Reproductive characteristics of Psilorhynchus homaloptera Hora and Mukerji, 1935 (Cyprinidae: Psilorhynchidae) in the lower Yarlung Zangbo River, Tibet*
2021-07-29PengchengLINHuamingHUFeiLIUMingzhengLIHuanzhangLIU
Pengcheng LIN , Huaming HU , Fei LIU , Mingzheng LI , Huanzhang LIU ,**
1 Institute of Hydrobiology, Chinese Academy of Sciences, Wuhan 430072, China
2 Key Laboratory of Aquatic Biodiversity and Conservation of Chinese Academy of Sciences, Chinese Academy of Sciences,Wuhan 430072, China
Abstract Psilorhynchus homaloptera mainly inhabits turbulent waters in the lower reach of the Yarlung Zangbo River, Tibet, China, and it is the only species of Psilorhynchus in China. However, many important aspects ofits biology remain poorly documented, which hinders the conservation eff orts. Therefore, we studied its reproduction using 801 specimens from the Motuo sector of the lower Yarlung Zangbo River from December 2015 to November 2016. The sex ratio was 0.68:1 (male:female) for the overall population.Females reached a larger total length and weight (134 mm and 18.6 g) than males did (113 mm and 12.2 g).Lengths at maturity were estimated 80.0 mm for males and 93.1 mm for females. Our analyses of gonad development and the size distribution of oocytes suggest that P. homaloptera is a single spawner with the peak spawning period between September and October. Furthermore, the estimated mean absolute fecundity was 557±204 eggs per fish, and mean relative fecundity was 50.9±10.9 eggs per g of female weight. The fecundity of P. homaloptera increased linearly with increasing total length, total weight, ovary weight, and age. This study on the reproductive biology of P. homaloptera demonstrates that the fish was in a vulnerable state due to the increasing anthropogenic activities in the lower Yarlung Zangbo River.
Keyword: reproductive biology; maturity; spawning period; fecundity; environmental adaptation
1 INTRODUCTION
Psilorhynchus, the only genus of the family Psilorhynchidae, is distributed in the lower reach of the Yarlung Zangbo River in Tibet, China, the north and northeast of the Brahmaputra drainage in India,east Nepal, and the Ayeyarwady drainage of north Myanmar, adjacent to China (Yue, 2000; Conway and Mayden, 2007; Arunachalam and Muralidharan,2008). Species ofPsilorhynchus, commonly known as torrent minnows or torrent stone carp, are generally small, have an arched back and flattened ventral surface, and live in mountainous rivers and streams with turbulent flows. So far, studies on this genus mainly covered its distribution, taxonomy, and phylogenetic position through morphology, osteology,and molecular analyses (Yue, 2000; Conway and Mayden, 2007; He et al., 2008; Conway, 2011).Biological studies ofPsilorhynchusare extremely limited. Conway et al. (2014) found a remarkable phenomenon of swimbladder sexual dimorphism inPsilorhynchus, wherein the male swimbladder is much larger and more complex than that of the female.Kaushik and Bordoloi (2017) reported the lengthweight relationships ofPsilorhynchusbalitora, and Li et al. (2018) and Dey et al. (2018) reported the length-weight relationships ofPsilorhynchushomaloptera.

Fig.1 Psilorhynchus homaloptera (107 mm in TL and 7.8 g in W) collected from the lower Yarlung Zangbo River in the study
As the only species ofPsilorhynchusdistributed in China,Psilorhynchushomalopteraoccurs in the Yarlung Zangbo-Brahmaputra River basin, living preferably in high-gradient streams with rocky bottom in the Himalayas (Talwar and Jhingran, 1991; Yue,2000; Shangningam and Vishwanath, 2014). This species mainly ingests algae and aquatic invertebrates(Yue and Chen, 1998). Due to overexploitation and hydro-engineering construction,P.homalopterahas been listed as an endangered species in the China Red Data Book (Yue and Chen, 1998). Understanding of the biological background—especially the reproductive characteristics, including sexual maturity, spawning period, and fecundity—is urgently needed for the population conservation and management of the fish species (Angermeier, 1995).However, little biological information has been published on this species, which further hinders the conservation eff orts.
Under this backdrop, we studied the reproductive characteristics ofP.homalopteracollected in the Motuo reach of the lower Yarlung Zangbo River. The aims of this study were to identify the following characteristics of the species: (1) size at sexual maturity (TL50); (2) the spawning period according to gonad development and the size distribution of oocytes; and (3) the absolute fecundity, relative fecundity, and regression analysis of relationships among total length, total weight, ovary weight, age,and fecundity. At last, proposals and suggestions were given to the conservation and management forP.homalopterain the region.
