Fate of low-molecular-weight organic phosphorus compounds in the P-rich and P-poor paddy soils
2021-07-24LIBaozhenAnnaGUNINAMostafaZHRANDaveyJONESPaulHILLHUYajunGETidaWUJinshui
LI Bao-zhen,Anna GUNINA,Mostafa ZHRAN,4,Davey L.JONES,Paul W.HILL,HU Ya-jun,GE Tida,WU Jin-shui,6
1 Key Laboratory of Agro-ecological Processes in Subtropical Region/Changsha Research Station for Agricultural &Environmental Monitoring,Institute of Subtropical Agriculture,Chinese Academy of Sciences,Changsha 410125,P.R.China
2 Department of Environmental Chemistry,University of Kassel,Nordbahnhofstrae 1a 37213,Witzenhausen,Germany
3 Department of Soil Biology and Biochemistry,Dokuchaev Soil Science Institute,Moscow 119017,Russian Federation
4 Soil and Water Research Department,Nuclear Research Center,Atomic Energy Authority,Abou-Zaabl 13759,Egypt
5 School of Natural Sciences,Bangor University,Bangor,Gwynedd LL57 2UW,UK
6 University of Chinese Academy of Sciences,Beijing 100049,P.R.China
Abstract Continuous application of organic fertilizers can cause accumulation of organic phosphorus (P) in soil,especially in the lowmolecular-weight organic phosphorus (LMWOP) forms.This organic P pool represents a potentially important source of P for both plants and microorganisms.To understand the effect of long-term fertilization (30 years) (P-rich soil) vs.fallowing(P-poor soil) on the bioavailability and fate of LMWOP in subtropical paddy soils,we determined the sorption and mineralization of 14C-labeled adenosine,adenosine monophosphate (AMP),adenosine diphosphate (ADP),and adenosine triphosphate(ATP) in each soil.The contents of carbon,nitrogen,and P in the P-rich soil were more than two times greater than those in the P-poor soil.The mineralization rates of the LMWOP compounds were faster in the P-rich soil compared to the P-poor soil,and followed the order AMP>ADP>ATP.Using sterilized soil,all forms of adenosine-P were strongly sorbed to the solid phase and reached saturation in a short time,with the adsorbance increasing with the number of phosphate groups.We concluded that the mineralization of LMWOP compounds was repressed slightly by sorption to the solid phase,but only in the short term.Thus,LMWOP compounds serve as readily available sources of C for microorganisms,making P available for themselves as well as for the plants.However,P accumulation and the progressive saturation of the P sorption sites in highly fertile soils may increase the potential risk of P runoff.
Keywords:rice paddy,phosphatase,phosphorus cycling,microbial community
1.Introduction
Phosphorus (P) is one of the most important nutrients for crops.The fate of the inorganic P pool,including its movement in the soil and its adsorption/desorption reaction,has been intensively studied (Kögel-Knabneret al.2010;Yanet al.2013,2017).Organic forms of P comprise 20–80%of the total P in the soils,and they represent a potential source of P for both plants and microorganisms (Chenet al.2003;Fransson and Jones 2007;Zhanget al.2014;Arrudaet al.2018;Huet al.2018).Fertile soils are generally rich in organic-P fractions,which are labile or moderately labile,including compounds such as nucleic acids,phospholipids,inositol phosphates,phosphoproteins,and P-containing metabolic compounds,such as adenosine phosphates (Leeet al.2004;Fransson and Jones 2007).These compounds may represent up to 50% of the organic P pool in soils(Turneret al.2003;Vatset al.2005;Liet al.2016).Thus,in order to develop sustainable agricultural systems by improving P utilization,it is essential to understand the factors that regulate the dynamics of the major forms of labile organic-P in soil.
