Virescentia guangxiensis (Batrachospermales, Rhodophyta):a new freshwater red algal species from South China*
2021-07-29KunpengFANGFangruNANJiaFENGJunpingQiLIUXudongLIUShulianXIE
Kunpeng FANG, Fangru NAN, Jia FENG, Junping LÜ, Qi LIU, Xudong LIU, Shulian XIE
School of Life Science, Shanxi Key Laboratory for Research and Development of Regional Plants, Shanxi University, Taiyuan 030006, China
Abstract Virescentia guangxiensis, a new species of Virescentia from Guangxi, South China, is described and illustrated based on morphological observations and phylogenetic analysis. This species was distinguished morphologically from other species by the presence of special expansion cells with a variable shape, obovoid, spherical, pear-shaped, located in the penultimate cells of primary or secondary fascicles, rarely terminal on primary fascicles, as well as by small whorls (250-350-μm wide) and short primary fascicles (5-7 cell stories). Phylogenetic analysis of molecular data from the rbc L and COI-5P loci supported the separation of the proposed new species from other species in the genus Virescentia. This is the first species of the order Batrachospermales reported in Guangxi and the second species of the genus Virescentia reported in China. This study expands the known species diversity and geographical distribution of freshwater Rhodophyta in China.
Key word:Virescentia; new species; rbc L; COI-5P; China
1 INTRODUCTION
Red algae (Rhodophyta) primarily inhabit marine environments, and freshwater red algae account for only 3% of species diversity in the division (Sheath and Vis, 2015). The Batrachospermales is an order that encompasses 70% of all freshwater red algal diversity, andBatrachospermumis the most speciesrich genus having at least 112 species (Kumano,2002). Based on morphological research and the application of molecular technology, seven sections ofBatrachospermumhave been raised to the genus level (Entwisle et al., 2009; Salomaki et al., 2014;Necchi et al., 2018, 2019a, b; Vis et al., 2020).Batrachospermales is widely distributed throughout China: 33 species have been discovered in China, of which 12 are endemic. Nonetheless, of these 33 species, molecular data are accessible for only 13, and the remaining species have only a morphological description (Xie et al., 2020).
GenusVirescentia, which belongs to the order Batrachospermales (Nemaliophycidae, Rhodophyta),was recently established as a genus ofBatrachospermumsectionVirescentiain 2018 based on morphological characteristics, geographic distribution, and DNA sequence data (Necchi et al., 2018). SectionVirescentiawas proposed by Sirodot based on a combination of characters: greenish thalli and carpogonia with cylindrical and stalked trichogynes (Sirodot, 1873).Seven new species of this section were described and illustrated in the monograph onBatrachospermumand represent a starting point for the study of this section (Sirodot, 1884). Subsequent morphometric analyses of type specimens and North American populations resulted in the proposal of many synonymies and the recognition of only two species,BatrachospermumhelminthosaBory andB.elegansSirodot (Sheath et al., 1994). Kumano (2002)recognized eleven species within the section. In recent years, morphological characteristics (size of carpogonia, carposporophytes, and carposporangia) of samples from Brazil, North America and Europe have been shown to diff er significantly, and DNA sequence data have suggested that there is considerable genetic variation in the genus and that it is partitioned geographically (Hanyuda et al., 2004; Rueness, 2010;De Castro Agostinho and Necchi, 2014). In order to resolve the phylogenetic relationships among the species of sectionVirescentia, the genusVirescentiawas established in 2018, and the species ofVirescentiawere circumscribed on the basis of morphological characteristics (shape of fascicles, occurrence of secondary fascicles, disposition of carpogonial branches, and size of carpogonia) (Necchi et al., 2018).
At present, there are six taxonomically accepted species of genusVirescentia(V.crispata(Kumano et Ratnasabapathy) Necchi, Agostinho et Vis;V.gulbenkiana(Reis) Necchi, Agostinho et Vis;V.helminthosa(Bory) Necchi, Agostinho et Vis;V.viride-americanaNecchi, Agostinho et Vis;V.viride-brasiliensis(Necchi et Agostinho) Necchi,Vis et Agostinho; andV.vogesiaca(Schultz ex Skuja)Necchi, Agostinho et Vis), of whichV.crispataandV.gulbenkianahave been distinguished in morphology only (Necchi et al., 2018). In China, only one taxon,V.helminthosa, has been reported in morphology(Shi, 2006; Xie et al., 2020). In this paper, a new species ofVirescentiafrom Guangxi, China,V.guangxiensis, is described and illustrated based on morphological and molecular evidence. This study expands the known species diversity and geographical distribution of freshwater Rhodophyta in China.