2 MATERIAL AND METHOD
2.1 Study area and fish collection
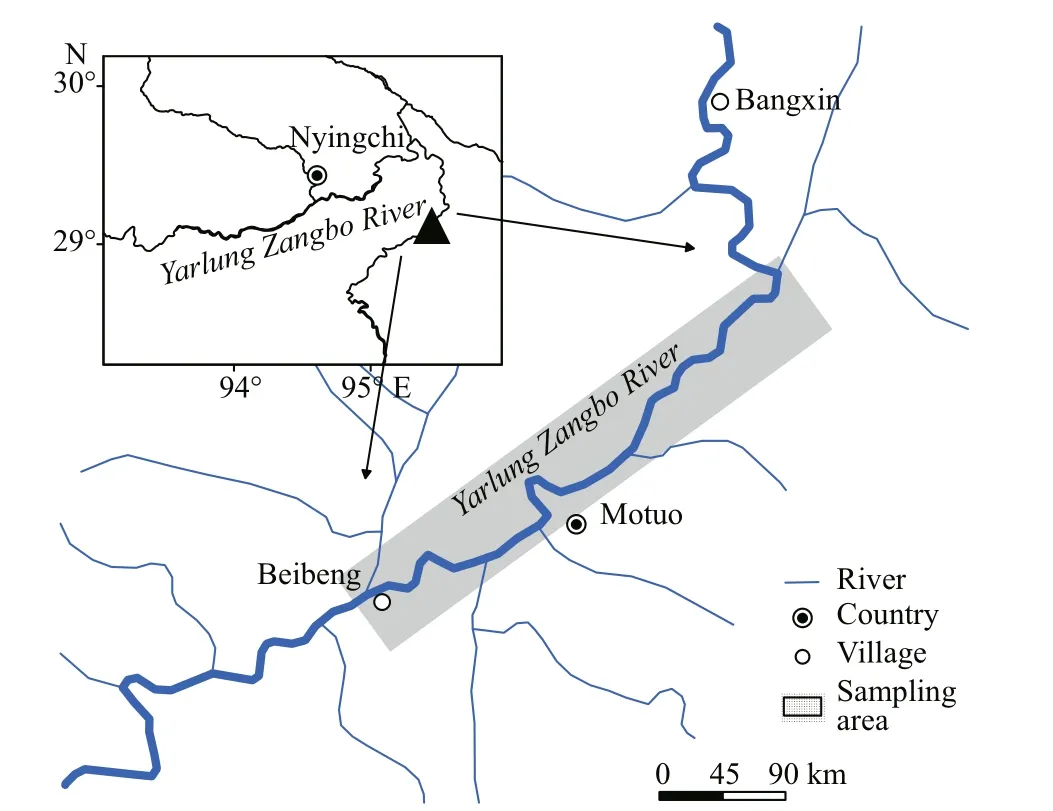
Fig.2 Points of sampling for Psilorhynchus homaloptera in the lower Yarlung Zangbo River from December 2015 to November 2016
Sampling was conducted in Motuo County, where the Yarlung Zangbo River flows across south Tibet and breaks through Yarlung Zangbo Grand Canyon,the deepest canyon in the world. Rivers and streams are in high gradient, and the substrates are mostly composed of cobbles and boulders with low proportions of gravel and sand. It is a corridor for warm, humid air currents from southeast Bengal Bay in the Indian Ocean to penetrate the Himalayas and Tibetan Plateau, so the climate in the canyon region is humid, with a mean annual air temperature of 16 °C and the annual precipitation of over 2 200 mm/a (Zhu et al., 2013).
In total, 801P.homalopteraindividuals were collected from December 2015 to November 2016 in the lower Yarlung Zangbo River and its tributaries(Figs.1 & 2). Sampling was not conducted in January or February 2016 because snowstorms made it inaccessible to Motuo. The specimens were captured using backpack electro-fishing gear and killed with MS-222. The total length (TL) of each captured individual was measured to the nearest 1 mm, and the total weight (W) and gonadal weight (WG) were measured to the nearest 0.01 g. Sex was determined macroscopically and the gonadal development stages were classified based on macroscopic features(Table 1). Water temperature at sampling locations was measured with thermometer in situ and monthly accumulated rainfall data were obtained from the Motuo Meteorological Bureau.
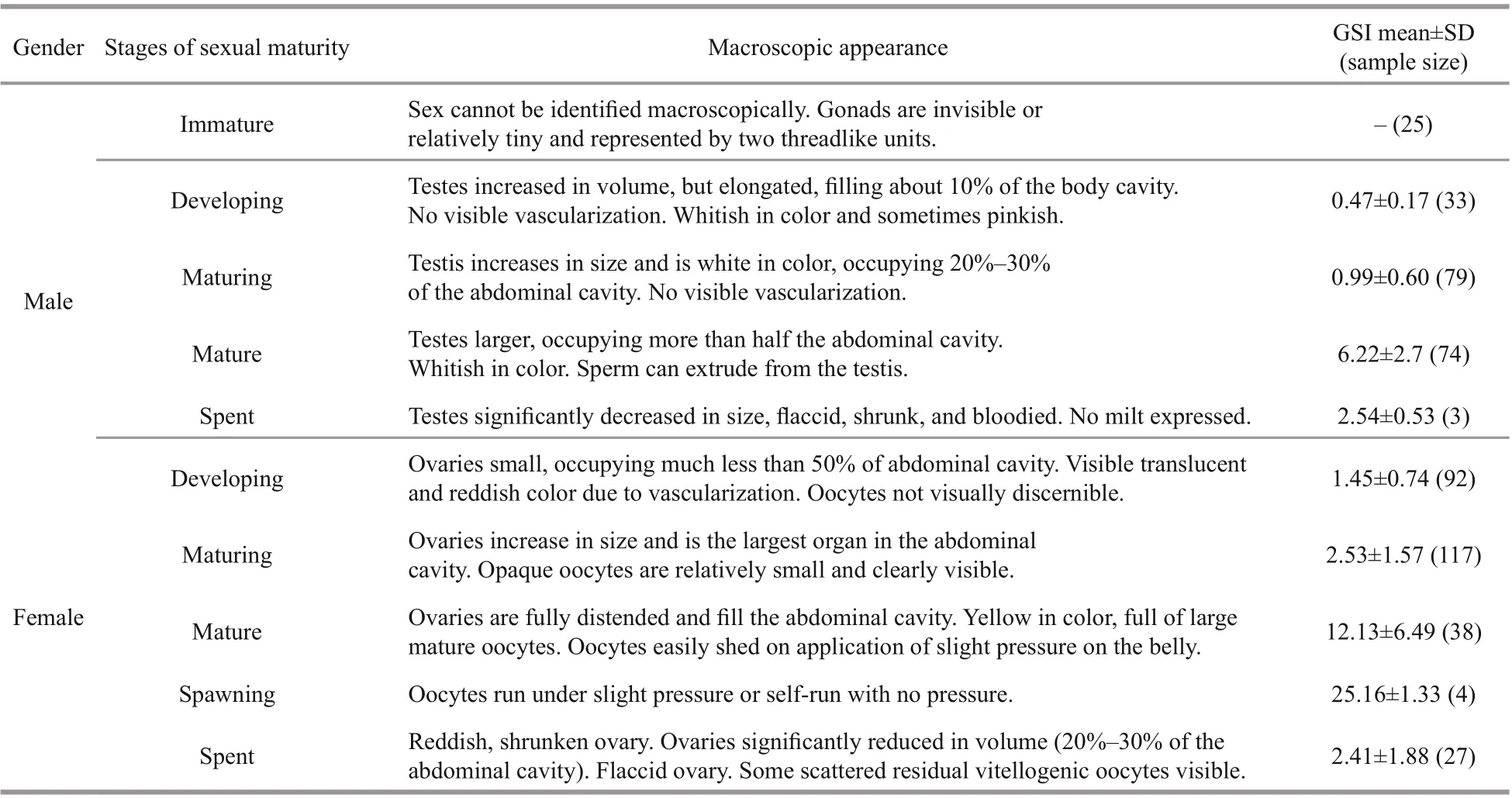
Table 1 Macroscopic maturity observation and corresponding gonado-somatic index (GSI) of Psilorhynchus homaloptera in the lower Yarlung Zangbo River, China
2.2 Size at sexual maturity