Cycling of organic-P in soil is largely regulated by the activity of plant roots and microorganisms (Chenet al.2003;Parham 2014;Yokoyamaet al.2017;Weiet al.2019a),which produce enzymes to mineralize P-containing organic compounds (e.g.,phosphatases),as well as being sources of these compounds (e.g.,in root and microbial turnover).For instance,ATP is one of the dominant forms of P in plant tissues (i.e.,ATP contents of pea shoots and roots are 113 and 98 mmol g–1;Smyth and Black 1984) and microbial cells (i.e.,ATP content in bacterial cells is 0.6–4.2 mmol L–1;Yaginumaet al.2014).ATP can be released from dead cells but is also excreted into the environment by Gram-positive and Gram-negative bacteria (Mempinet al.2013) and by plant roots (from actively growing root regions where cell expansion is occurring;Kimet al.2006;Geet al.2019).The role of the ATP released by living bacterial cells is still under discussion,but ATP is a possible source of nutrients or a signaling molecule for bacterial communities (Mempinet al.2013).ATP contains not only C and P,but also N,therefore the products of its degradation can serve as sources of multiple nutrients for both microorganisms and plants.
The availability of P in the soil depends on a combination of environmental and biological factors,including:i) the amount and type of clay minerals,i.e.,presence of gibbsite and goethite increases the sorption of inorganic P,making it unavailable for plants (Liet al.2016);ii) oxygen content,i.e.,frequent changes in soil moisture (pulse redox conditions)mobilizes labile forms of organic P (Yevdokimovet al.2016;Guet al.2018;Weiet al.2019b);and iii) pH conditions,i.e.,the acidification of the rhizosphere by root exudates (i.e.,by carboxylic acids) increases P availability (Bending 2017).The type of fertilizer applied also directly and indirectly affects soil microbial community composition and activity(Yaoet al.2016;Weiet al.2017,2019a;Yuet al.2019),which in turn is responsible for P mineralizationviathe production of hydrolytic enzymes.However,it is still not clear what regulates the turnover of dissolved organic-P(DOP) in rice paddy soils,because:i) The Eh and pH are frequently fluctuating (Yanet al.2017),ii) the contents of Al and Fe are high,and iii) these soils are always fertilized with either organics,mineral fertilizers,or a combination of the two (Lanet al.2012;Donget al.2014).For low-molecularweight compounds (sugars,carboxylic and amino acids),it is known that microbial utilization overcomes sorption on the mineral matrix (Fischeret al.2010;Guninaet al.2014).However,it is not clear whether the same patterns could be observed for organic-P compounds present in soil solutions in paddy environments.
Thus,the present research hypothesized that the sorption and mineralization of organic P substrates in soils will be affected by:i) the number of phosphate groups within the organic-P compound,because this part of molecule can be sorbed to the mineral phase,and ii) soil fertility level(contents of total carbon (C),nitrogen (N) and P),because it directly affects microbial activity.To test these hypotheses,the base compound (adenosine) with either 1,2,or 3 phosphate groups - namely adenosine monophosphate(AMP),adenosine diphosphate (ADP),and adenosine triphosphate (ATP) (all labeled with14C) - were obtained,and their fates in P-rich (fertilized) and P-poor (fallow) paddy soils were studied in a short-term laboratory experiment.
2.Materials and methods
2.1.Soil samples
Soil samples were collected from the Changsha Research Station for Agricultural and Environmental Monitoring(113°19´52´´E,28°33´04´´N) in Jingjin County,Hunan Province,China.Two soil samples were collected from:i)fertilized soil,as the P-rich soil from a site that was cultivated for rice (OryzasativaL.) and fertilized with a combination of pig manure and inorganic fertilizer that contained 120 kg N ha–1,40 kg P2O5ha–1,and 100 kg K2O ha–1in every growing season,and ii) a P-poor (control) soil,which was under fallow for the same period of time.Soil samples were collected from a depth of 0–20 cm in four replicates,sieved (<2 mm),and stored dry prior to analysis.The soil was a typical Stagnic Anthrosol developed from granitic red parent material,and had 6.1% clay,61.1% silt,and 32.8%sand for the control,and 5.5% clay,50.6% silt,and 43.9%sand for the P-rich soil.The main characteristics of the soils are presented in Table 1.Importantly,the Olsen-P content was significantly higher in the P-rich than in the P-poor soil(42.45vs.3.87 mg kg–1).

