NEK家族在细胞周期调控中的作用
2021-07-28李园园郭磊韩之明
李园园,郭磊,韩之明,3
综 述
NEK家族在细胞周期调控中的作用
李园园1,郭磊2,韩之明1,3
1. 中国科学院动物研究所,干细胞与生殖生物学国家重点实验室,北京 100101 2. 广东省第二人民医院生殖医学中心,生殖力保护实验室,广州 510317 3. 北京干细胞与再生医学研究院,北京 100101
NIMA相关激酶(NIMA-related kinases, NEKs)是丝氨酸/苏氨酸激酶,在细胞周期调控中发挥着重要的作用,参与了中心体分离、纺锤体组装、染色质凝集、核膜破裂、纺锤体组装检验点信号、胞质分裂、纤毛形成及DNA损伤反应等多种细胞活动。本文结合近年来在该激酶家族的相关研究,从NEK家族的组成、结构特征及其在有丝分裂和减数分裂过程中的作用等多个方面展开综述,以期为进一步研究NEKs在细胞周期调控中的作用提供重要基础,也为肿瘤的临床诊断和治疗提供理论依据。
NIMA相关激酶;有丝分裂;减数分裂
细胞是生命活动的基本单位。细胞周期是一个非常复杂和精细的调节过程,该过程受到细胞内外各种因素的精密调控,细胞周期的紊乱与许多疾病的发生发展相关。研究显示,许多蛋白激酶家族,如细胞周期蛋白依赖性激酶(cyclin-dependent kinases, CDK)、Aurora激酶、Polo样激酶(polo-like kinase, PLK)和NIMA相关激酶(NIMA-related kinases, NEKs),都参与了细胞周期调控的过程。近年的研究发现,NEK家族蛋白在细胞周期调控的过程中扮演了重要的角色,参与中心体复制和分离、纺锤体形成、染色体在赤道板上的排列、纺锤体检验点(spindle assembly checkpoint,SAC)调控、纤毛形成及DNA损伤反应(DNA damage response, DDR)等多种细胞活动。本文主要综述了NEK家族成员的生物学特性及其在细胞周期调控中的作用,同时对NEK家族的未来研究方向进行了探讨,以期让相关科研人员更充分、更全面地了解NEK家族的研究进展,为进一步研究其在细胞周期调控中的作用提供有力的支撑,也为深入了解肿瘤发生机制及抗肿瘤药物设计提供研究基础。
1 NEK家族及其生物学特性
1.1 NEK家族的发现
NIMA (never in mitosis A)最早是在对曲霉属真菌的有丝分裂突变体的研究中发现的[1,2]。20世纪80年代中期,Osmani等[3]通过调控基因的mRNA表达水平证明参与了曲霉有丝分裂的G2/M期转换。进一步的研究证明,的过表达可以促进有丝分裂的提前发生,有丝分裂过程中NIMA与CDK1-cyclin B复合体是同等重要的调节因子[4,5]。在对曲霉的研究中发现,NIMA的降解是细胞完成正确的有丝分裂进程所必需的[6]。一系列的研究表明,NIMA激酶在曲霉和酵母()中参与了染色质凝集、纺锤体组装和胞质分裂等多个细胞周期过程[7~11]。20世纪90年代初期,Letwin等[12]从小鼠()中分离出,发现编码一种与NIMA相关的蛋白激酶,在结构、组成和表达上与NIMA存在较高的一致性,从而提出了在哺乳动物中可能存在一个基因家族。随后的研究发现了小鼠和人()的细胞中均存在与相关的基因,证明了高等哺乳动物确实存在NEK家族[13,14]。研究已证明,NEKs存在于多种生物体中,从原生生物如衣藻()[15]、疟原虫()[16]等到多细胞真核生物如果蝇()[17]、非洲爪蟾()[18]、小鼠[13]和人[14]。
1.2 NEK家族成员的结构特征
人类NEK家族由11种NIMA相关激酶组成[19,20],这些激酶具有与曲霉NIMA相似的氨基末端催化区域,是含有典型的丝氨酸/苏氨酸激酶序列的高度保守的激酶结构域,其氨基末端和羧基末端的调节结构域在序列组成和长度上有显著差异。一般来讲,NEK家族的氨基末端激酶区域是中度保守的,与NIMA的激酶区域的氨基酸序列有40%~50%的同源性。NEK10的激酶区域位于整个氨基酸序列的中段,与NEK家族典型的氨基末端催化区域不同。在NEK家族中,人NEK2和NIMA的同源性最高,能达到44%[21]。除此之外,NEK6和NEK7的激酶区域的序列一致性达到了85%以上[22]。人类NEK家族的催化区域均含有一个His-Arg-Asp(HRD)基序,在激活环中都有一个丝氨酸/苏氨酸残基,而这个残基很可能是激活修饰的作用位点。在一些NEK家族成员中,这个残基是自磷酸化的,而其他成员则是通过一个上游激酶进行磷酸化修饰的[23~26]。就磷酸化识别序列而言,NIMA的第3位残基具有对苯丙氨酸的强烈偏好[27],人类NEK家族也具有相似的偏好,例如NEK2和NEK6的第3位残基更喜欢疏水残基,尤其偏爱苯丙氨酸或亮氨酸[28,29]。
NEK家族成员具有保守的氨基末端催化区域,而羧基末端区域在长度、序列和结构上都存在很大差异(图1)。其常见的特点就是寡聚化序列,通常是一种卷曲螺旋结构,可通过自磷酸化而被激活。一般而言,自磷酸化通常是在激酶结构域的激活环内进行,但是也可发生在蛋白质的其他区域,例如NEK8和NEK9羧基末端的非催化区域可以通过自磷酸化调控自身的定位和激活[23,30]。研究发现,包括曲霉NIMA和脊椎动物NEK2在内的几种NEKs均显示在非催化区域内存在靶向蛋白质降解的破坏基序[6,31],例如NEK2含有一个KEN (Lys-Glu-Asn)- box和羧基末端MR (methionine-arginine dipeptide)- tail,均能被后期促进复合物/环状体(anaphase- promoting complex/cyclosome, APC/C)所识别,其中MR-tail还可介导NEK2与APC/C的核心亚基CDC20直接作用,从而导致NEK2以一种不依赖于纺锤体组装检验点的方式进行降解[32]。在NEK家族中,NEK6和NEK7仅由一个催化区域和短的氨基末端延伸区域组成[33,34],而后者可能与底物识别有关[35]。NEK6和NEK7是NEK9的下游激酶,可以和NEK9蛋白中RCC1域和coiled-coil域之间的一个序列结合[25]。