The size at sexual maturity was defined as the length at which 50% of the population reached sexual maturity (Lt50), and this definition was applied to estimate maturity macroscopically for each sex(Somerton, 1980). Gonad development in females and males were classified into six stages according to macroscopic gonad development characteristics of the common Cyprinidae fish in Brulé et al. (2003) and Huo et al. (2013). A logistic formula for determining the proportion (P) of mature fish was applied to assess the size at 10-mm (TL) size intervals:P=(1+e-k(TLmid-TL50))-1(Chen and Paloheimo, 1994), where TLmidis the midpoint of the TL class, TL50is the mean TL at sexual maturity,Pis the proportion of mature fish,andkis the slope.
2.3 Spawning period
The spawning period was evaluated through comprehensive consideration of monthly variations in the gonado-somatic index (GSI), monthly proportions of macroscopic maturity stages, and the size distribution of oocytes.
The GSI of mature individuals was calculated using the equation GSI=WG/W×100 (Brewer et al.,2008). ANOVA followed by Tukey’s post hoc test was applied to test whether there were significant diff erences in GSI among months (Zar, 1999; Ma et al., 2012).
2.4 Fecundity
To study individual fecundity, 34 females were processed using the gravimetric method (Bagenal and Braum, 1978). Subsamples from anterior, middle, and posterior parts of the ovaries were sampled. Fecundity was estimated asF=n×W/w, wherenis the number of oocytes in the subsamples,Wis the weight of ovaries,andwis the weight of sample parts. To assess absolute fecundity, all oocytes that had begun vitellogenesis were counted as potentially ripe eggs. The relative fecundity was calculated as the number of ripe oocytes per gram of total weight (Adebisi, 1987). Regression analysis was applied to study the relationships between the TL,W, ovary weight (Wo), age (A), and fecundity ofP.homaloptera. Age was estimated from scales.
2.5 Statistical analysis
The data were described as means±standard deviation (SD). Data and images were processed using Microsoft Excel 2010 and OriginPro 2016.SPSS software (version 16.0) was used for statistical analyses, andP<0.05 was considered significantly diff erent.