Table 1 Chemical characteristics for the P-rich and P-poor paddy soils at 0–20 cm depth1)
2.2.Chemical analyses
The pH and electrical conductivity (EC) were determined with a standard electrode with a soil/water ratio of 1:2.5(w/v).Soil moisture content was determined by drying at 105°C for 24 h,and total C and N were obtained by the dry combustion method (Vario MAX,Elementar Analysen System GmbH,Germany).Total P content in soil was measured by a molybdate blue method after the soil samples were digested in a mixture of nitric and perchloric acids.Olsen-P was extracted into 0.5 mol L–1NaHCO3(pH 8.5)solution by shaking at 205 r min–1for 30 min (Dinget al.2012).Soil dissolved organic C (DOC) was extracted with K2SO4(0.5 mol L–1) and measured with a liquid-TOC analyzer (Phoenix-8000).Soil microbial biomass C (MBC)was determined by chloroform fumigation-extraction (Wuet al.1990).
2.3.Organic-P mineralization
Five grams of dry soil was placed into a 50 mL centrifuge tube,hydrated to 50% of WHC,and later saturated to over 100% by placing a water table 1 cm above the soil surface,and pre-incubated for 2 weeks at 20°C.There were 20 tubes for each soil.After pre-incubation,0.5 mL of a14C-labeled solution (0.2 kBq mL–1) of either [8-14C]-adenosine (Sigma-Aldrich Corp,USA),[U-14C]-adenosine monophosphate (AMP;NEN-Dupont,USA),[8-14C]-adenosine diphosphate (ADP;NEN-Dupont),or [8-14C]-adenosine triphosphate (ATP;NEN-Dupont) were added to each soil type at five concentrations:10,50,100,500 and 1 000 µmol L–1.To collect the14CO2producted from mineralization of the added compounds,a 1 mol L–1NaOH trap (1 mL) was placed inside the tube which was then tightly sealed and incubated in the dark at 20°C for 168 h.The NaOH traps were replaced after 1,3,6,24,48,72 and 168 h.The amount of14CO2captured was determined by liquid scintillation counting (Wallac EG &G,Milton Keynes,UK).After 168 h,25 mL of 0.5 mol L–1KH2PO4was added to all samples and the tubes were shaken for 30 min in order to extract any free14C organic compounds still remaining in the soil.Subsequently,a 1 mL aliquot of the 0.5 mol L–1KH2PO4extract was removed and centrifuged (18 000×g for 5 min) to remove microorganisms/particles from the solution.The radioactivity of the solution was determined as described above.
2.4.Organic-P sorption
Sorption experiments were conducted using the soils sterilized by autoclave at 121°C for 30 min (Serrasolsas and Khanna 1993).The autoclaving eliminated microorganisms that could degrade the14C-labelled substrates and denatured any phosphatases that existed in the soil (Fransson and Jones 2007;Robertset al.2007).Solid phase sorption of each substrate was determined by shaking 2.5 g of soil with 5 mL of each14C-labeled compound for periods of up to 3 h.The same14C-labeled compounds listed above were used,namely:[8-14C]-adenosine,[U-14C]-AMP,[8-14C]-ADP,or[8-14C]-ATP.The14C-labeled solutions (0.2 kBq mL–1) were added at five concentrations:10,50,100,500 and 1 000µmol L–1into the soil.After shaking for either 0.25,1,3,or 6 h,an aliquot of solution (350 µL) was removed from the soil suspensions.Aliquots were centrifuged at 18 000×g for 5 min and14C activity in the supernatant was determined as described above.
2.5.Statistical and data analysis
The mineralization of substrates is bi-phasic,with a rapid first phase followed by a second slow phase (Hillet al.2011).The14CO2efflux from the soil after the addition of14C-labelled substrates was best fitted to a first order double exponential decay equation (Hillet al.2011):
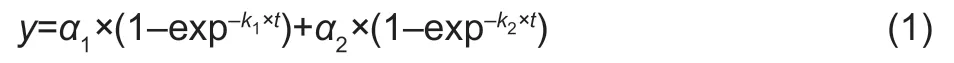
wherek1andk2are the coefficients describing the fast and slow mineralization phases,respectively,anda1anda2describe the sizes of the pools.Thet1/2of the soil solution substrate pool (a1) was calculated as:

Freundlich isotherm was fitted to the data from the sorption experiment and the amount of sorbed compounds was calculated as follows:


whereSis the amount of sorbed (µmol g–1) compounds,aandbare empirically derived parameters (Robertset al.2007),ESCis the equilibrium solution concentration at the end of the experiment (µmol L–1),The partition coefficient(Kd) was calculated as follows:All data represented the means of four replicates with their standard errors.The analysis of significant differences was performed by one-way ANOVA at the 95% confidence level (P<0.05).Residuals were checked for normality and homogeneity.All analyses were conducted with SigmaPlot 10.0 (SPSS Inc.,Chicago,IL).