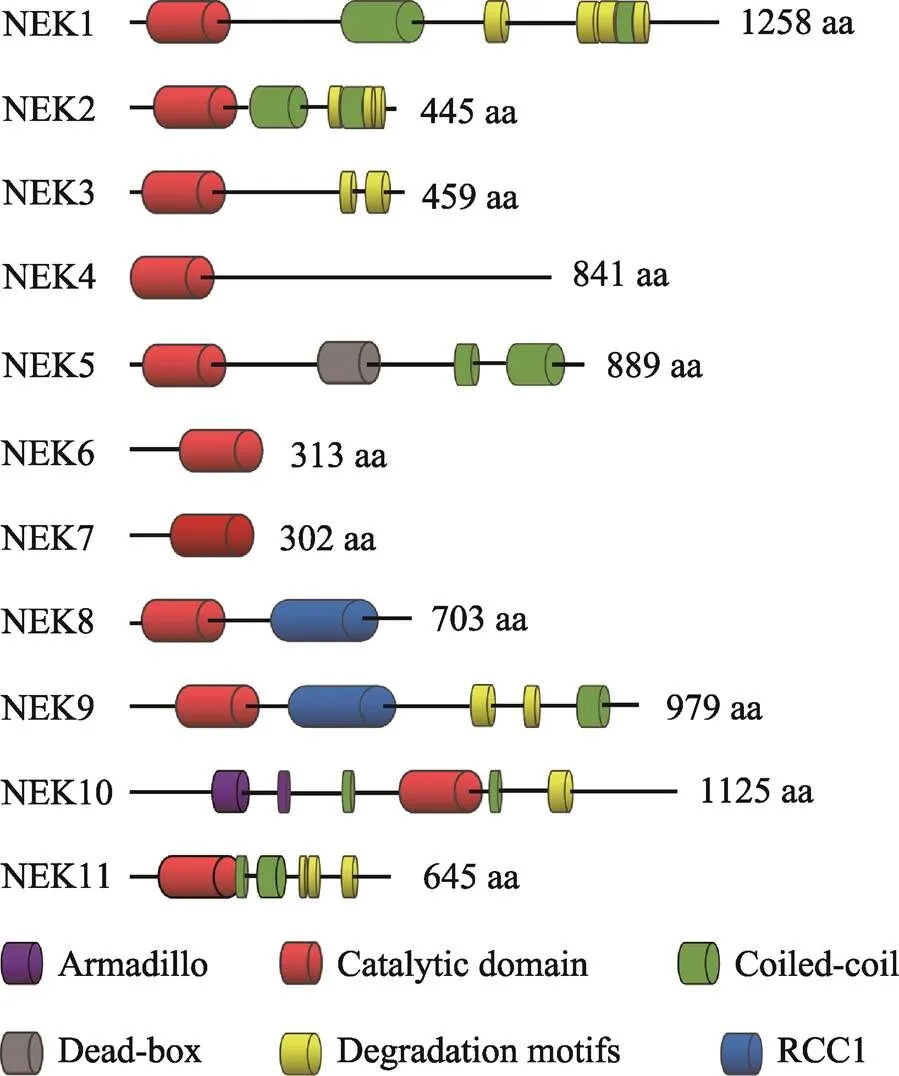
图1 人NEK家族的结构特征
2 NEK家族在细胞周期调控中的作用
作为蛋白激酶,NEK家族参与了细胞周期、细胞分裂、纤毛形成和DNA损伤反应等多种细胞活动(表1)。人和哺乳动物NEK家族在细胞有丝分裂和减数分裂过程中的作用主要有以下几个方面。
2.1 NEK家族在有丝分裂中的作用
的过表达可以诱导处于细胞周期任何阶段的曲霉细胞、酵母细胞、非洲爪蟾卵母细胞或人类细胞进入有丝分裂[82,83],研究发现,人类NEK家族参与细胞周期进程和分化过程中的多个事件。在有丝分裂中,NEK2、NEK6、NEK7和NEK9相互配合调控双极纺锤体的形成、染色质凝集、核膜破裂和胞质分裂等。NEK3除参与调控有丝分裂外,还可促进催乳素依赖性信号传导[45],而NEK1、NEK4、NEK5、NEK7、NEK8、NEK10和NEK11均与DNA损伤应答有关。
2.1.1 有丝分裂起始
有丝分裂的起始和退出是由CDK1、cyclins、有丝分裂相关激酶和磷酸酶驱动的细胞周期转换。在高等真核生物中,有丝分裂的起始导致多个细胞结构的改变,例如中心体分离、微管生长和收缩、核膜破裂以及染色质凝集等[84]。尽管没有研究证明NEK家族是有丝分裂起始所必需的,但是已确定NEK2、NEK6、NEK7和NEK9参与调控了细胞从间期进入M期的中心体的分离、纺锤体的组装、核孔复合物的去组装和核膜破裂等。
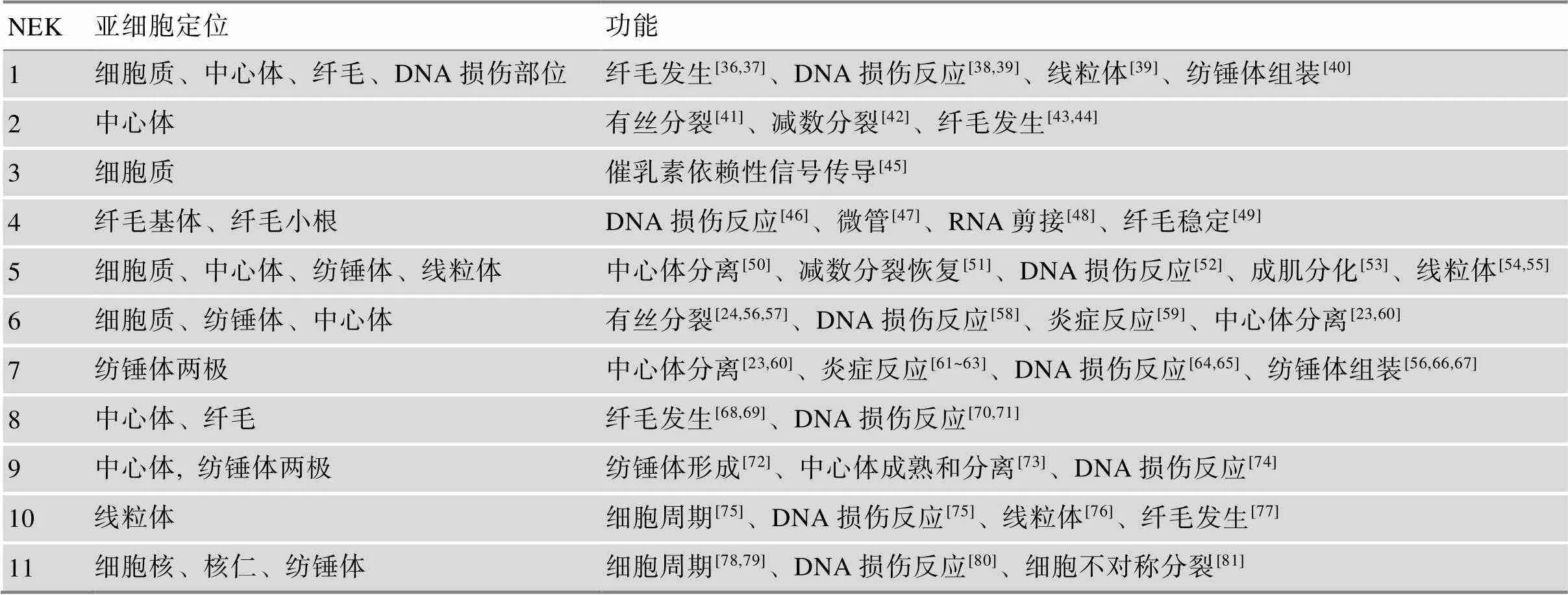
表1 人和哺乳动物NEK家族的亚细胞定位和功能
研究发现,一些NEK家族成员在从真菌到人类的微管组织中心均有定位[9,17,85~87]。在人类细胞中,NEK2作为中心体的核心组分,参与调控中心体的分离[41,88,89]。在有丝分裂间期,两个中心粒由一些蛋白质连接体结合在一起,而该连接体是由卷曲螺旋蛋白组成的,包括C-Nap1、rootletin、Cep68、centlein和LRRC45,而NEK2不仅可通过磷酸化连接蛋白[90~94]和中心粒相关蛋白GAS2L1[95,96],还可通过失活驱动蛋白KIFC3[97],共同调控有丝分裂前期的中心体分离和双极纺锤体形成。在有丝分裂间期,NEK2与蛋白激酶MST-2和磷酸酶PP1形成三聚体结构,维持在一个去磷酸化的失活状态。当有丝分裂启动时,PLK1可通过磷酸化MST-2破坏这种结构,导致NEK2的激活。除此之外,NEK2也可通过自磷酸化而被激活[98]。在有丝分裂过程中,NEK5与NEK2的定位模式相似。人基因的敲降导致分裂间期NEK2减少、中心粒周围物质(pericentriolar material, PCM)缺失、微管生长缓慢以及中心体连接蛋白rootletin被过度募集到中心体上,从而导致中心体的过早分离,分离的中心体之间相对较接近[50],这个现象与过表达人基因的结果是一致的[41,91],而且同时敲降和基因后中心体的过早分离被加重。我们推测,NEK5可能与NEK2协同调控中心体的分离。
研究发现,在有丝分裂的G1期和S期,NEK7可通过调控PCM的募集促进中心体的复制[99]。人基因的敲降导致PCM组分和原中心粒组装相关蛋白PLK4、CPAP、SAS-6以及STIL不能被募集到中心体,从而调控中心体的复制[100],而人基因和基因的过表达能够诱导额外的中心体形成[101]。在有丝分裂中,人、和基因的敲降导致前期中心体的分离失败、分裂中期形成脆弱的纺锤体、纺锤体两极的距离减小以及微管密度降低[23,56,66]。事实上,对于这些纺锤体的缺陷最简单的解释是中心体和纺锤体两极的微管成核作用减少。研究显示,NEK9能与启动微管成核的γ-tubulin环状复合体(γ-tubulin ring complex, γ-TuRC)的多个组分互作,如磷酸化γ-TuRC的衔接蛋白NEDD1[73,102],后者的激活促进了γ-tubulin被募集到中心体上,而的缺失会导致纺锤体组装延迟、双极纺锤体的形成减少和微管结构异常[102]。此外,NEK6和NEK7均定位到纺锤体两极,NEK6在有丝分裂的中期和后期定位到纺锤体微管上[56],NEK7可将γ-tubulin募集到纺锤体的两极[66]。研究结果提示,这些激酶对微管成核的调控可能不仅是通过纺锤体两极和纺锤体本身,还有可能是通过augmin复合体将γ-TuRCs募集到纺锤体的两极[103]。除此之外,这些激酶调控纺锤体形成的另一种途径可能是通过磷酸化微管相关蛋白进行的,例如Eg5作为一种驱动蛋白,参与了有丝分裂双极纺锤体的形成和维持过程,而Eg5被募集到纺锤体微管上的过程依赖于CDK1对Eg5的磷酸化作用[104,105]。研究发现,NEK6也可磷酸化Eg5[106],这一发现有助于阐明NEK6或NEK9在双极纺锤体的形成和维持中的作用[23,106]。另一项研究显示,EML4作为一种促进微管稳定性的微管相关蛋白参与微管动力学的调控,NEK6和NEK7可通过磷酸化EML4降低其与微管的亲和力,从而促进染色体中板聚合[107]。NEK6和NEK7还可以直接将微管蛋白磷酸化,这一发现提示NEK6和NEK7可能通过磷酸化微管蛋白直接参与微管动力学的调控[56]。这些研究均表明,NEK6、NEK7和NEK9在纺锤体的形成中发挥了重要作用。
NEK2、NEK6、NEK7和NEK9除影响纺锤体形成之外,也发挥其他的功能。例如,NEK2的剪接异构体NEK2C定位在细胞核中,这可能与NEK2在细胞核中的功能有关[108]。研究显示,Nup98是核孔复合体(nuclear pore complexes, NPCs)的组成成分,CDK1和NIMA可磷酸化Nup98,从而促进Nup98从NPCs的解离。CDK1还可磷酸化NEK9的Ser869位点,进而激活NEK9,而NEK6和NEK7可通过与激活的NEK9结合而被激活[23]。因此,我们推测NEKs也可能参与NPCs的解体和核膜破裂[109]。除此之外,NEK9还可与BICD2相互作用。而BICD2作为一种动粒蛋白相关蛋白,在有丝分裂前期可与动力蛋白结合,促进核孔复合体的去组装[110]。这些研究结果均表明,NEK家族在有丝分裂起始中发挥重要作用。