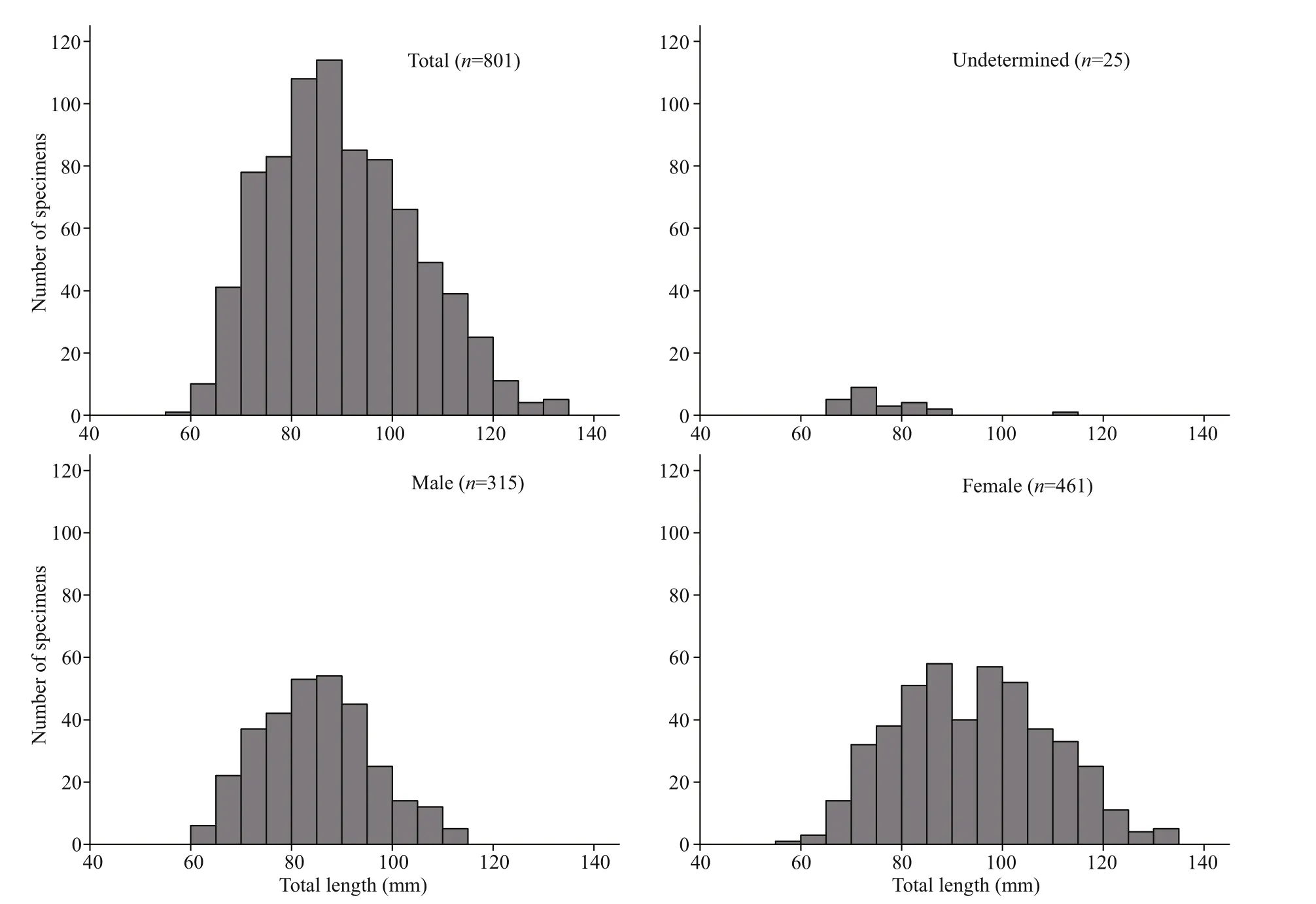
Fig.3 Length-frequency distribution for female ( n=461), male ( n=315), and unsexed ( n=25) Psilorhynchus homaloptera captured in the lower Yarlung Zangbo River
3 RESULT
3.1 Length-frequency distribution and sex ratio
The total length ofP.homalopteraspecimens ranged from 57 to 134 mm, and the total weight ranged from 1.7 to 18.6 g. Of the 801 individuals caught, 315 were males, with 60-113 mm in TL; 461 were females, with 57-134 mm in TL; and 25 were undetermined, with 61-111 mm in TL (Fig.3). Lengthfrequency distributions were significantly diff erent between males and females (Kolmogorov-Smirnov test,Z=4.215,P<0.001). The number of males (289)in the <100-mm TL class was nearly equal to that of females (301), while there were more females (160)in the >100-mm TL class than there were males (26)(Fig.3).
The male: female sex ratio (M/F) was 0.68:1 for the population, which was significantly diff erent from 1:1 (χ2=27.469,P<0.05, Table 2). There were nosignificant diff erences in the M/F from the expected 1:1 ratio in March, April, June, July or September(P>0.05), while the sex ratio was strongly biased towards females during the other months (P<0.05).a. from April to November 2016; b. from January to November 2016. GSI values are expressed as means±standard deviation.