2 MATERIAL AND METHOD
2.1 Sample collection
Algal specimens were collected in December 2019 from the spring water of Baimo Cave in Bama County,Guangxi, South China (24°18′2″N, 107°5′58″E,altitude 260.5 m). Spring water comes from Baimo Cave and is also the source of the Panyang River. It is located on the shaded side of the river that receives weak sunlight, and spring water gushes out all year round and flows into the Panyang River. Water temperature (WT), pH, atmospheric pressure (AP),dissolved oxygen (DO), electronic conductivity (EC),and total dissolved solids (TDS) were measured using handheld meters (YSI Professional Plus Multiparameter Water Quality Instrument 19E102487,YSI Incorporated, Brannum Lane Yellow Springs,Ohio, USA). The samples were washed with sterile water to remove impurities and stored at -80 °C after quick-freezing in liquid nitrogen. Specimens for DNA analysis were preserved in silica desiccant. Some voucher specimens were preserved in 4%formaldehyde for morphological analyses; the remaining materials were pressed as herbarium voucher specimens and preserved at the Herbarium of Shanxi University (SXU) (Thiers, 2020), Shanxi University, Taiyuan, Shanxi, China.
2.2 Morphological observation and sequence amplification
Morphological analyses were performed using an Olympus BX-43 microscope equipped with a chargecoupled device (DP72; Olympus, Tokyo, Japan) for photography.
For DNA extraction, tissue dried in silica gel was homogenized by grinding in liquid nitrogen with a mortar and pestle, and total DNA was extracted following the protocol of Saunders (1993) with modifications described in Vis and Sheath (1997). A 1 282-bp fragment of the plastid-encoded ribulose-1,5-bisphosphate carboxylase-oxygenase large subunit gene (rbcL) and a 664-bp barcode region near the 5′ end of thecox1 gene (COI-5P) were chosen for amplification in 20-μL volumes containing 15.3-μL ddH2O, 2-μL 10× buff er, 2.0-μL 2.5-mmol/L dNTPs,0.2-μL Taq DNA polymerase (all from Sangon Biotech Co., Ltd., China), 2.0 μL of each primer(10 mmol/L), and 0.5 μL of genomic DNA. TherbcL gene was polymerase chain reaction (PCR) amplified using the F160 andrbcLR primers (Vis et al., 1998)with the following cycle conditions: 95 °C for 2 min;35 cycles of 93 °C for 1 min, 47 °C for 1 min, and 72 °C for 2 min; and a final hold for 2 min at 72 °C.The COI-5P gene was PCR amplified using the GazF1 and GazR1 primers (Saunders, 2005) with the following cycle conditions: 94 °C for 2 min; 35 cycles of 94 °C for 30 s, 47 °C for 30 s, and 72 °C for 1 min;and a final hold for 7 min at 72 °C.
2.3 Sequencing and data analysis
The PCR products were purified using a SanPrep column DNA gel purification kit (Sangon, China) and then submitted to BGI Tech Corporation (Beijing,China) for sequencing with both PCR primers using an ABI 3730XL sequencer. Contigs were assembled and edited using Sequencher 4.10.1 and submitted to GenBank.
For phylogenetic analyses, sequence data for order Batrachospermales were downloaded from GenBank(Supplementary Table S1), andThoreahispidawas used as the outgroup (Vis and Entwisle, 2000;Entwisle et al., 2009; Chapuis et al., 2017). Sequenceswere aligned in ClustalX 2.0 (Thompson et al., 1997),and pairwise distances between specimens of Batrachospermaceae were computed in MEGA 5.0(Tamura et al., 2011). The aligned sequences were used to construct phylogenetic trees. Appropriate models of sequence evolution for our datasets were determined with a hierarchical likelihood ratio test performed in Modeltest version 3.7 (Posada and Buckley, 2004). The parameters for therbcL maximum likelihood (ML) analyses were as follows:GTR+I+G model; gamma distribution=0.666 3;proportion ofinvariable sites=0.475 9; base frequencies A=0.385 4, C=0.105 6, G=0.136 3, and T=0.372 7;and rate matrix A-C=3.506 1, A-G=5.628 0, AT=0.796 7, C-G=3.047 8, and C-T=18.856 4. The parameters for the COI-5P ML analyses were as follows: GTR+I+G model; gamma distribution=0.471 3; proportion ofinvariable sites=0.411 1; base frequencies A=0.334 3, C=0.093 0, G=0.098 1, and T=0.474 6; and rate matrix A-C=1.600 0, AG=9.313 4, A-T=0.144 5, C-G=3.795 6, and CT=20.916 5. Phylogenetic inferences were made using the neighbor-joining method (NJ) in MEGA 5.0(Tamura et al., 2011) and Bayesian inference (BI) in MrBayes version 3.2 (Ronquist and Huelsenbeck,2003), and ML trees were constructed using PHYML(Felsenstein, 1981; Guindon and Gascuel, 2003).Both BI and ML analyses were performed using a general time reversible model with a gamma distribution. The BI analysis was run for 5 000 000 generations until the standard deviation of the split frequencies was less than 0.01. The ML analysis was performed using the rapid bootstrapping and best tree search algorithm for 1 000 repetitions. The resulting phylogenetic trees were edited using Figtree V.1.4.2(http://tree.bio.ed.ac.uk/software/figtree/).