2.1.2 细胞周期检验点
细胞周期阻滞可发生在细胞周期的G1/S、S期和G2/M期,是由内源性因素(如停滞的复制叉)或者外源性因素(包括紫外线(UV)辐射、电离辐射(IR)、活性氧(ROS)和某些化疗药物)所造成的DNA损伤引起的。细胞周期由一系列的检验点所监控,当DNA出现损伤时,这些检验点蛋白被激活,进而导致细胞周期的延迟或阻滞。检验点的激活是由PIKK (phosphatidylinositol-3 kinase-related kinase)家族成员共济失调毛细血管扩张突变(ataxia telangiectasia mutated, ATM)蛋白和共济失调毛细血管扩张突变与相关(ataxia telangiectasia mutated andrelated, ATR)蛋白及其效应激酶CHK1/2 (checkpoint kinase 1/2)启动的,ERK1/2 (extracellular signal- regulated kinase 1/2)和p38及其下游激酶MK2 (MAPK activated protein kinase 2)在细胞周期阻滞中也发挥重要作用。在NEK家族中,NEK2和NEK6作为DNA损伤反应的靶点,是受DNA损伤抑制的[58,111],而其他的NEK家族成员在DNA损伤修复中发挥重要作用。
在有丝分裂的G1/S和G2/M转换中,NEK1在DNA损伤修复中起作用[112~115]。当敲除的细胞暴露于IR和UV辐射时,CHK1和CHK2不能被激活。此外,NEK1的激活不依赖于ATM和ATR。这些研究结果提示,NEK1可能是作为损伤信号的独立传感器发挥作用。
研究发现,NEK2不仅可与SAC蛋白相互作用,还可促进动粒复合蛋白HEC1的Ser165位点磷酸化[116~118]。除此之外,在紊乱的染色体动粒上可检测到磷酸化HEC1 (Ser165)的表达,而HEC1可将MPS1和MAD1/MAD2复合体募集到动粒上[119]。由此推测,NEK2可能参与纺锤体组装检验点SAC蛋白完整性的调控。
研究还发现,NEK8可通过RAD51蛋白和DNA损伤修复调控复制叉的稳定性[71],而NEK10和NEK11参与调控G2/M期的DNA损伤反应检验点。当细胞暴露于UV辐射时,NEK10与MEK1、RAF1形成一个三聚体的结构,NEK10可通过促进MEK1的激活,进而导致G2/M期阻滞和ERK1/2的磷酸化[75],敲降人基因可以抑制MEK1和ERK1/2的磷酸化。当发生DNA损伤和遗传毒性应激时,NEK11活性显著增加,而当抑制ATM和ATR激酶时,NEK11不能被激活[79,80]。当细胞暴露于IR辐射时,ATR和ATM激活CHK1,CHK1的激活促进NEK11和CDC25A的磷酸化,而NEK11的激活可进一步磷酸化CDC25A,这一过程促进SCF泛素连接酶复合物与CDC25A的结合,从而促进CDC25A的降解,最终导致G2/M期阻滞[79],使细胞有充足的时间进行DNA修复,不会过早进入有丝分裂。
2.1.3 胞质分裂
胞质分裂发生在细胞分裂后期姐妹染色单体分离之后, 是细胞周期和生物个体发育过程中的一个重要环节, 直接关系到遗传物质和细胞质组分能否在2个子细胞中正常分配。胞质分裂是由许多亚细胞结构和生物分子相互协调作用的结果。动物细胞胞质分裂过程主要包括分裂沟的定位、胞质分裂结构收缩的组装、分裂沟的产生和收缩、分裂沟膜泡的融合以及中间体的形成和剪切。
在真核生物中,NEK家族也参与胞质分裂的调控。在裂殖酵母中,Grallert等[11]发现FIN1在胞质分裂中起重要作用。在果蝇中,NEK2定位在有丝分裂后期的中体上,它的过表达可导致actin和anillin在卵裂沟的形成部位发生错位[17]。人NEK2剪接异构体NEK2B的敲降可导致细胞无法完成胞质分裂而形成多核细胞[120]。NEK6和NEK7也定位在有丝分裂后期的中体上,在胞质分裂中NEK6的激酶活性达到最大[56,66,106]。人或基因敲降的细胞可成功进入中期,但不能完成胞质分裂,而且人或的等位基因突变体细胞也经常出现胞质分裂的失败[56,66]。研究还发现,来自小鼠敲除胚胎的胚胎成纤维细胞也表现出胞质分裂失败的缺陷[121]。除此之外,NEK6和NEK9还可介导与胞质分裂有关的驱动蛋白MKLP2和KIF14的定位和募集[122]。以上证据均表明,NEK家族可能通过胞质分裂相关因子的定位和活性改变调控胞质分裂[56,122]。
2.2 NEK家族在减数分裂中的作用
如上所述,NEK家族在有丝分裂过程中发挥重要的调节作用。减数分裂作为一种特殊的细胞分裂方式,是真核生物和二倍体生物有性生殖和配子产生所必需的。在减数分裂中,染色体的错误分离有可能导致非整倍体受精卵或后代的产生。与有丝分裂相比,人们对NEK家族在减数分裂中的作用了解较少。近些年的研究发现,一些NEK家族成员,如NEK1、NEK2、NEK5、NEK9和NEK11,在减数分裂中也发挥重要的作用。