3.Results
3.1.Soil characteristics
The two paddy soils used in the experiments differed significantly in their chemical characteristics (Table 1).The EC,and total N,C,and P in the P-rich soil wereca.two times higher compared to those in the P-poor soil,whereas Oslen-P was 11 times higher.
3.2.Mineralization of substrates
Mineralization curves of adenosine and the three organic P substrates showed a biphasic14CO2evolution (Fig.1).The total amount of ADP mineralized in the P-rich soil was 1.5 times greater than in the P-poor soil (Fig.1) with similar values being observed for adenosine,AMP,and ATP.Generally,less than 1% of the initially applied14C was recovered in a 0.5 mol L–1K2SO4extract at the end of a 7-day incubation period,which suggested a nearly complete utilization of all compounds by the microbial community.
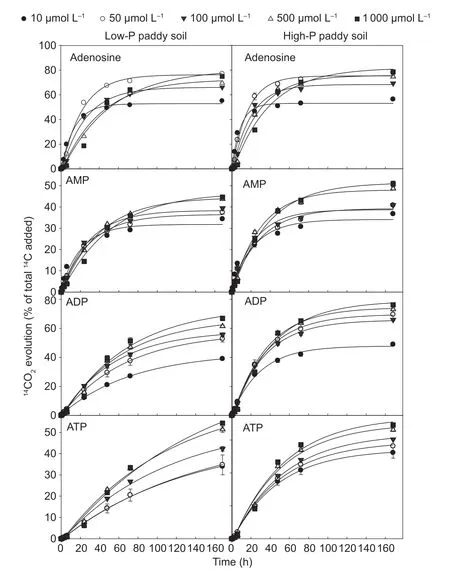
Fig.1 Time-dependent mineralization of 14C-labeled adenosine,AMP,ADP and ATP at concentrations ranging from 10 to 1 000 µmol L–1 in P-rich and P-poor paddy soils in comparison to their non-phosphorylated counterparts (10 to 1 000 µmol L–1 of adenosine).Values represent mean±SE (n=3).
There was a significant divergence in mineralization rates among the substrates during the initial utilization phase(first 48 h):for the first hour,mineralization rates followed the order AMP>ADP>adenosine>ATP in both paddy soils(Fig.2).After 48 h,the mineralization rates followed a different trend:adenosine>ADP>ATP>AMP in both soils(Fig.2).Moreover,the mineralization rates increased for adenosine and ATP,but decreased for AMP in both soils.These results indicated that the soil microorganisms utilized the AMP and ADP as C or P sources first,and then adenosine and ATP.Moreover,the mineralization rate of P-containing substrates was always higher in the P-rich soil than in the P-poor soil as a function of incubation time.
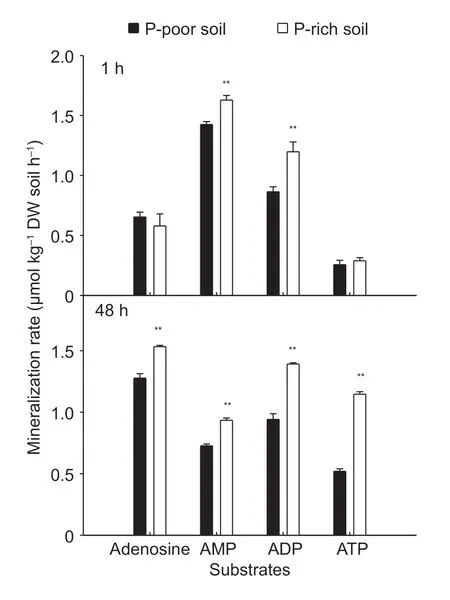
Fig.2 Mineralization rates of 14C-labeled adenosine,AMP,ADP,and ATP at a concentration of 1 000 µmol L–1 after 1 h and 48 h of incubation in the P-rich and P-poor paddy soils.Values represent mean±SE (n =4).** represent significant differences of same substrate between two paddy soils at the P<0.01 level.