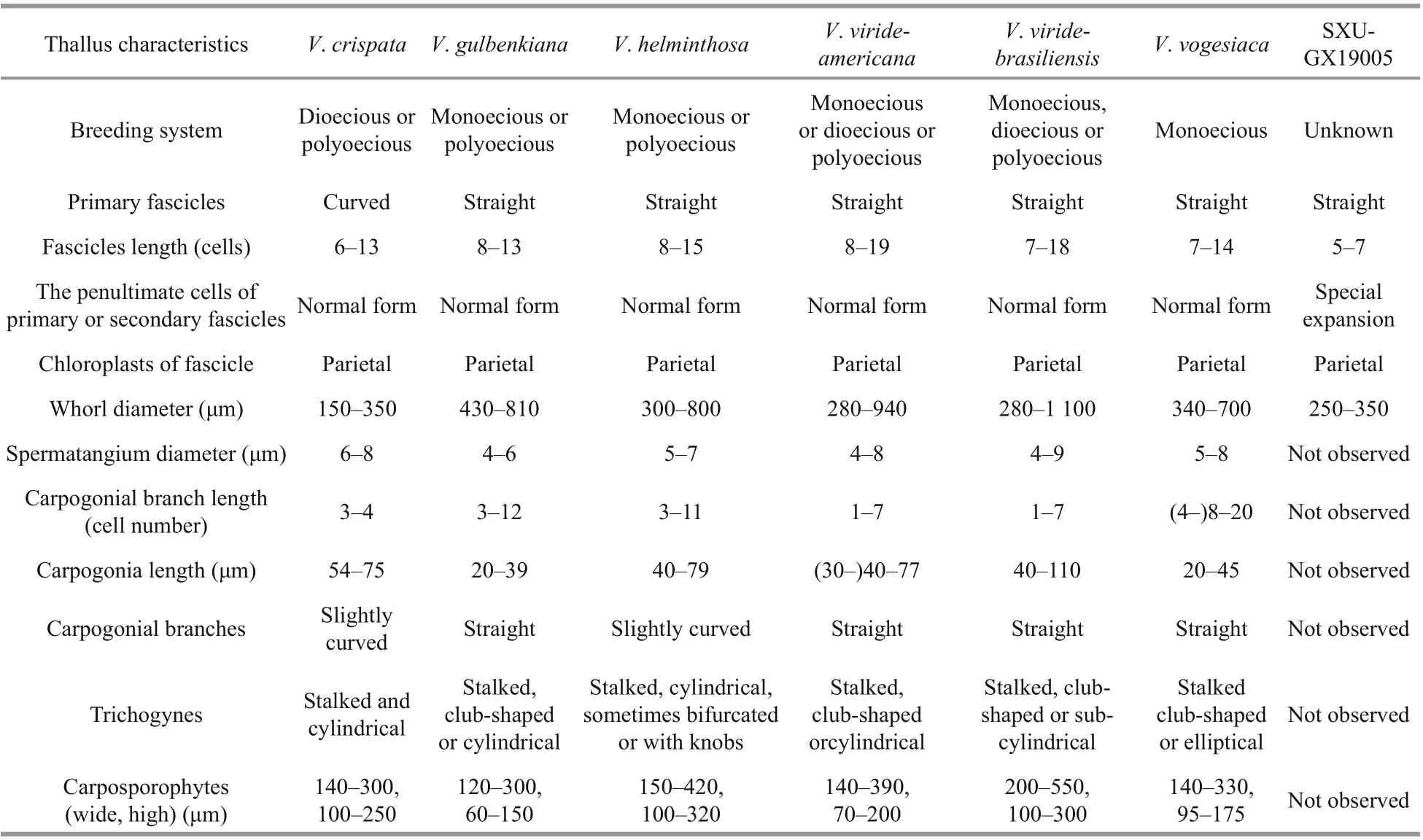
Table 1 Morphological comparison of sample in this study with six species of Virescentia previously reported
3 RESULT
3.1 Morphological observation
Vegetative structures were identified and illustrated(Fig.1) based on the morphological and morphometric characters described in previous literature (Sirodot,1884; Kumano, 2002; De Castro Agostinho and Necchi, 2014; Necchi et al., 2018), and a morphological comparison of the present sample with six previously reported species ofVirescentiais provided (Table 1).Because neither spermatangia nor carpogonia were observed, distinctions in these structures between the present specimen and others were unclear. The specimen broadly overlapped previously described species for most morphometric and morphological characters: greenish thalli, irregular branching, welldeveloped whorls, few and sparse secondary fascicles,and a brownish main axis. The specimen was distinguished based on some uncertain morphological characters, including the presence of special expansion cells with a variable shape, obovoid (15-25 μm in diameter, 27-34-μm long), spherical (18-25 μm in diameter), or pear-shaped (15-20 μm in diameter,20-30-μm long), located in the penultimate cells of primary or secondary fascicles, or rarely terminal on primary fascicles, which were not observed in other species ofVirescentia. In addition, its small whorls(250-350-μm wide) and short primary fascicles (5-7 cell stories, branched 2-3 times) were also diff erent.