在哺乳动物生殖细胞中,NEK1高表达,并参与减数分裂中纺锤体形成的调控[36]。在敲除小鼠的精母细胞和卵母细胞中,第一次减数分裂的纺锤体组装和染色体排列异常,调控纺锤体动力相关蛋白-肌球蛋白X (myosin X, MYO10)和α-adducin的定位和表达改变[64,123,124]。我们推测,NEK1可能通过与MYO10和α-adducin的相互作用调控纺锤体的形成。在小鼠卵母细胞中,NEK2是微管组织中心的组成成分,它的敲降导致第一次减数分裂纺锤体两极的异常和染色体排列异常[42],研究证明centrobin/Nip2是NEK2的作用底物,在微管组织中心发挥重要作用[125,126],而且在卵母细胞中敲降与敲降的表型是一致的[42]。这些结果提示,NEK2可能通过磷酸化centrobin参与调控卵母细胞减数分裂I中纺锤体组装。在小鼠精母细胞减数分裂过程中,NEK2可磷酸化染色质结构蛋白HMGA2,通过降低后者与DNA的亲和力调控染色质的凝集[127]。我们最近的一项研究发现,NEK5在减数分裂G2/M转换过程中发挥了重要作用,在敲降的卵母细胞中MPF活性降低,导致了卵母细胞减数分裂恢复的失败[51]。同时,我们还发现NEK5定位在MI~MII期纺锤体上,推测NEK5也可能参与减数分裂纺锤体的组装。在敲降的小鼠卵母细胞中,纺锤体组装和染色体排列异常,γ-tubulin在纺锤体两极的定位异常,SAC被激活[128]。在小鼠卵母细胞中敲降影响了MI期纺锤体的迁移,导致卵母细胞的均等分裂[81]。上述研究结果表明,在生殖细胞中NEK1、NEK2、NEK5和NEK9等是保证减数分裂正常进行和染色体正确分离的关键蛋白,其表达的改变会导致纺锤体组装相关因子的定位和活性改变进而干扰纺锤体组装和减数分裂细胞周期进程。
3 结语与展望
自发现以来,NEK家族一直是细胞生物学的研究热点,研究证明NEK家族在细胞周期调控中发挥着关键的作用,但其在减数分裂中的功能和分子机制还有待于进一步深入的研究。细胞周期高度有序的运转是通过G1/S期转换、G2/M转换和中/后期转换等多个过程的调控来实现的。细胞周期紊乱是肿瘤发生的主要原因,细胞周期相关蛋白的表达异常在肿瘤细胞增殖中扮演着重要角色。因此,对NEK家族的生物学功能及其在细胞周期调控中作用的研究,不仅可以更深入地了解细胞周期过程及调控机制,还有助于阐明NEK家族在肿瘤发生发展中的作用机制,对肿瘤的临床诊断和治疗也具有重要意义。
[1] Morris NR. Mitotic mutants of., 1976, 26(3): 237–254.
[2] Oakley BR, Morris NR. A mutation inthat blocks the transition from interphase to prophase., 1983, 96(4): 1155–1158.
[3] Osmani SA, May GS, Morris NR. Regulation of the mRNA Levels of nimA, a Gene Required for the G2-M Transition in., 1987, 104(6): 1495–1504.
[4] Osmani SA, Pu RT, Morris NR. Mitotic induction and maintenance by overexpression of a G2-specific gene that encodes a potential protein kinase., 1988, 53(2): 237–244.
[5] Osmani AH, McGuire SL, Osmani SA. Parallel activation of the NIMA and p34cdc2 cell cycle-regulated protein kinases is required to initiate mitosis in., 1991, 67(2): 283–291.
[6] Pu RT, Osmani SA. Mitotic destruction of the cell cycle regulated NIMA protein kinase of Aspergillus nidulans is required for mitotic exit., 1995, 14(5): 995–1003.
[7] Krien MJ, Bugg SJ, Palatsides M, Asouline G, Morimyo M, O'Connell MJ. A NIMA homologue promotes chromatin condensation in fission yeast., 1998, 111(Pt7): 967–976.
[8] Wu L, Osmani SA, Mirabito PM. A role for NIMA in the nuclear localization of cyclin B in., 1998, 141(7): 1575–1587.
[9] De Souza CP, Osmani AH, Wu LP, Spotts JL, Osmani SA. Mitotic histone H3 phosphorylation by the NIMA kinase in., 2000, 102(3): 293–302.
[10] Grallert A, Hagan IM.NIMA-related kinase, Fin1, regulates spindle formation and an affinity of Polo for the SPB., 2002, 21(12): 3096–3107.
[11] Grallert A, Krapp A, Bagley S, Simanis V, Hagan IM. Recruitment of NIMA kinase shows that maturation of thespindle-pole body occurs over consecutive cell cycles and reveals a role for NIMA in modulating SIN activity., 2004, 18(9): 1007–1021.
[12] Letwin K, Mizzen L, Motro B, Ben-David Y, Bernstein A, Pawson T. A mammalian dual specificity protein kinase, Nek1, is related to the NIMA cell cycle regulator and highly expressed in meiotic germ cells., 1992, 11(10): 3521–3531.