3.3.Half-life of adenosine substrate-derived C in soil
The half-life (t1/2) of C derived from each adenosine substrate increased with increasing the applied concentration in both soils (Table 2).Thet1/2of the adenosine substrates increased to 3–10 times with increasing the number of phosphate groups for both soils depending on the concentration (Table 2).Thet1/2of ADP- and ATP-derived C was 1.5 and 3 times slower in the P-rich soil than in the P-poor soil,whereast1/2of AMP-C and adenosine-C was the same in both soils.Thus,the increasingt1/2of adenosine compounds with increasing the number of phosphate groups can reflect the effect of sorption on the mineral matrix,which can partially affect microbial mineralization.The fastert1/2of the studied compounds in the P-rich soil indicated higher microbial activity than in the P-poor soil.
3.4.Sorption of organic-P compounds
The sorption of the14C-labeled adenosine compounds to the solid phase was time-dependent and followed the same pattern in both paddy soils:ATP=ADP>AMP>adenosine (Fig.3).The sorption was very fast for all compounds and reached the maximum after 15 min.The Freundlich sorption isotherm showed that saturation was not reached over the applied concentration range of the substances (10 to 1 000 µmol L–1) (Fig.4).The Freundlich parameteraincreased with the number of phosphate groups,whereas parameterbdecreased in both paddy soils (Table 3).The solid-tosolution partition coefficientsKdfor AMP,ADP,and ATP were concentration-dependent and decreased with substrate concentration in both soils,but there was no dependency for adenosine (Table 3).Moreover,theKdincreased with an increasing number of phosphate groups from adenosine to ATP.Additionally,Kdwas always greater for ATP in the P-poor soil than in the P-rich soil,except when ATP was applied at concentrations above 500 µmol L–1.The results showed that organic P sorption increased with the number of phosphate groups and decreased with increased nutrient availability.
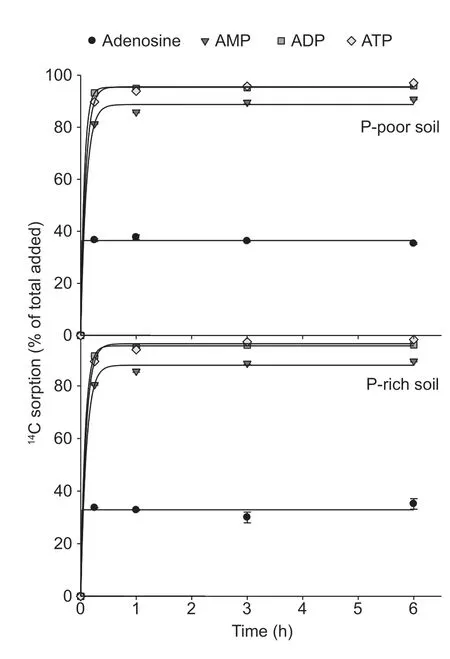
Fig.3 Sorption of 14C-labeled adenosine,AMP,ADP,and ATP in P-poor and P-rich paddy soils.The initial concentration was 500 µmol L–1.Values represent mean±SE (n=3).
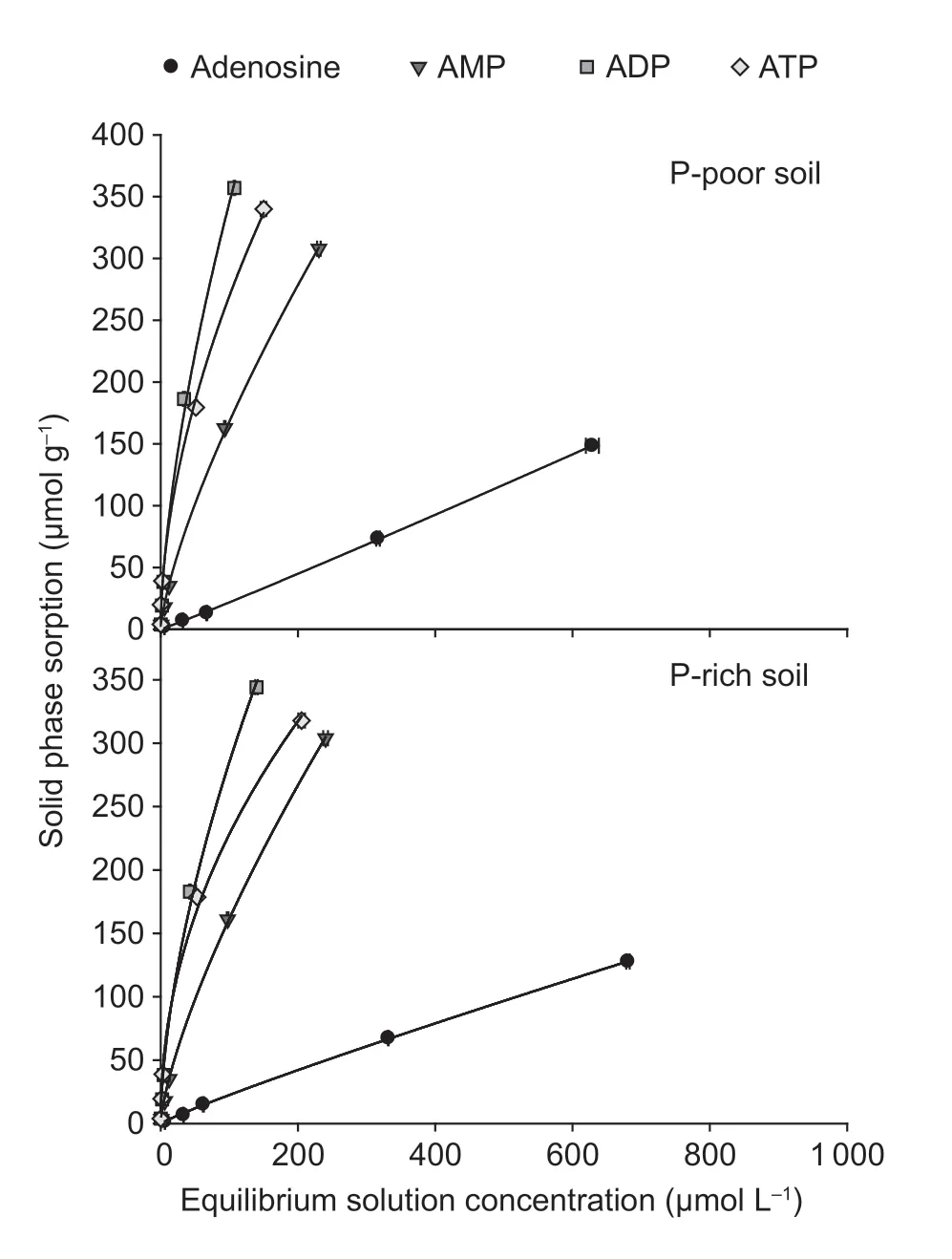