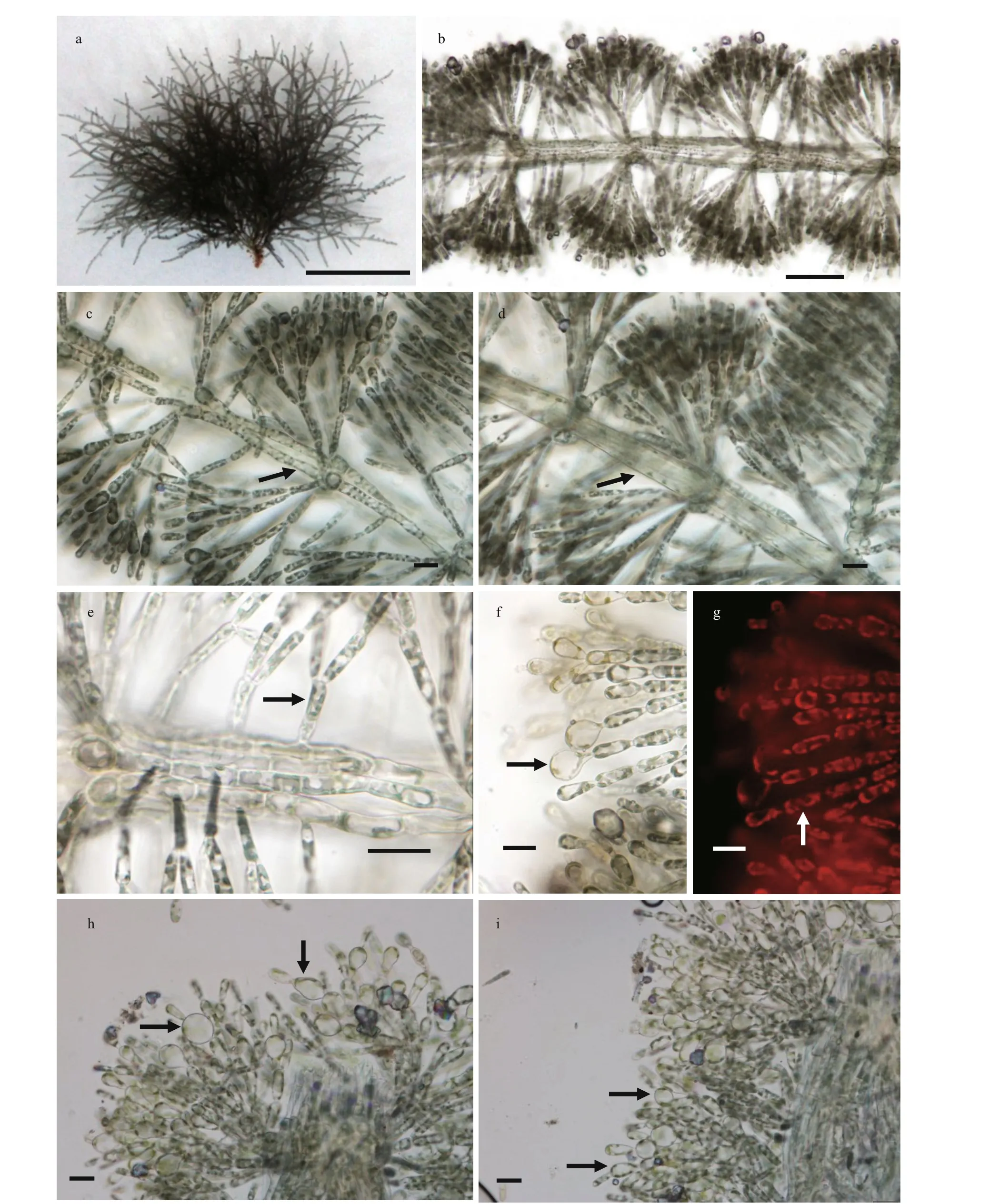
Fig.1 Morphological structures of V. guangxiensis
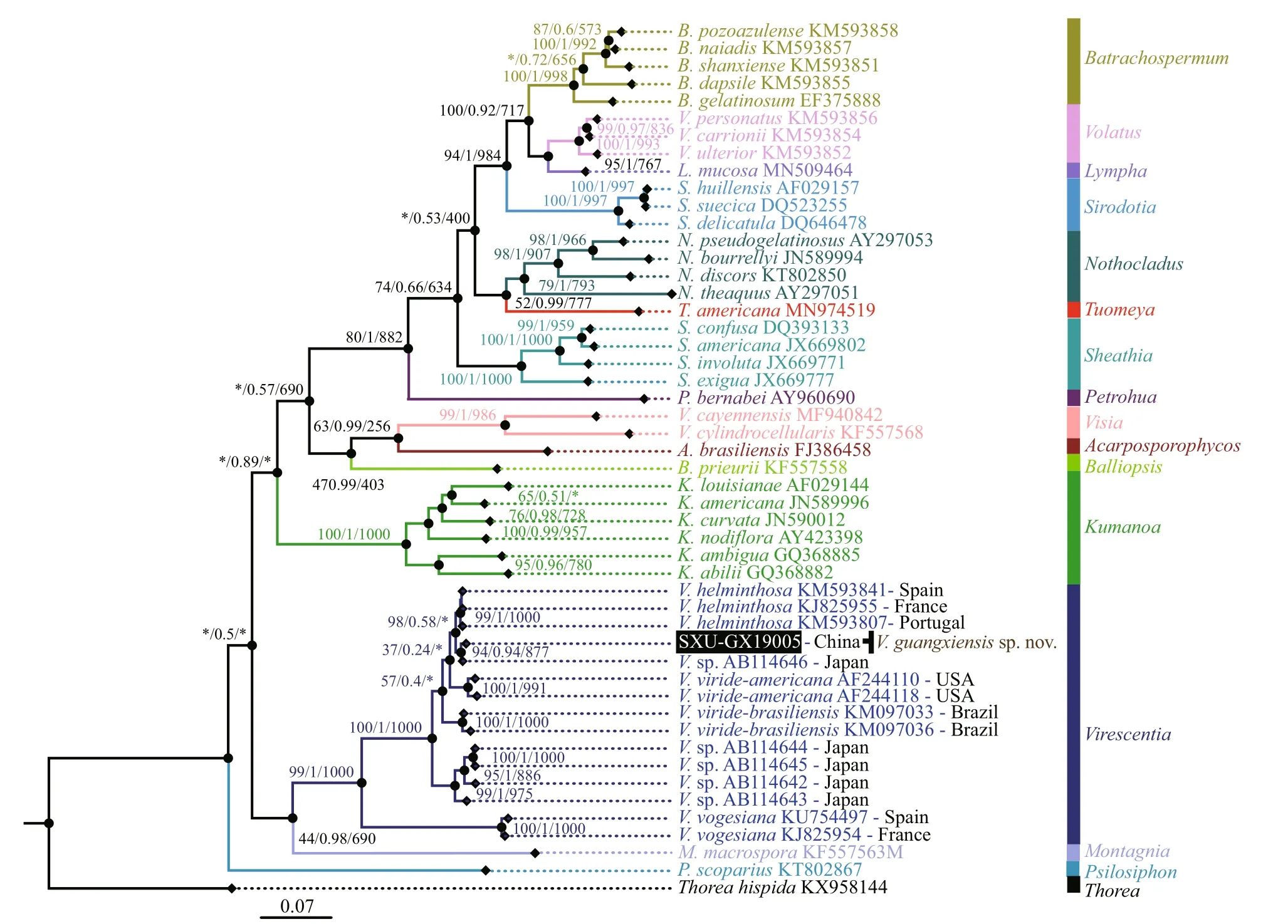
Fig.2 Phylogenetic tree (BI) based on the rbc L gene sequences showing the relationships of the specimen from this study(black box) and other Batrachospermales species
3.2 Molecular analysis
Sequence data for species in the order Batrachospermales were collected (49rbcL representing 40 taxa from 15 genera and 32 COI-5P representing 29 taxa from 11 genera). TherbcL alignment in this study was 1 210 bp in length with 505 variable sites (among which 436 were parsiminformative). For COI-5P, the alignment was 664 bp in length with 331 variable sites (among which 285 were parsim-informative).