[13] Sonn S, Khang I, Kim K, Rhee K. Suppression of Nek2A in mouse early embryos confirms its requirement for chromosome segregation., 2004, 117(Pt 23): 5557–5566.
[14] Bowers AJ, Boylan JF. Nek8, a NIMA family kinase member, is overexpressed in primary human breast tumors., 2004, 328: 135–142.
[15] Bradley BA, Wagner JJ, Quarmby LM. Identification and sequence analysis of six new members of the NIMA-related kinase family in., 2004, 51(1): 66–72.
[16] Dorin D, Le Roch K, Sallicandro P, Alano P, Parzy D, Poullet P, Meijer L, Doerig C. Pfnek-1, a NIMA-related kinase from the human malaria parasite Plasmodium falciparum Biochemical properties and possible involvement in MAPK regulation., 2001, 268(9): 2600–2608.
[17] Prigent C, Glover DM, Giet R. Drosophila Nek2 protein kinase knockdown leads to centrosome maturation defects while overexpression causes centrosome fragmentation and cytokinesis failure., 2005, 303(1): 1–13.
[18] Uto K, Nakajo N, Sagata N. Two structural variants of Nek2 kinase, termed Nek2A and Nek2B, are differentially expressed intissues and development., 1999, 208(2): 456–464.
[19] Fry AM, O'Regan L, Sabir SR, Bayliss R. Cell cycle regulation by the NEK family of protein kinases., 2012, 125(Pt 19): 4423–4433.
[20] Moniz L, Dutt P, Haider N, Stambolic V. Nek family of kinases in cell cycle, checkpoint control and cancer., 2011, 6(1): 18.
[21] Fry AM. The Nek2 protein kinase: a novel regulator of centrosome structure., 2002, 21(40): 6184–6194.
[22] Kandli M, Feige E, Chen A, Kilfin G, Motro B. Isolation and characterization of two evolutionarily conserved murine kinases (Nek6 and Nek7) related to the fungal mitotic regulator, NIMA., 2000, 68(2): 187–196.
[23] Bertran MT, Sdelci S, Regué L, Avruch J, Caelles C, Roig J. Nek9 is a Plk1-activated kinase that controls early centrosome separation through Nek6/7 and Eg5., 2011, 30(13): 2634–2647.
[24] Belham C, Roig J, Caldwell JA, Aoyama Y, Kemp BE, Comb M, Avruch J. A mitotic cascade of NIMA family kinases. Nercc1/Nek9 activates the Nek6 and Nek7 kinases., 2003, 278(37): 34897–34909.
[25] Roig J, Mikhailov A, Belham C, Avruch J. Nercc1, a mammalian NIMA-family kinase, binds the Ran GTPase and regulates mitotic progression., 2002, 16(13): 1640–1658.
[26] Rellos P, Ivins FJ, Baxter JE, Pike A, Nott TJ, Parkinson DM, Das S, Howell S, Fedorov O, Shen QY, Fry AM, Knapp S, Smerdon SJ. Structure and regulation of the human Nek2 centrosomal kinase., 2007, 282(9): 6833–6842.
[27] Lu KP, Kemp BE, Means AR. Identification of substrate specificity determinants for the cell cycle-regulated NIMA protein kinase., 1994, 269(9): 6603–6607.
[28] Alexander J, Lim D, Joughin BA, Hegemann B, Hutchins JR, Ehrenberger T, Ivins F, Sessa F, Hudecz O, Nigg EA, Fry AM, Musacchio A, Stukenberg PT, Mechtler K, Peters JM, Smerdon SJ, Yaffe MB. Spatial exclusivity combined with positive and negative selection of phosphorylation motifs is the basis for context-dependent mitotic signaling., 2011, 4(179): ra42.
[29] Lizcano JM, Deak M, Morrice N, Kieloch A, Hastie CJ, Dong L, Schutkowski M, Reimer U, Alessi DR. Molecular basis for the substrate specificity of NIMA-related kinase-6 (NEK6). Evidence that NEK6 does not phosphorylate the hydrophobic motif of ribosomal S6 protein kinase and serum- and glucocorticoid-induced protein kinase in vivo., 2002, 277(31): 27839–27849.
[30] Zalli D, Bayliss R, Fry AM. The Nek8 protein kinase, mutated in the human cystic kidney disease nephronophthisis, is both activated and degraded during ciliogenesis., 2012, 21(5): 1155–1171.
[31] Hames RS, Wattam SL, Yamano H, Bacchieri R, Fry AM. APC/C-mediated destruction of the centrosomal kinase Nek2A occurs in early mitosis and depends upon a cyclin A-type D-box., 2001, 20(24): 7117– 7127.
[32] Hayes MJ, Kimata Y, Wattam SL, Lindon C, Mao G, Yamano H, Fry AM. Early mitotic degradation of Nek2A depends on Cdc20-independent interaction with the APC/C., 2006, 8(6): 607–614.
[33] 李梅章, 褚嘉祐, 杨昭庆, 余龙. 一个基因家族新成员的克隆和鉴定., 2001, 23(2): 97–102. Li MZ, Zhu JY, Yang ZQ, YL. Isolating and identifying a novel member belonging togene family., 2001, 23(2): 97–102.
[34] Kimura M, Okano Y. Identification and assignment of the human NIMA-related protein kinase 7 gene (NEK7) to human chromosome 1q31.3., 2001, 94(1–2): 33–38.
[35] Vaz Meirelles G, Ferreira Lanza DC, da Silva JC, Santana Bernachi J, Paes Leme AF, Kobarg J. Characterization of hNek6 interactome reveals an important role for its short N-terminal domain and colocalization with proteins at the centrosome., 2010, 9(12): 6298–6316.
[36] Upadhya P, Birkenmeier EH, Birkenmeier CS, Barker JE. Mutations in a NIMA-related kinase gene, Nek1, cause pleiotropic effects including a progressive polycystic kidney disease in mice., 2000, 97(1): 217–221.
[37] Thiel C, Kessler K, Giessl A, Dimmler A, Shalev SA, von der Haar S, Zenker M, Zahnleiter D, Stöss H, Beinder E, Abou Jamra R, Ekici AB, Schröder-Kress N, Aigner T, Kirchner T, Reis A, Brandstätter JH, Rauch A. NEK1 mutations cause short-rib polydactyly syndrome type majewski., 2011, 88(1): 106–114.
[38] Melo-Hanchuk TD, Slepicka PF, Meirelles GV, Basei FL, Lovato DV, Granato DC, Pauletti BA, Domingues RR, Leme AFP, Pelegrini AL, Lenz G, Knapp S, Elkins JM, Kobarg J. NEK1 kinase domain structure and its dynamic protein interactome after exposure to Cisplatin., 2017, 7(1): 5445.
[39] Singh V, Khalil MI, De Benedetti A. The TLK1/Nek1 axis contributes to mitochondrial integrity and apoptosis prevention via phosphorylation of VDAC1., 2020, 19(3): 363–375.
[40] Brieño-Enríquez MA, Moak SL, Holloway JK, Cohen PE. NIMA-related kinase 1 (NEK1) regulates meiosis I spindle assembly by altering the balance between α-Adducin and Myosin X., 2017, 12(10): e0185780.
[41] Fry AM, Meraldi P, Nigg EA. A centrosomal function for the human Nek2 protein kinase, a member of the NIMA family of cell cycle regulators., 1998, 17(2): 470–481.
[42] Sonn S, Oh GT, Rhee K. Nek2 and its substrate, centrobin/Nip2, are required for proper meiotic spindle formation of the mouse oocytes., 2011, 19(1): 15–20.