Fig.4 Sorption isotherms for 14C-labeled adenosine,AMP,ADP,and ATP in P-poor and P-rich paddy soils.Values represent mean±SE (n=3).
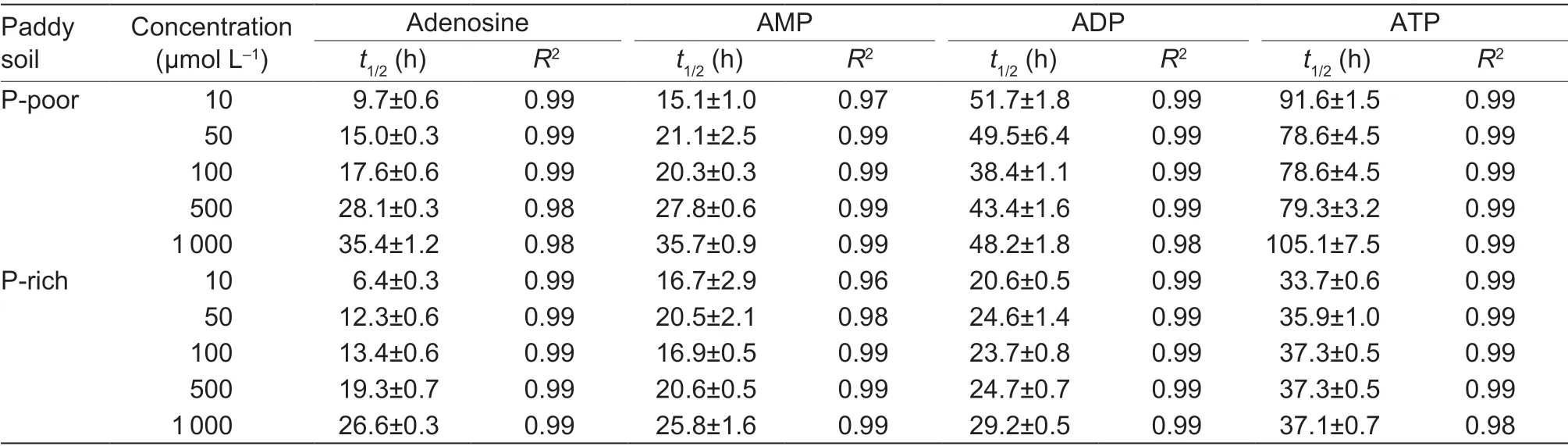
Table 2 Half-life (t1/2) of 14C-labeled adenosine,AMP,ADP and ATP in P-poor and P-rich soils1)
4.Discussion
Long-term fertilization can improve soil fertility of paddy surface soils (Abdiet al.2014;Yanet al.2017;Liuet al.2019;Wanget al.2019;Zhanget al.2019) by increasing the total C,N,and P contents,as well as increasing the size of the microbial biomass (Table 1).Mineralization of organic-P compounds in both paddy soils was similar for the fast stage(0–24 h) followed by a slower mineralization stage (24–168 h),which was consistent with the report of a previous study(Fransson and Jones 2007).The results indicated that high rates of phosphatase activity and dephosphorylation of the added compounds occur almost immediately,which makes adenosine available for uptake by soil microbes (Fransson and Jones 2007).The adsorption capacity of organic P is closely related to the number of phosphate groups (Condron 2005).Consistent with our first hypothesis,theKdwas the lowest for adenosine,followed by AMP and ADP,and the highest for ATP.Higher adsorption leads to lower microbial accessibility (Cabritaet al.2002;Giannecchiniet al.2005;Tozziet al.2006).As a result,the mineralization rates of organic P substrates decreased with the increasing number of phosphate groups,regardless of soil fertility.The mineralization rates of ADP and ATP were accelerated over time,potentially indicating that phosphatase activity was increased with prolonged incubation times.
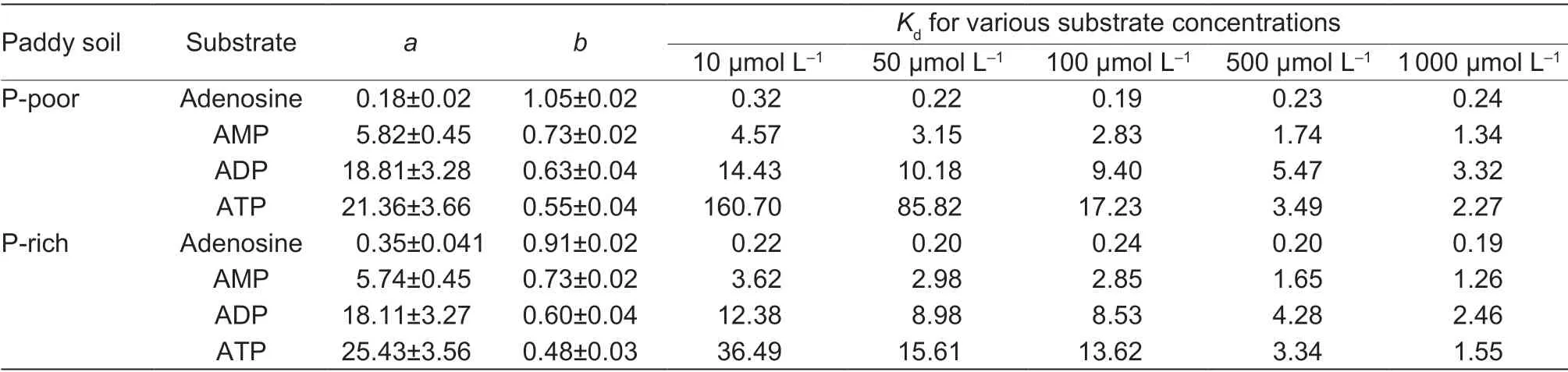
Table 3 Parameters of Freundlich sorption isotherm fits to the sorption of different 14C-labeled substrates to the solid phase at different concentrations in P-poor and P-rich soils1)
As expected by our second hypothesis,the mineralization rates of added LMWOP compounds were profoundly affected by soil fertility,which was significantly higher in the P-rich than P-poor soil.Many studies have found that the addition of exogenous nutrients or increasing of the soluble orthophosphate concentration in soil could potentially increase phosphatase activity and accelerate the mineralization process