binding molecules such as cIAP1,cIAP2,and TRAF1,which results in the activation of the classical NF-kappa B and mitogenactivated protein kinase pathways leading to cell proliferation[106,108].In contrast to TNFR1,which has a ubiquitous expression,TNFR2 is expressed by some limited cells such as T cells especially Tregs[101],neural cells including neural progenitor cells[109],MSCs[7,8],and interestingly by ECs,particularly by EPCs[68,100].
Our previous investigations on Tregs have revealed that the TNFα-TNFR2 signaling pathway is in complete control of their immunosuppressive effect.In this setting,we have shown that when Tregs are harvested from TNFR2 KO mice or this receptor is blocked by using an anti-TNFR2 monoclonal antibody,the Treg immunosuppressive function is entirely hampered[101].Similarly,in the absence of the ligand TNFα,Tregs could not perform their proper immunosuppressive function[101].Observing the importance of this signaling pathway and with regards to the crucial role of the TNFα-TNFR2 axis in MSC and EPC biology,we decided to evaluate the implication of this signaling pathway in these cells as well.Since both cell populations have shown some level of immunomodulatory functions,we first evaluated if the blockade of this axis impacts this important effect.Our results demonstrated that the interference in the TNFα-TNFR2 signaling pathway(either by blocking the receptorviamonoclonal antibody or using T cells harvested from TNFα KO mice)in EPCs regardless of their sources has led to the complete loss of their immunosuppressive function[68].Moreover,this blockade reduced EPC immunoregulatory function because they were significantly less able to produce IL-10,TGFβ,and HLA-G anti-inflammatory cytokines[68].
Observing this effect encouraged evaluating the implication of this signaling pathway in MSCs.In this setting,we isolated MSCs from TNFR2 KO mice and compared their immunomodulatory effect with MSCs collected from wild-type mice.Our results showed for the first time that the TNFR2 expression by MSCs is crucial for their ability to suppress the proliferation and decrease the activation phenotype of different T cell populations[8].Moreover,inhibiting the TNF-TNFR2 signaling pathway in MSCs resulted in decreased production of anti-inflammatory cytokines TGFβ and IL-10 and increased production of proinflammatory cytokines,INFγ,IL-2,TNFα,and IL-17 by T cells[8].We found that when TNFR2 KO MSCs were compared to wild-type MSCs,they were significantly less capable of converting CD3+CD25-T convs to CD4+Foxp3+Tregs and CD8+Foxp3+Tregs[8].Besides,the newly induced Tregs had even less capacity to suppress T convs when set in a new mix lymphocyte reaction assay[7].Further investigations to reveal the importance of the TNFR2 receptor on other cells has demonstrated that the expression of this receptor is also necessary by T cells for their efficient conversion into Tregs by MSCs[89].
Although the TNFR2 molecule plays an important regulatory role in MSCs,its blockade caused a partial deficiency in the MSC suppressive property.This effect was unlike EPCs in which their immunoregulatory effect was entirely TNFα-TNFR2 dependent,making MSCs more ‘’intelligent’’ or flexible stem cells(Figure 3).MSCs from the TNFR2 KO mice still had some level of immunomodulatory and regenerative functions[7],meaning that even in the absence of prosurvival signals,MSCs could adapt themselves to the new inflammatory environment.

Figure 3 The comparison between mesenchymal stem cell and endothelial progenitor cell immunosuppressive activity in the presence and blockade of the tumor necrosis factor alpha-tumor necrosis factor receptor 2 signaling pathway.Because the immunosuppressive function of mesenchymal stem cells(MSCs)is greater than endothelial progenitor cells(EPCs),we have kept them as the reference(5/5).In this case,in the presence of the tumor necrosis factor-alpha(TNFα)-tumor necrosis factor receptor 2(TNFR2)signaling pathway(i.e.normal condition),MSCs have the highest immunosuppressive effect(5/5).On the other hand,in the presence of this signaling,EPCs have a less immunosuppressive effect(3/5).Interestingly,while blockade of this axis led to a complete loss of immunosuppressive function in EPC(0/5),MSCs kept suppressing T cells with less efficiency(2/5),showing that this axis is partially controlling their immunoregulatory properties.
CONCLUSION
Thanks to recent investigations,we currently know that besides their strong regenerative functions,EPCs and MSCs also have some levels of immunomodulatory features that make them interesting choices for cell therapy of immunological disorders.Moreover,due to their immunosuppressive effect,they could persist longer when administered in an allogenic combination.
Nevertheless,these two cell types do not demonstrate the same level of immunoregulatory effect and their mechanisms of action are also dissimilar.In this review,we have compared for the first time the immunosuppressive effect of MSCs and EPCs against T cells.We have shown that MSCs regardless of their sources are more immunosuppressive and immunomodulatory than CB-EPCs and APB-EPCs.Moreover,while induction of Foxp3+Tregs from T convs is an essential mechanism of action for MSC indirect immunoregulatory function,EPCs induce neither CD4+Foxp3+nor CD8+Foxp3+Treg populations.
The TNFα-TNFR2 immune checkpoint signaling pathway has emerged as a novel target for immune therapy of immunological disorders including cancer and transplantation[110-112].Thanks to the recent publications,particularly on other immunosuppressive cells such as Tregs,we currently know that this signaling pathway plays a crucial role in EPC and MSC immunoregulatory functions.A comparison between these two cell types demonstrates that hampering in the TNFα-TNFR2 axis leads to the complete disruption of EPCs and has a significant impact on MSC immunomodulatory functions.Due to its protective and anti-inflammatory role,activation of the TNFR2 axis has been suggested as a therapeutic approach in several degenerative,inflammatory,and cardiovascular disorders.The stimulation through the TNFR2 molecule has been shown as a promising approach to increase the proangiogenic effect of TNFR2 expressing cells leading to improved ischemia conditions[113,114],myocardial infarction[115,116],and Alzheimer’s disease[117].Similar outcomes were reported regarding improved Treg immunosuppressive function,which could potentially improve graftvshost disease[118]or autoimmune disorders[110].Conversely,the TNFα-TNFR2 axis was used as a potential target for Treg elimination in cancer conditions in which an elevated immune response is required[111,119,120].This is indeed very interesting since the Foxp3 molecule is a transcription factor(i.e.intranuclear)and not easily accessible for Treg elimination.Therefore,targeting TNFR2(with cytoplasmic expression)seems to be an efficient alternative to hamper immunosuppression in Tregs and also in other immunomodulatory cells such as EPCs and MSCs.
杂志排行
World Journal of Stem Cells的其它文章
- Inter-regulatory role of microRNAs in interaction between viruses and stem cells
- Mesenchymal stem cells for enhancing biological healing after meniscal injuries
- Modulating poststroke inflammatory mechanisms:Novel aspects of mesenchymal stem cells,extracellular vesicles and microglia
- Antler stem cells and their potential in wound healing and bone regeneration
- Therapeutic prospects of mesenchymal stem/stromal cells in COVID-19 associated pulmonary diseases:From bench to bedside
- Mesenchymal stem cells as a potential therapeutic tool to cure cognitive impairment caused by neuroinflammation