[43] Endicott SJ, Basu B, Khokha M, Brueckner M. The NIMA-like kinase Nek2 is a key switch balancing cilia biogenesis and resorption in the development of left- right asymmetry., 2015, 142(23): 4068–4079.
[44] Viol L, Hata S, Pastor-Peidro A, Neuner A, Murke F, Wuchter P, Ho AD, Giebel B, Pereira G. Nek2 kinase displaces distal appendages from the mother centriole prior to mitosis., 2020, 219(3): e201907136.
[45] Miller SL, DeMaria JE, Freier DO, Riegel AM, Clevenger CV. Novel association of Vav2 and Nek3 modulates signaling through the human prolactin receptor., 2005, 19(4): 939–949.
[46] Nguyen CL, Possemato R, Bauerlein EL, Xie A, Scully R, Hahn WC. Nek4 regulates entry into replicative senescence and the response to DNA damage in human fibroblasts., 2012, 32(19): 3963–3977.
[47] Doles J, Hemann MT. Nek4 status differentially alters sensitivity to distinct microtubule poisons., 2010, 70(3): 1033–1041.
[48] Basei FL, Meirelles GV, Righetto GL, Dos Santos Migueleti DL, Smetana JH, Kobarg J. New interaction partners for Nek4.1 and Nek4.2 isoforms: from the DNA damage response to RNA splicing., 2015, 13: 11.
[49] Coene KL, Mans DA, Boldt K, Gloeckner CJ, van Reeuwijk J, Bolat E, Roosing S, Letteboer SJ, Peters TA, Cremers FP, Ueffing M, Roepman R. The ciliopathy- associated protein homologs RPGRIP1 and RPGRIP1L are linked to cilium integrity through interaction with Nek4 serine/threonine kinase., 2011, 20(18): 3592–3605.
[50] Prosser SL, Sahota NK, Pelletier L, Morrison CG, Fry AM. Nek5 promotes centrosome integrity in interphase and loss of centrosome cohesion in mitosis., 2015, 209(3): 339–348.
[51] Li YY, Guo L, Li H, Li J, Dong F, Yi ZY, Ouyang YC, Hou Y, Wang ZB, Sun QY, Lu SS, Han ZM. NEK5 regulates cell cycle progression during mouse oocyte maturation and preimplantation embryonic development., 2019, 86(9): 1189–1198.
[52] Melo-Hanchuk TD, Slepicka PF, Pelegrini AL, Menck CFM, Kobarg J. NEK5 interacts with topoisomerase IIβ and is involved in the DNA damage response induced by etoposide. J Cell Biochem, 2019, 120(10): 16853– 16866.
[53] Shimizu K, Sawasaki T. Nek5, a novel substrate for caspase-3, promotes skeletal muscle differentiation by up-regulating caspase activity., 2013, 587(14): 2219–2225.
[54] Ferezin CC, Basei FL, Melo-Hanchuk TD, de Oliveira AL, Peres de Oliveira A, Mori MP, de Souza-Pinto NC, Kobarg J. NEK5 interacts with LonP1 and its kinase activity is essential for the regulation of mitochondrial functions and mtDNA maintenance., 2021, 11(3): 546–563.
[55] Melo Hanchuk TD, Papa PF, La Guardia PG, Vercesi AE, Kobarg J. Nek5 interacts with mitochondrial proteins and interferes negatively in mitochondrial mediated cell death and respiration., 2015, 27(6): 1168–1177.
[56] O'Regan L, Fry AM. The Nek6 and Nek7 protein kinases are required for robust mitotic spindle formation and cytokinesis., 2009, 29(14): 3975– 3990.
[57] Yin MJ, Shao L, Voehringer D, Smeal T, Jallal B. The serine/threonine kinase Nek6 is required for cell cycle progression through mitosis., 2003, 278(52): 52454–52460.
[58] Lee MY, Kim HJ, Kim MA, Jee HJ, Kim AJ, Bae YS, Park JI, Chung JH, Yun J. Nek6 is involved in G2/M phase cell cycle arrest through DNA damage-induced phosphorylation., 2008, 7(17): 2705–2709.
[59] Gerçeker E, Boyacioglu SO, Kasap E, Baykan A, Yuceyar H, Yildirim H, Ayhan S, Ellidokuz E, Korkmaz M. Never in mitosis gene A-related kinase 6 and aurora kinase A: New gene biomarkers in the conversion from ulcerative colitis to colorectal cancer., 2015, 34(4): 1905–1914.
[60] Sdelci S, Bertran MT, Roig J. Nek9, Nek6, Nek7 and the separation of centrosomes., 2011, 10(22): 3816–3817.
[61] Gross O, Thomas CJ, Guarda G, Tschopp J. The inflammasome: an integrated view., 2011, 243(1): 136–151.
[62] Zhao N, Li CC, Di B, Xu LL. Recent advances in the NEK7-licensed NLRP3 inflammasome activation: Mechanisms, role in diseases and related inhibitors., 2020, 113: 102515.
[63] Sun ZZ, Gong W, Zhang Y, Jia ZJ. Physiological and pathological roles of mammalian NEK7., 2020, 11: 606996.
[64] de Souza EE, Meirelles GV, Godoy BB, Perez AM, Smetana JH, Doxsey SJ, McComb ME, Costello CE, Whelan SA, Kobarg J. Characterization of the human NEK7 interactome suggests catalytic and regulatory properties distinct from those of NEK6., 2014, 13(9): 4074–4090.
[65] Tan R, Nakajima S, Wang Q, Sun H, Xue J, Wu J, Hellwig S, Zeng X, Yates NA, Smithgall TE, Lei M, Jiang Y, Levine AS, Su B, Lan L. Nek7 protects telomeres from oxidative DNA damage by phosphorylation and stabilization of TRF1., 2017, 65(5): 818–831.e5.
[66] Kim S, Lee K, Rhee K. NEK7 is a centrosomal kinase critical for microtubule nucleation., 2007, 360(1): 56–62.
[67] de Souza EE, Hehnly H, Perez AM, Meirelles GV, Smetana JH, Doxsey S, Kobarg J. Human Nek7- interactor RGS2 is required for mitotic spindle organization., 2015, 14(4): 656–667.
[68] Sohara E, Luo Y, Zhang J, Manning DK, Beier DR, Zhou J. Nek8 regulates the expression and localization of polycystin-1 and polycystin-2., 2008, 19(3): 469–476.
[69] Zalli D, Bayliss R, Fry AM. The Nek8 protein kinase, mutated in the human cystic kidney disease nephronophthisis, is both activated and degraded during ciliogenesis., 2012, 21(5): 1155–1171.
[70] Choi HJ, Lin JR, Vannier JB, Slaats GG, Kile AC, Paulsen RD, Manning DK, Beier DR, Giles RH, Boulton SJ, Cimprich KA. NEK8 links the ATR- regulated replication stress response and S phase CDK activity to renal ciliopathies., 2013, 51(4): 423–439.
[71] Abeyta A, Castella M, Jacquemont C, Taniguchi T. NEK8 regulates DNA damage-induced RAD51 foci formation and replication fork protection., 2017, 16(4): 335–347.
[72] Kaneta Y, Ullrich A. NEK9 depletion induces catastrophic mitosis by impairment of mitotic checkpoint control and spindle dynamics., 2013, 442(3–4): 139–146.