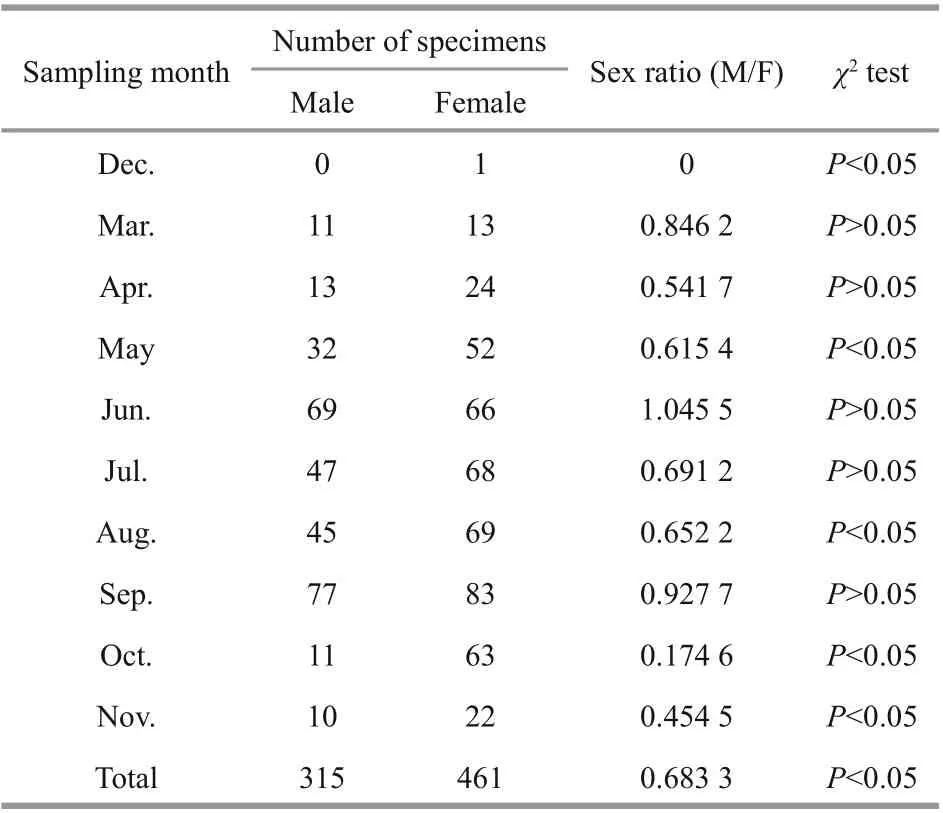
Table 2 Sex ratio of males to females (M/F) for Psilorhynchus homaloptera in the lower Yarlung Zangbo River from December 2015 to November 2016

Fig.4 Logistic functions for Psilorhynchus homaloptera fitted to the cumulative percentage of sexually mature individuals in relation to size
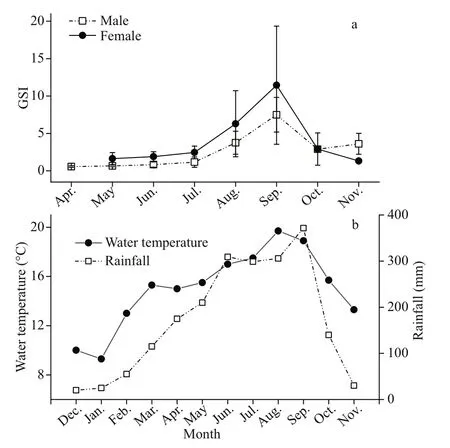
Fig.5 Monthly gonado-somatic index (GSI) of Psilorhynchus homaloptera males and females and the monthly water temperature and rainfall in the lower Yarlung Zangbo River
3.2 Size at sexual maturity
The size at sexual maturity revealed distinct diff erences between males and females (Fig.4).Estimated TL50values were 80.0 mm for males and 93.1 mm for females. The logistic functions are expressed as follows:P=(1+e-0.097(TLmid-80.0))-1(n=315,R2=0.914) for males andP=(1+e-0.067(TLmid-93.1))-1(n=461,R2=0.879) for females. The observed minimum total length of mature individuals was 65 mm for males and 75 mm for females.
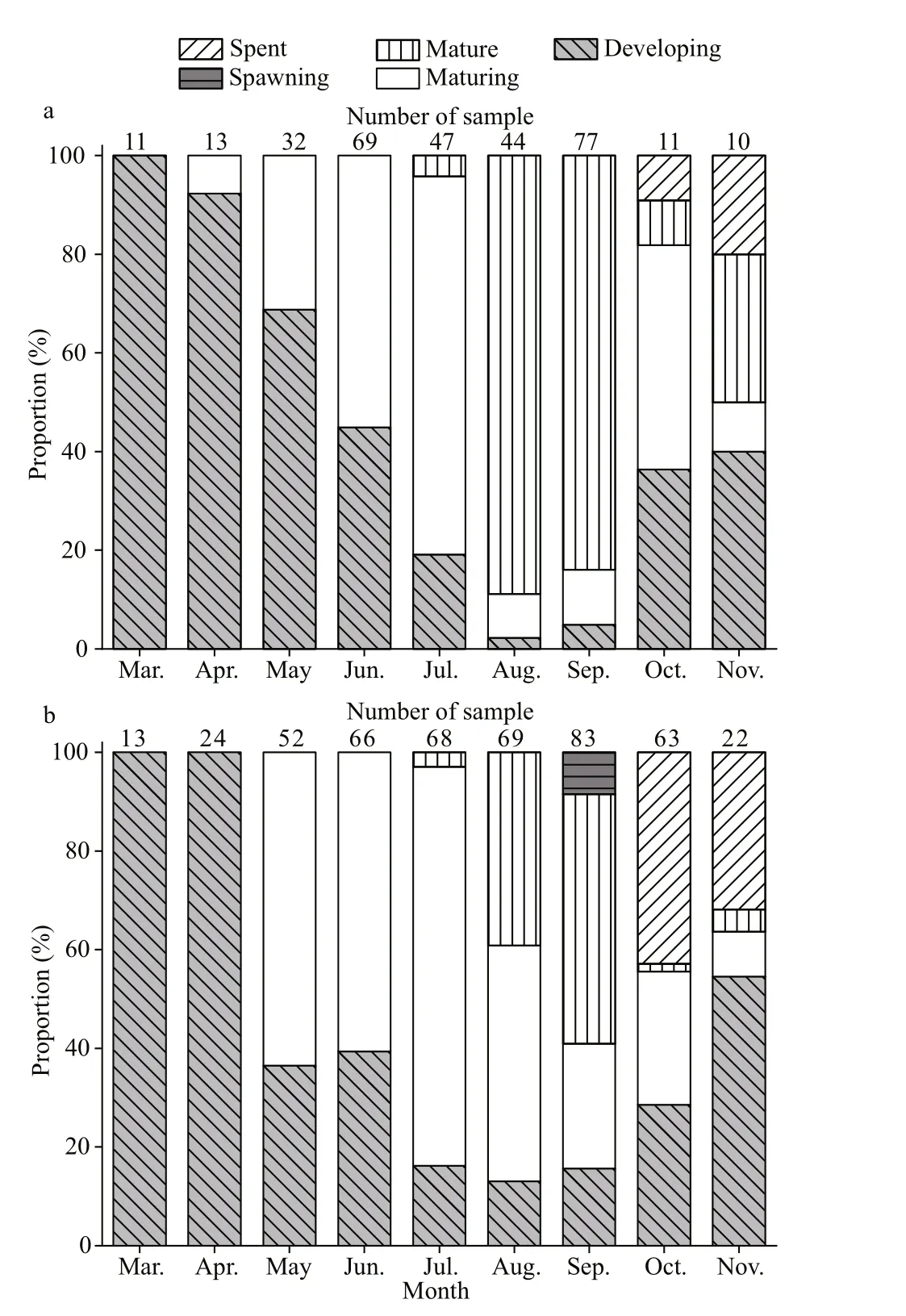
Fig.6 Monthly distribution of macroscopic gonad maturity stages in Psilorhynchus homaloptera fish from December 2015 to November 2016
3.3 Spawning period
In the September, GSI showed the highest values(females: 11.45; males: 7.49), a relatively high percentage of mature stages (females: 50.60%;males: 88.31%), and the highest egg size (mean oocyte diameter: 1.928±0.219 mm) (Figs.5-7). The GSI of September was significantly higher than that of any other month (ANOVA, Tukey’s post hoc,P<0.05). Spawning ovaries occurred in September.Spent ovaries and testicles both appeared from October to November (Fig.6). The mean oocyte diameter (1.928±0.219 mm) in September was significantly higher than those between July and August (Fig.7, ANOVA, Tukey’s post hoc,P<0.05).GSI revealed an increasing tendency in May and August for both