Separate phylogenetic trees based on therbcL and COI-5P sequences were constructed using three methods (NJ, BI, and ML). All showed similar topologies, diff ering only in theVirescentiaclade(nodes with “*”) forrbcL in the ML tree and COI-5P in the NJ tree with low support values. The specimen SXU-GX19005 collected in this study was clearly placed within theVirescentiaclade. In therbcL phylogeny (Fig.2), this specimen was placed in a well-supported clade (94/0.94/877) withVirescentiasp. (AB114646) from Japan and was sister toV.helminthosa(from Spain, France, and Portugal)with a distance of 0.77% (9.3 bp). Distance among the threeV.helminthosasamples was 0.55% (6.7 bp), and that between SXU-GX19005 andVirescentiasp.AB114646 was 0.33% (4 bp). Similarly, in the COI-5P phylogeny (Fig.3), SXU-GX19005 was separated from other species in theVirescentiaclade(67/0.86/631) with a distance of 4.97% (33 bp) fromV.helminthosa, 7.38% (49 bp) fromV.viridebrasiliensis, and 8.96% (59.5 bp) fromV.virideamericana. These results suggested that SXUGX19005 is a new species, namedVirescentiaguangxiensis.
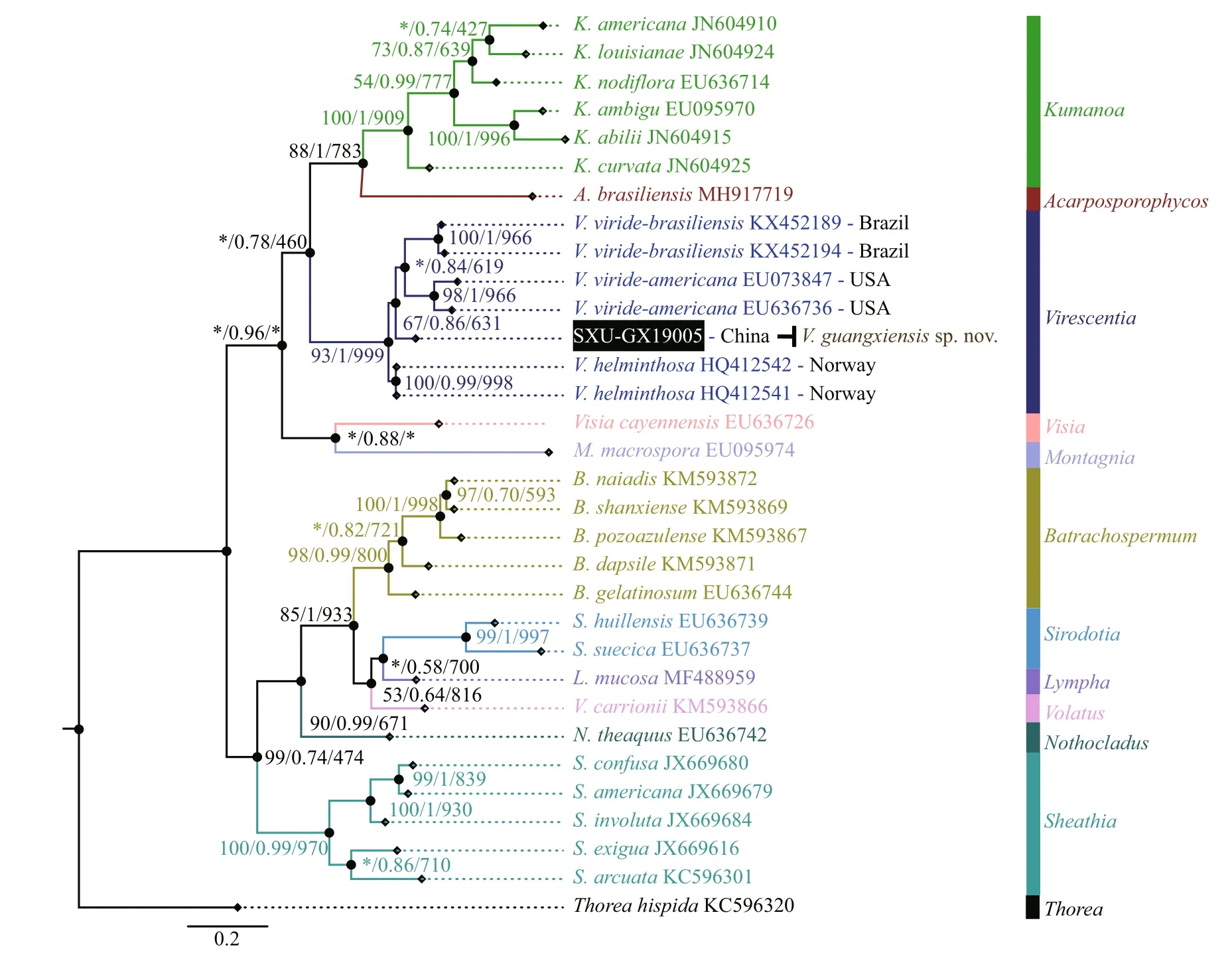
Fig.3 Phylogenetic tree (BI) based on the COI-5P gene sequences showing the relationships of the specimen from this study(black box) and other Batrachospermales species
3.3 Taxonomy
VirescentiaguangxiensisFANG, NAN, et XIE sp.nov. (Fig.1a-i)
Description: plants moderately mucilaginous,greenish in color, 2-3-cm high. Whorls contiguous or separated, barrel-shaped or spherical, 250-350-μm wide, internodes 140-230 μm, main axis sometimes brownish colored, axial cells 20-40 μm in diameter,cortical filament cells cylindrical, 6-10 μm in diameter, 30 μm or more long. Primary fascicles straight, 5-7 cell stories, dichotomously branched,2-3 times branched. Proximal cells cylindrical or barrel-shaped, 6-10 μm in diameter, 30-40-μm long,and distal cells ellipsoidal or obovoid, 5-8 μm in diameter with chloroplasts, parietal, often dissected into spirally arranged ribbons. Secondary fascicles few and sparse, unbranched or dichotomously branched, shorter than or as long as primary fascicles.Cells with special morphological characteristics,special expansion and variable in shape, obovoid (15-25 μm in diameter, 27-34-μm long), spherical (18-25 in diameter), pear-shaped (15-20 μm in diameter,20-30-μm long), located in the penultimate cells of primary or secondary fascicles, rarely terminal on primary fascicles. Neither spermatangia nor carpogonia were observed.