[73] Sdelci S, Schutz M, Pinyol R, Bertran MT, Regue L, Caelles C, Vernos I, Roig J. Nek9 phosphorylation of NEDD1/GCP-WD contributes to Plk1 control of gamma-tubulin recruitment to the mitotic centrosome., 2012, 22(16): 1516–1523.
[74] Smith SC, Petrova AV, Madden MZ, Wang H, Pan Y, Warren MD, Hardy CW, Liang D, Liu EA, Robinson MH, Rudra S, Wang J, Ehdaivand S, Torres MA, Wang Y, Yu DS. A gemcitabine sensitivity screen identifies a role for NEK9 in the replication stress response., 2014, 42(18): 11517–11527.
[75] Moniz LS, Stambolic V. Nek10 mediates G2/M cell cycle arrest and MEK autoactivation in response to UV irradiation., 2011, 31(1): 30–42.
[76] Peres de Oliveira A, Basei FL, Slepicka PF, de Castro Ferezin C, Melo-Hanchuk TD, de Souza EE, Lima TI, Dos Santos VT, Mendes D, Silveira LR, Menck CFM, Kobarg J. NEK10 interactome and depletion reveal new roles in mitochondria., 2020, 18: 4.
[77] Porpora M, Sauchella S, Rinaldi L, Delle Donne R, Sepe M, Torres-Quesada O, Intartaglia D, Garbi C, Insabato L, Santoriello M, Bachmann VA, Synofzik M, Lindner HH, Conte I, Stefan E, Feliciello A. Counterregulation of cAMP-directed kinase activities controls ciliogenesis., 2018, 9(1): 1224.
[78] Noguchi K, Fukazawa H, Murakami Y, Uehara Y. Nucleolar Nek11 is a novel target of Nek2A in G1/S-arrested cells., 2004, 279(31): 32716–32727.
[79] Melixetian M, Klein DK, Sørensen CS, Helin K. NEK11 regulates CDC25A degradation and the IR-induced G2/M checkpoint., 2009, 11(10): 1247– 1253.
[80] Noguchi K, Fukazawa H, Murakami Y, Uehara Y. Nek11, a new member of the NIMA family of kinases, involved in DNA replication and genotoxic stress responses., 2002, 277(42): 39655–39665.
[81] Guo L, Wang ZB, Wang HH, Zhang T, Qi ST, Ouyang YC, Hou Y, Sun QY. Nek11 regulates asymmetric cell division during mouse oocyte meiotic maturation., 2016, 474(4): 667–672.
[82] O'Connell MJ, Norbury C, Nurse P. Premature chromatin condensation upon accumulation of NIMA., 1994, 13(20): 4926–4937.
[83] Lu KP, Hunter T. Evidence for a NIMA-like mitotic pathway in vertebrate cells., 1995, 81(3): 413–424.
[84] Hégarat N, Rata S, Hochegger H. Bistability of mitotic entry and exit switches during open mitosis in mammalian cells., 2016, 38(7): 627–643.
[85] Wloga D, Camba A, Rogowski K, Manning G, Jerka- Dziadosz M, Gaertig J. Members of the NIMA-related kinase family promote disassembly of cilia by multiple mechanisms., 2006, 17(6): 2799–2810.
[86] Krien MJ, West RR, John UP, Koniaras K, McIntosh JR, O'Connell MJ. The fission yeast NIMA kinase Fin1p is required for spindle function and nuclear envelope integrity., 2002, 21: 1713–1722.
[87] Mahjoub MR, Montpetit B, Zhao L, Finst RJ, Goh B, Kim AC, Quarmby LM. The FA2 gene ofencodes a NIMA family kinase with roles in cell cycle progression and microtubule severing during deflagellation., 2002, 115: 1759–1768.
[88] Andersen JS, Wilkinson CJ, Mayor T, Mortensen P, Nigg EA, Mann M. Proteomic characterization of the human centrosome by protein correlation profiling., 2003, 426(6966): 570–574.
[89] O'regan L, Blot J, Fry AM. Mitotic regulation by NIMA-related kinases., 2007, 2(25): 1–12.
[90] Bahe S, Stierhof YD, Wilkinson CJ, Leiss F, Nigg EA. Rootletin forms centriole-associated filaments and functions in centrosome cohesion., 2005, 171(1): 27–33.
[91] Faragher AJ, Fry AM. Nek2A kinase stimulates centrosome disjunction and is required for formation of bipolar mitotic spindles., 2003, 14(7): 2876–2889.
[92] Fry AM, Mayor T, Meraldi P, Stierhof YD, Tanaka K, Nigg EA. C-Nap1, a novel centrosomal coiled-coil protein and candidate substrate of the cell cycle-regulated protein kinase Nek2., 1998, 141(7): 1563– 1574.
[93] Yang J, Adamian M, Li T. Rootletin interacts with C-Nap1 and may function as a physical linker between the pair of centrioles/basal bodies in cells., 2006, 17(2): 1033–1040.
[94] Fang G, Zhang D, Yin H, Zheng L, Bi X, Yuan L. Centlein mediates an interaction between C-Nap1 and Cep68 to maintain centrosome cohesion., 2014, 127(Pt 8): 1631–1639.
[95] Au FKC, Hau BKT, Qi RZ. Nek2-mediated GAS2L1 phosphorylation and centrosome-linker disassembly induce centrosome disjunction., 2020, 219(5): e201909094.
[96] Au FK, Jia Y, Jiang K, Grigoriev I, Hau BK, Shen Y, Du S, Akhmanova A, Qi RZ. GAS2L1 is a centriole- associated protein required for centrosome dynamics and disjunction., 2017, 40(1): 81–94.
[97] Hata S, Pastor Peidro A, Panic M, Liu P, Atorino E, Funaya C, Jäkle U, Pereira G, Schiebel E. The balance between KIFC3 and EG5 tetrameric kinesins controls the onset of mitotic spindle assembly., 2019, 21(9): 1138–1151.
[98] Meirelles GV, Perez AM, de Souza EE, Basei FL, Papa PF, Melo Hanchuk TD, Cardoso VB, Kobarg J. “Stop Ne(c)king around”: How interactomics contributes to functionally characterize Nek family kinases., 2014, 5(2): 141–160.
[99] Loncarek J, Hergert P, Magidson V, Khodjakov A. Control of daughter centriole formation by the pericentriolar material., 2008, 10(3): 322–328.
[100] Gupta A, Tsuchiya Y, Ohta M, Shiratsuchi G, Kitagawa D. NEK7 is required for G1 progression and procentriole formation., 2017, 28(15): 2123– 2134.
[101] Kim S, Kim S, Rhee K. NEK7 is essential for centriole duplication and centrosomal accumulation of pericentriolar material proteins in interphase cells., 2011, 124(Pt 22): 3760–3770.
[102] Roig J, Groen A, Caldwell J, Avruch J. Active Nercc1 protein kinase concentrates at centrosomes early in mitosis and is necessary for proper spindle assembly., 2005, 16(10): 4827–4840.
[103] Goshima G, Kimura A. New look inside the spindle: microtubule-dependent microtubule generation within the spindle., 2010, 22(1): 44–49.
[104] Blangy A, Lane HA, d'Hérin P, Harper M, Kress M, Nigg EA. Phosphorylation by p34cdc2 regulates spindle association of human Eg5, a kinesin-related motor essential for bipolar spindle formation in vivo., 1995, 83(7): 1159–1169.
[105] Sawin KE, Mitchison TJ. Mutations in the kinesin-like protein Eg5 disrupting localization to the mitotic spindle., 1995, 92(10): 4289–4293.