sexes (Fig.5a). Mean oocyte diameter also showed an increasing trend in July and October(Fig.7). Therefore, we concluded thatP.homalopterais a single spawner that spawns between September and October, as indicated by mean monthly GSI values, maturity stages, and mean oocyte diameter.During August and September, monthly water temperature and rainfall reached the maximum (Fig.5b).
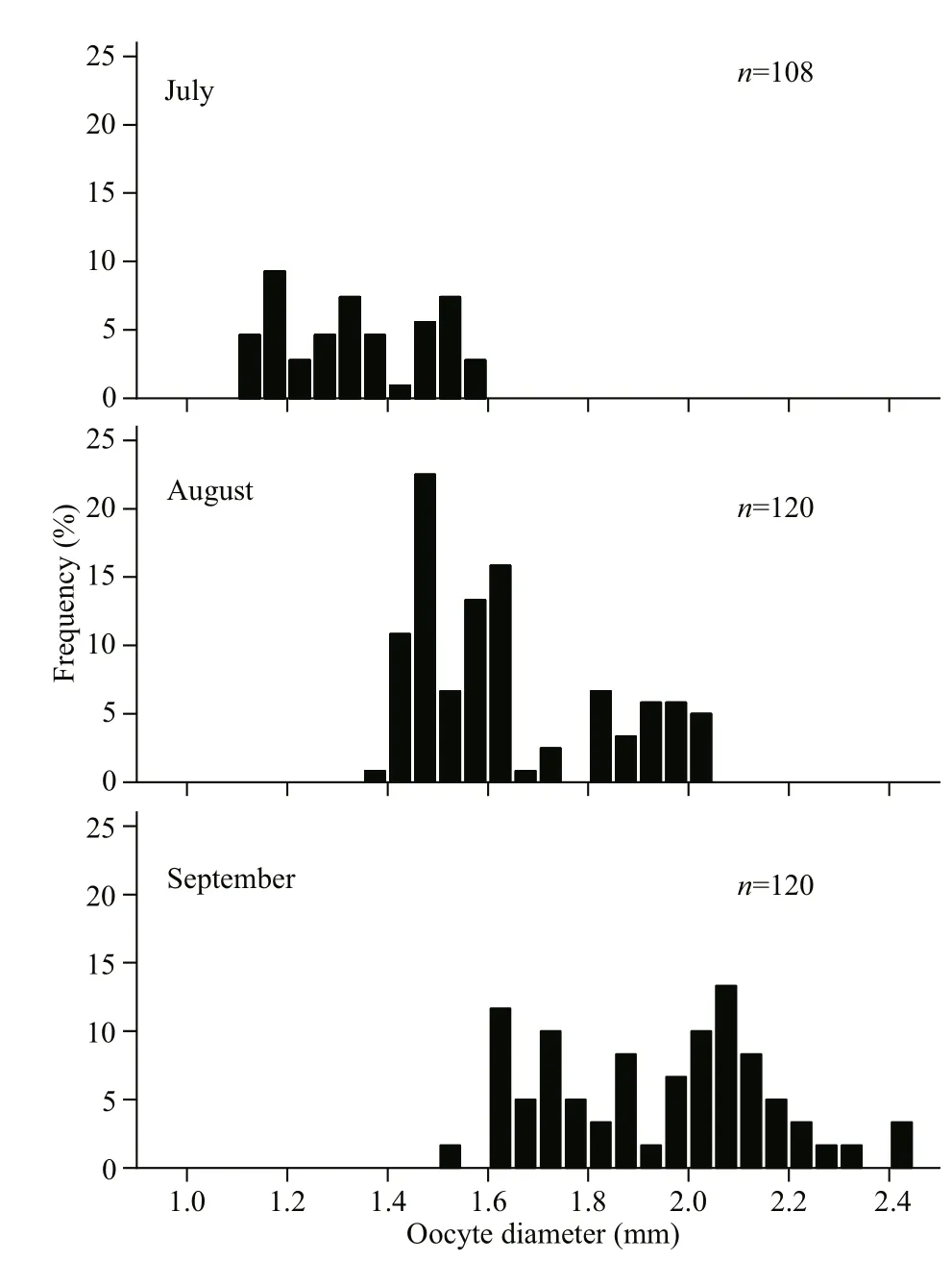
Fig.7 Monthly size-frequency of Psilorhynchus homaloptera oocytes from July to September 2016
3.4 Fecundity
The mean absolute fecundity was 557±204 eggs per fish, with 191-1 007 eggs per fish and 95-134 mm in TL. The relative fecundity was calculated as 50.9±10.9 eggs per g of female weight, with 24.2-73.8 eggs per g of female weight.
The fecundity ofP.homalopteraincreased linearly with the increasing total length, total weight, ovary weight, and age (Fig.8), and the fitted regression equations were as follows:
Total length:F=-976.648+13.855TL,n=34,R2=0.554;
Total weight:F=-44.359+55.393W,n=34,R2=0.653;
Ovary weight:F=314.878+110.603W0,n=34,R2=0.539;
Age:F=53.937+131.435A,n=25,R2=0.550.
Total weight was more highly correlated with fecundity than total length, ovary weight, or age were.
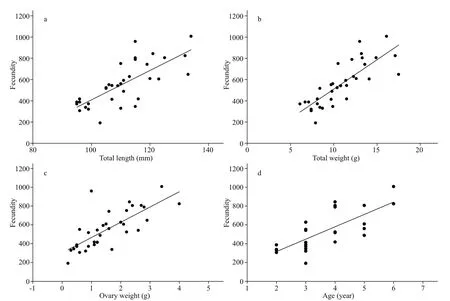
Fig.8 Relationships among total length (a), total weight (b), ovary weight (c), age (d), and fecundity of Psilorhynchus homaloptera
4 DISCUSSION
Reproductive biology is necessary to comprehend life history and stock assessment. It can also be important for understanding why some species might be more susceptible to exploitation from fisheries or other environmental stresses and essential for population conservation and management planning(Cortés, 2000). However, information on the biological features ofP.homalopterafrom the lower Yarlung Zangbo River remains deficient. These data will help fill current gaps regarding the biology and reproductive strategy of this species.