Diagnosis: the new species diff ers from other species in that it has special expansion cells located in the penultimate cells of primary or secondary fascicles. Diagnostic DNA sequence:rbcL and COI-5P (accession number: MT533618 forrbcL and MT533619 for COI-5P).
Typelocality: China―Guangxi, Bama County(24°18′2″N, 107°5′58″E, ASL: 260.5 m): the spring water comes from Baimo Cave and is also the source of the Panyang River (WT=21.0 °C, pH=7.79,AP=9.086×104Pa, DO=6.66 mg/L, EC=310.1 μS/cm,TDS=218.4 mg/L).
Holotypeheredesignated: deposited in Herbarium of Shanxi University (SXU), Shanxi University,Taiyuan, Shanxi Province, China. SXU-GX19005,Dried material prepared from reference strain GX15,Shanxi University Herbarium (SXU), Shanxi University, Taiyuan, Shanxi Province, China,December 2019 by Kunpeng FANG.
Etymology: the species epithet refers to the type locality (Guangxi, China).
4 DISCUSSION
Phylogenies based on therbcL and COI-5P sequences revealed the genusVirescentiato be monophyletic. WithinVirescentia, species clades corresponded to geographic regions, and each clade represented a species as follows:V.helminthosa(Europe),V.vogesiaca(Europe),V.viride-americana(North America),V.viride-brasiliensis(South America) andV.guangxiensis(Asia). This result was consistent with all previous molecular systematic studies (Vis et al., 1998; Hanyuda et al., 2004;Entwisle et al., 2009; De Castro Agostinho and Necchi, 2014, Necchi et al., 2018). Members of the genus can be recognized by a combination of characters: the presence of well-developed whorls;carpogonial branches well diff erentiated from the fascicles, straight, rarely curved; carpogonia with cylindrical, club-shaped or elliptical, stalked trichogynes; and axial, large, and dense carposporophytes (Necchi et al., 2018). Among the six taxonomically accepted species,V.helminthosais characterized by slightly curved carpogonial branches andV.crispataby audouinelloid and curved primary and secondary fascicles. BothV.gulbenkianaandV.vogesiacahave short carpogonia (20-45 μm), but the secondary fascicles are few and sparse inV.gulbenkianaand abundant inV.vogesiaca.V.viride-americanaandV.viride-brasiliensisare morphologically similar and distinguished only by geographic distribution and DNA sequence data (rbcL and COI-5P) (Necchi et al., 2018).