[106] Rapley J, Nicolàs M, Groen A, ReguéL, Bertran MT, Caelles C, Avruch J, Roig J. The NIMA-family kinase Nek6 phosphorylates the kinesin Eg5 at a novel site necessary for mitotic spindle formation., 2008, 121(Pt 23): 3912–3921.
[107] Adib R, Montgomery JM, Atherton J, O'Regan L, Richards MW, Straatman KR, Roth D, Straube A, Bayliss R, Moores CA, Fry AM. Mitotic phosphorylation by NEK6 and NEK7 reduces the microtubule affinity of EML4 to promote chromosome congression., 2019, 12(594): eaaw2939.
[108] Wu W, Baxter JE, Wattam SL, Hayward DG, Fardilha M, Knebel A, Ford EM, da Cruz e Silva EF, Fry AM. Alternative splicing controls nuclear translocation of the cell cycle-regulated Nek2 kinase., 2007, 282(36): 26431–26440.
[109] Laurell E, Beck K, Krupina K, Theerthagiri G, Bodenmiller B, Horvath P, Aebersold R, Antonin W, Kutay U. Phosphorylation of Nup98 by multiple kinases is crucial for NPC disassembly during mitotic entry., 2011, 144(4): 539–550.
[110] Holland PM, Milne A, Garka K, Johnson RS, Willis C, Sims JE, Rauch CT, Bird TA, Virca GD. Purification, cloning, and characterization of Nek8, a novel NIMA-related kinase, and its candidate substrate Bicd2., 2002, 277(18): 16229–16240.
[111] Fletcher L, Cerniglia GJ, Nigg EA, Yend TJ, Muschel RJ. Inhibition of centrosome separation after DNA damage: a role for Nek2., 2004, 162(2): 128–135.
[112] Pelegrini AL, Moura DJ, Brenner BL, Ledur PF, Maques GP, Henriques JA, Saffi J, Lenz G. Nek1 silencing slows down DNA repair and blocks DNA damage-induced cell cycle arrest., 2010, 25(5): 447–454.
[113] Chen YM, Chen PL, Chen CF, Jiang XZ, Riley DJ. Never-in-mitosis related kinase 1 functions in DNA damage response and checkpoint control., 2008, 7(20): 3194–3201.
[114] Chen YM, Chen CF, Riley DJ, Chen PL. Nek1 kinase functions in DNA damage response and checkpoint control through a pathway independent of ATM and ATR., 2011, 10(4): 655–663.
[115] Polci R, Peng AM, Chen PL, Riley DJ, Chen YM. NIMA-related protein kinase 1 is involved early in the ionizing radiation-induced DNA damage response., 2004, 64(24): 8800–8803.
[116] Du J, Cai X, Yao J, Ding X, Wu Q, Pei S, Jiang K, Zhang Y, Wang W, Shi Y, Lai Y, Shen J, Teng M, Huang H, Fei Q, Reddy ES, Zhu J, Jin C, Yao X. The mitotic checkpoint kinase NEK2A regulates kinetochore microtubule attachment stability., 2008, 27(29): 4107–4114.
[117] Lou Y, Yao JH, Zereshki A, Dou Z, Ahmed K, Wang HM, Hu JB, Wang YZ, Yao XB. NEK2A interacts with MAD1 and possibly functions as a novel integrator of the spindle checkpoint signaling., 2004, 279(19): 20049–20057.
[118] Wei R, Ngo B, Wu GK, Lee WH. Phosphorylation of the Ndc80 complex protein, HEC1, by Nek2 kinase modulates chromosome alignment and signaling of the spindle assembly checkpoint., 2011, 22(19): 3584–3594.
[119] Martin-Lluesma S, Stucke VM, Nigg EA. Role of Hec1 in spindle checkpoint signaling and kinetochore recruitment of Mad1/Mad2., 2002, 297(5590): 2267–2270.
[120] Fletcher L, Cerniglia GJ, Yen TJ, Muschel RJ. Live cell imaging reveals distinct roles in cell cycle regulation for Nek2A and Nek2B., 2005, 1744(2): 89–92.
[121] Salem H, Rachmin I, Yissachar N, Cohen S, Amiel A, Haffner R, Lavi L, Motro B. Nek7 kinase targeting leads to early mortality, cytokinesis disturbance and polyploidy., 2010, 29(28): 4046–4057.
[122] Cullati SN, Kabeche L, Kettenbach AN, Gerber SA. A bifurcated signaling cascade of NIMA-related kinases controls distinct kinesins in anaphase., 2017, 216(8): 2339–2354.
[123] Chan PC, Hsu RYC, Liu CW, Lai CC, Chen HC. Adducin-1 is essential for mitotic spindle assembly through its interaction with myosin-X., 2014, 204(1): 19–28.
[124] Hsu WH, Wang WJ, Lin WY, Huang YM, Lai CC, Liao JC, Chen HC. Adducin-1 is essential for spindle pole integrity through its interaction with TPX2., 2018, 19(8): e45607.
[125] Jeong Y, Lee J, Kim K, Yoo JC, Rhee K. Characterization of NIP2/centrobin, a novel substrate of Nek2, and its potential role in microtubule stabilization., 2007, 120(Pt 12): 2106–2116.
[126] Lee J, Kim S, Jeong Y, Rhee K. Centrobin/Nip2 expression in vivo suggests its involvement in cell proliferation., 2009, 28(1): 31–36.
[127] Di Agostino S, Fedele M, Chieffi P, Fusco A, Rossi P, Geremia R, Sette C. Phosphorylation of high-mobility group protein A2 by Nek2 kinase during the first meiotic division in mouse spermatocytes., 2004, 15(3): 1224–1232.
[128] Yang SW, Gao C, Chen L, Song YL, Zhu JL, Qi ST, Jiang ZZ, Wang ZW, Lin F, Huang H, Xing FQ, Sun QY. Nek9 regulates spindle organization and cell cycle progression during mouse oocyte meiosis and its location in early embryo mitosis., 2012, 11(23): 4366–4377.
Roles of NEK family in cell cycle regulation
Yuanyuan Li1, Lei Guo2, Zhiming Han1,3
As a serine/threonine kinase, NIMA-related kinases (NEKs) play important roles in the regulation of cell cycle, and involve in several cellular activities such as centrosome separation, spindle assembly, chromatin condensation, nuclear envelope breakdown, spindle assembly checkpoint signaling, cytokinesis, cilia formation and DNA damage response. In this review, we summarize the component, structural characteristics and functions of NEK family in mitosis and meiosis based on the relevant researches in recent years, providing a reference for the further study on the roles of NEKs in the regulation of cell cycle and a theoretical basis for the clinical diagnosis and treatment of tumors.
NIMA-related kinases; mitosis; meiosis
2021-03-27;
2021-05-12
国家重点研发计划资助项目(编号:2018YFC1004000,2019YFA0109900)和国家自然科学基金项目(编号:31970509)资助[Supported by the National Key R&D Program of China (Nos. 2018YFC1004000, 2019YFA0109900), and the National Natural Science Foundation of China (No. 31970509)]
李园园,博士,专业方向:发育生物学。E-mail: liyuanyuan891116@163.com
韩之明,博士,副研究员,专业方向:发育生物学。E-mail: hanzm@ioz.ac.cn
10.16288/j.yczz.20-421
2021/6/25 13:16:28
URI: https://kns.cnki.net/kcms/detail/11.1913.R.20210625.1123.002.html
(责任编委: 史庆华)