In the present study, the predominance of females in the overall population and the population oflarger adults reflects females’ greater longevity. Similar results were observed in Schizothoracinae fishes(Schizothoraxo’connori,Schizothoraxwaltoni,Schizothoraxcurvilabiatus) andGarratibetanafrom the Yarlung Zangbo River (Ma et al., 2012; Zhou et al., 2015; Wang, 2018; Gong, 2019) and other subtropical fishes (Lemes et al., 2017; Zaniboni-Filho et al., 2017). This phenomenon may be related to factors such as growth and mortality rates, migration,physiological changes aff ected by temperature change, feeding regime, and reproductive cycle(Zaniboni-Filho et al., 2017).
Reproductive eff ort of a fish can be measured as the egg mass per female, which is the product of fecundity and egg size (Duarte and Alcaraz, 1989).Investigating fecundity and egg size allows for the examination of energetic trade-off s between reproduction and survival. The relative fecundity ofP.homalopteravaried from 24.2 to 73.8 eggs per g of female weight, with a mean value of 50.9±10.9 eggs per g of female weight. This value is slightly higher than that of other species in the lower Yarlung Zangbo River. Wang (2018) and Gong (2019) show that the relative fecundity ofSchizothoraxcurvilabiatusandGarratibetanaare 5-14 and 7-24 eggs per gram,respectively. Moreover, it can be concluded that the relative fecundity ofP.homalopterais much lower than those of other cyprinids in the adjacent waters,e.g., 163 in the common carpCyprinuscarpio(Sivakumaran et al., 2003), 198 inLabeogoniusfrom Brahmaputra (Chowdhary and Shahid, 2018), 275 inBarilusbendelisisfrom Garhwal Himalaya (Saxena et al., 2016), and 692 in a widespread cyprinid fishHemiculterleucisculusfrom Alma-Gol wetlands(Patimar et al., 2008).