of organic P (Fox and Comerford 1992;Marklein and Houlton 2012;Weiet al.2018).The results most likely attributed to the stimulatory effect of increased P availability on phosphatase activity.In additional,the LMWOP was much more accessible to microorganisms in P-rich soil,supported by the greaterKdof LMWOPs in P-poor soil than in P-rich soil (Table 3).Also,organic matter accumulation could compete with P for absorption sites,therefore decreasing P adsorption capacity and increasing P availability in soil (Mikuttaet al.2006;Lindegren and Persson 2009;Pavinatoet al.2009;Finket al.2016).In our study,the SOC in P-rich soil was 2 times higher than that in P-poor soil (Table 1).Consequently,P sorption in the P-rich soil was smaller than in the P-poor soil (the strongest for ATP) (Table 3).However,long-term fertilization in soil enhances P accumulation and decreases the soil P saturation capacity (Wanget al.2012;Yanet al.2013,2017).This can also have a negative consequence for highly fertile paddy soils,leading to an increased risk of P runoff during rice cultivation (Zhanget al.2003;Shanet al.2005;Wanget al.2012;Yanet al.2017).
The sorption of organic-P on the solid phase was largely completed within 15 min (Fig.3).The sorption did not greatly interfere with the mineralization of AMP,especially at the low substrate concentrations where sorption to the solid phase was the strongest (Table 3).These results were consistent with previous findings (Fransson and Jones 2007),which showed that microorganisms can promote desorption of adenosine compounds from the mineral matrix and utilize them.P-solubilizing microorganisms are common in soil,which produce organic acids and H+during the metabolism of organic C (Aloriet al.2017).Thet1/2of ADP- and ATPderived C were two times faster in the P-rich soil than in the P-poor soil.However,a small variation fort1/2of both compounds between the applied concentrations was found(Table 2).These observations indicated that phosphatase activity was high in both soils,with an additional increase in the production of phosphatases in the P-rich soil.Thet1/2of AMP-C was even higher at some of the applied concentrations,which suggested that phosphatase activity did not limit the utilization of LMWOP compounds if only one phosphate group was present (Fransson and Jones 2007).