Virescentiaguangxiensis, described here from China, represents previously undescribed diversity withinVirescentia. Its morphological characteristics(greenish thalli, irregular branching, well-developed whorls, few and sparse secondary fascicles, and brownish main axis) are consistent with other species from genusVirescentia. Unfortunately, neither spermatangia nor carpogonia were observed, but this species can still be distinguished from others based on additional morphological characters. In particular, it exhibited special expansion cells with a variable shape, obovoid, spherical, or pear-shaped, located in the penultimate cells of primary or secondary fascicles, and rarely terminal on primary fascicles,which were not observed in otherVirescentiaspecies.In addition, small whorls (250-350-μm wide) and short primary fascicles (5-7 cell stories, branched 2-3 times) were also discriminative for this species. TheVirescentiasp. specimen from Japan had only onerbcL sequence (rbcL-AB114646), which was similar to that of the specimen from this study, with a divergence of only 4 bp. Nonetheless, it was lack of voucher specimens for morphological observation(Hanyuda et al., 2004). Whether it is the same species as the specimens in this study is still uncertain. The remaining four sequences of otherVirescentiasp.specimens from Japan formed an independent branch on the tree, representing a second potential species,consistent with previous studies (Necchi et al., 2018).
At present, only oneVirescentiaspecies,V.helminthosa, is recognized by morphology in China and distributed in two provinces, Chongqing and Zhejiang. This species has typical characteristics ofV.helminthosa(whorls composed of straight fascicles and carpogonial branches slightly curved),but it lacks supporting molecular data (Shi, 2006; Xie et al., 2020). By contrast,V.guangxiensiswas collected from the type locality Guangxi, which is far from Zhejiang and Chongqing. Furthermore,V.guangxiensishas small whorls (250-350-μm wide)and axial cells (20-40 μm in diameter), short primary fascicles and internodes (140-230 μm), and missing terminal hairs compared withV.helminthosa(whorls 290-440-μm wide, axial cells 60-80 μm, 6-12 primary fascicles, and 330-90-μm internodes). Thus,V.guangxiensisis diff erent fromV.helminthosaand is the second species of genusVirescentiareported in China.
Guangxi Province is located in south China and exhibits a subtropical and tropical monsoon climate with an annual average temperature of 17.5-23.5 °C.Karst landforms are widespread in Guangxi,accounting for 37.8% of the total land area, and the groundwater is rich in calcium bicarbonate and magnesium bicarbonate. Only two taxa of order Acrochaetiales have been reported in Guangxi for Subclass Nemaliophycidae (Shi, 2006). Therefore,Virescentiaguangxiensisis the first species of order Batrachospermales reported in Guangxi. The type locality Baimo Cave is located in Bama County,Hechi City. Bama County is a famous“Changshouzhixiang” (hometown oflongevity) in China. “Bai Mo” means spring water outlet in the Zhuang language, and the spring in Baimo Cave is the source of the Panyanghe River. The rare morphological characteristic (special expansion cells) of the new species may be a result of environmental adaptation.However, there are no reports of similar characters in other freshwater red algae, and further research is required to determine the cause of this morphological character.
5 CONCLUSION
Morphological comparison and molecular analysis both supported the proposal of a new freshwater red algal speciesVirescentiaguangxiensis. It is characterized morphologically by the presence of special expansion cells with a variable shape, obovoid,spherical, or pear-shaped, located in the penultimate cells of primary or secondary fascicles, rarely terminal on primary fascicles, as well as by small whorls and short primary fascicles. Phylogenetic analysis of sequence data from therbcL and COI-5P loci supported the separation ofV.guangxiensisfrom other species of genusVirescentia, justifying the proposal of the new species.V.guangxiensisis the first species of order Batrachospermales reported in Guangxi and the second species of genusVirescentiareported in China. The description of this new species expands the known species diversity and geographical distribution of freshwater Rhodophyta in China.
6 DATA AVAILABILITY STATEMENT
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
7 ACKNOWLEDGMENT
Dr. E. W. Christina (The Pennsylvania State University) is acknowledged for the English editing.
杂志排行
Journal of Oceanology and Limnology的其它文章
- Evaluating the eff ect ofinput variables on quantifying the spatial distribution of croaker Johnius belangerii in Haizhou Bay, China*
- Metabolomics analysis for skin ulceration syndrome of Apostichopus ja ponicus based on UPLC/Q-TOF MS*
- Four new species of free-living marine nematode from the sea areas of China*
- Molecular characterization and expression of the SiUCP2 gene in sea urchin Strongylocentrotus intermedius*
- Reproductive characteristics of Psilorhynchus homaloptera Hora and Mukerji, 1935 (Cyprinidae: Psilorhynchidae) in the lower Yarlung Zangbo River, Tibet*
- Identification of potential sex-related genes in Siniperca chuatsi*