Egg diameter also is an important factor for determining fish reproductive strategy. Most developed oocytes ofP.homalopteraare 1.9 mm in diameter. Coburn (1986) reported that the egg sizes of 71 species of eastern North American cyprinids ranged from approximately 0.7 mm—in several species ofNotropis,Hybognathushankinsoni, andHybopsisaestivalis—to 2.0 mm inCampostomaanomalum. Therefore, the reproductive traits ofP.homalopteraare characterized by relatively low fecundity and large eggs. Large eggs generally have greater fitness than do smaller ones in a given environment. Thus, the trade-off between relatively low fecundity and large eggs can increase the off spring fitness, since more energy is invested in each off spring(Wootton, 1998). Large eggs enable the production of relatively larger and well-developed larvae. These individuals have a greater ability to swim against currents in hillstreams, giving them access to a wider range of potential prey (McDowall, 2009). These characteristics are associated with the equilibrium strategy guild proposed by Winemiller (1995), which largely coincides with theKstrategy for adapting to low-resource habitats. The species in this guild make great eff orts to ensure the survival of their off spring by providing them with enough yolk and/or by way of direct parental care during the early stages oflife(Winemiller, 1995; Wootton, 1998).
The estimation of GSI and egg diameter distribution ofP.homalopterarevealed that the fish spawns once a year, mainly from September and October, indicating thatP.homalopterais a single spawner with a short spawning period. These results are confirmed by the back-calculation of otolith daily increments fromP.homalopteralarva that we captured in November(unpublished data). Many studies indicate that freshwater fish spawning could be aff ected by riverine physical or chemical conditions, such as photoperiod(Jones and Petreman, 2015), water temperature(Chang et al., 2017), water discharge (King et al.,2016), dissolved oxygen (Sear et al., 2014), and climate (Strüssmann et al., 2010). In the present study,the final maturation and ovulation ofP.homalopteraseem to be well synchronized with the highest water temperature and rainfall, which occurs in August and September (Fig.5). Heavy rainfall causes flooding,and this with the increase in water temperature have been shown to have a positive influence on gonadal recrudescence in many riverine fishes. In the Murray River, Australia, golden and silver perch (Macquariaambigua,Bidyanusbidyanus) exhibit more spawning activities during the flood period, when water temperatures are above 20 °C; furthermore, fish native to this region may show the highest spawning outcomes a) when the magnitude of floods increases following lower antecedent flow conditions and b) at water temperatures of 18-20 °C (King et al., 2009,2016).
In addition, the spawning period ofP.homalopterais diff erent from those ofSchizothoraxcurvilabiatusandGarratibetana(February to April) in the lower Yarlung Zangbo River (Wang, 2018; Gong, 2019).This may be explained by the food limitation hypothesis (Kramer, 1978; Le Pape and Bonhommeau,2015). As a transitional zone from the Tibetan Plateau to Indian peninsula, the Motuo reach has a humid subtropical climate with a high altitude gradient.During the rainy season, flooding and the consequent fast current may reduce algal biomass, regardless of nutrient enrichment, and further reduce food availability and quality. After that (autumn), the moist forest habitat attracts insects and fruits fall into the river, so the standing crops of benthic algae may increase when they are not scoured by rainy season floods (Kramer, 1978). Therefore,P.homalopteraspawning in autumn may be controlled by the juvenile food availability.
Above all,P.homaloptera’s unique reproduction tactics may be attributed to the tradeoff between investing in higher quality, larger larvae and reducing their quantity to adapt the low-food-resource and volatile environmental conditions. Accordingly,reasonable conservation measures are needed to maintain the fish stock for future generations, asP.homalopterais being heavily threatened in the wild. Minimum landing size limits can be set to allow juvenile fish to survive long enough to reproduce.Another measure is to close this region to fishing during the spawning period and establish a nature reserve to protect the spawning ground. Moreover,long-term monitoring is essential to getting more detailed information on the life history traits ofP.homalopteraand maintaining the species’ stock in the natural habitat.
5 CONCLUSION
Overall, the present study indicates thatP.homaloptera, an important protected species in the Yarlung Zangbo River, is autumn-spawning fish whose spawning occurs between September and October. Males and females are 65 and 75 mm,respectively, at sexual maturity. The fecundity of the species is low and its eggs are large. These reproduction tactics can be regarded as an adaptation to the riverine conditions in the lower Yarlung Zangbo River. In view of our results, reasonable seasonal and space fishing management strategies for this species should be established. Long-term ecological studies are also necessary to reveal the life history traits forP.homaloptera.
6 DATA AVAILABILITY STATEMENT
The datasets generated and/or analyzed from the current study are available from the corresponding author upon reasonable request.
杂志排行
Journal of Oceanology and Limnology的其它文章
- Evaluating the eff ect ofinput variables on quantifying the spatial distribution of croaker Johnius belangerii in Haizhou Bay, China*
- Metabolomics analysis for skin ulceration syndrome of Apostichopus ja ponicus based on UPLC/Q-TOF MS*
- Four new species of free-living marine nematode from the sea areas of China*
- Virescentia guangxiensis (Batrachospermales, Rhodophyta):a new freshwater red algal species from South China*
- Molecular characterization and expression of the SiUCP2 gene in sea urchin Strongylocentrotus intermedius*
- Identification of potential sex-related genes in Siniperca chuatsi*