5.Conclusion
Long-term fertilization of paddy soils not only increased the contents of essential nutrients (C,N,and P) but also improved LMWOP mineralization.Mineralization rates of adenosine phosphates were the highest for AMP and the lowest for ATP in both soils,indicating that the number of phosphate groups can partially affect this process.However,the microbial activity was higher in the P-rich than P-poor soil,as indicated by the faster mineralization rates of ADP and ATP.The sorption of AMP was lower than ADP and ATP,and each respective adenosine phosphate showed the same sorption trend in both soils.Thus,these results suggested that on the one hand soil fertilization can improve the capacity of the microbial community to mineralize LMWOP,and thus promote plant nutrition;but on the other hand,it can increase the risk of P runoff.
Acknowledgements
This work was funded by the Natural Science Foundation of Hunan Province,China (2020JJ4563),the National Natural Science Foundation of China (4181101348),the Innovation Groups of Natural Science Foundation of Hunan Province (2019JJ10003),the Chinese Academy of Sciences President’s International Fellowship Initiative to Anna Gunina(2019VCC0003),and the Talented Young Scientist Program(TYSP) to Mostafa Zhran supported by the China Science and Technology Exchange Center (Egypt-19-004).We thank Dr.Zhu Zhenke and Dr.Wei Xiaomeng of the Institute of Subtropical Agriculture,Chinese Academy of Sciences for their advice on writing and data analyses.
Declaration of competing interest
The authors declare that they have no conflict of interest.
杂志排行
Journal of Integrative Agriculture的其它文章
- Errata regarding previously published articles
- Mental accounting and consumption of self-produced food
- Do credit constraints affect households’ economic vulnerability?Empirical evidence from rural China
- An entirely new approach based on remote sensing data to calculate the nitrogen nutrition index of winter wheat
- Changes in soil organic carbon pools following long-term fertilization under a rain-fed cropping system in the Loess Plateau,China
- Peanut yield,nutrient uptake and nutrient requirements in different regions of China
