新型水泥基材料富镁C3A对氮磷的共去除
2021-07-23邹友琴李勇丽郝鹏飞欧阳思达朱衷榜
邹友琴,李勇丽,郝鹏飞,欧阳思达,朱衷榜,章 萍
新型水泥基材料富镁C3A对氮磷的共去除
邹友琴,李勇丽,郝鹏飞,欧阳思达,朱衷榜,章 萍*
(南昌大学资源环境与化工学院,江西 南昌 330031)
将镁掺杂进水泥基材料铝酸三钙(C3A)制得新型富镁铝酸三钙(Mg@C3A)应用于水体氨氮(NH4+-N)和磷(PO43-)的共去除.通过批量实验,考察了Mg@C3A投加量、氮磷浓度、溶液pH值、温度等因素对NH4+-N、PO43-共去除的影响,并阐述了共去除机制.结果表明:Mg@C3A是由Mg掺杂C3A同构体和表面MgO组成,其中Mg的引入未改变C3A晶体结构和基本形貌.Mg@C3A材料对NH4+和PO43−具有良好的共去除效果.当Mg@C3A的投加量为3g/L,NH4+和PO43−的最大去除量分别为38.4,78.9mg/g;温度升高有利于Mg@C3A 对NH4+和PO43−的共去除,而高pH值可促进NH4+的去除.Mg@C3A材料对NH4+的去除主要是OH-的中和作用和鸟粪石的沉淀作用主导,PO43-主要是与Mg2+或Al3+结合形成鸟粪石或磷酸铝被去除.
富镁铝酸三钙(Mg@C3A);铵根(NH4+);磷酸根(PO43-);共去除
鸟粪石(MgNH4PO4·6H2O)结晶法是脱氮除磷的传统工艺之一[1-3],其是通过外加镁源将废水中的NH4+和PO43-与Mg2+形成鸟粪石晶体(MgNH4PO4·6H2O)沉淀而实现氮、磷共去除[4-6].然而,鸟粪石结晶法应用中受多种因素如氮磷浓度、溶液pH值、温度等影响.研究表明当NH4+和PO43-的摩尔配比小于1.2:1,溶液pH值较低,环境温度较高均不利于鸟粪石的生成,导致NH4+的去除率降低[7-9].因此,寻找合适的含镁化合物,且探明其去除氮磷的影响机制,对提高鸟粪石结晶法在水体氮磷共去除的应用性尤为重要.
铝酸三钙(Ca3Al2O6,简称C3A),水泥熟料的主要矿物之一.C3A易发生水化反应,可释放大量Ca2+、Al3+及OH-[10-12],目前已作为环境材料被应用于含氨氮、铅及锌等水体污染修复中[13-15].研究发现,C3A晶格中的钙可被镁等离子取代而形成金属掺杂的C3A复合材料,且所掺杂的金属不影响C3A的水化行为[16-17].因此,若将镁掺杂进C3A制得的镁掺杂水泥基材料Mg@C3A投加至氮、磷废水,其水化过程中能释放大量Mg2+、Ca2+、Al3+与OH-,通过诱导Mg2+与NH4+、PO43-生成鸟粪石,OH-中和部分NH4+,Ca2+、Al3+与PO43-结合形成含磷化合物,从而有望实现水体氮磷的共去除.然而,目前利用镁掺杂水泥基材料Mg@C3A对水体氮磷共去除的研究尚未报道.
本研究采用固相反应合成了Mg@C3A复合材料,通过X射线衍射(XRD)、拉曼光谱(Raman)、及扫描电镜(SEM)等表征探究Mg@C3A的晶相组成与形貌特征;通过批量实验,考察Mg@C3A投加量、氮磷初始浓度、溶液pH值、温度等因素对其共去除氮磷效果的影响,并深入阐述共去除机制,以期为拓宽水泥基材料应用于水污染修复提供理论与技术支持.
1 材料与方法
1.1 Mg@C3A的制备
采用固相反应法,将氢氧化镁、氢氧化铝和碳酸钙以MgO/(CaO+Al2O3)质量比为15%进行称量,均匀混合后进行压片处理.将压片后的样品置于1623K的马弗炉内煅烧3.5h.煅烧后的样品经球磨粉碎均匀后,在333K条件下烘干.重复上述步骤2~3次即可获得Mg@C3A.
1.2 去除实验
将已知量的Mg@C3A和20mL已知浓度的氮磷共存溶液置于50mL锥形瓶中,在搅拌速度为150r/min的气浴恒温振荡器中进行去除反应.考察Mg@C3A投加量(1~5g/L)、初始NH4+浓度(10~ 1000mg/L)、初始PO43-浓度(10~1000mg/L)、反应温度(298~318K)、pH值(4.0~10.0)等参数对共去除氮磷效果的影响.实验设置3个平行.反应结束后,将混合物离心分离,取上清液测定溶液的剩余铵根和磷酸根的浓度.
Mg@C3A对氨氮和磷酸根的去除量与去除率的计算方法按照式(1)与式(2)进行.
e=(0-e)/(1)
=(0-e)/0(2)
式中:e为Mg@C3A对氨氮和磷酸根的去除容量, mg/g;为Mg@C3A对氨氮和磷酸根的去除率,%;0和e分别为氨氮或磷酸根的初始和剩余浓度, mg/L;为反应溶液体积,L;为Mg@C3A质量,g.
1.3 固体产物表征
样品的物相结构采用德国BRUKER公司D8ADVANCE型X射线衍射仪(XRD)进行检测,测试条件为Cu靶Ka辐射源,管电压40kV,= 0.15418nm,管电流50mA,扫描速度6o/min;样品的微观形貌和晶体结构采用日本Hitachi公司Nano- S450型场发射扫描电子显微镜(SEM),工作电压为15kV;样品的物质组成采用Virsa型拉曼光谱仪(英国Renishaw公司)分析,激发波长633nm,波数范围100~1000cm-1,激发能9mW.溶液中NH4+-N与PO43-浓度采用紫外–可见光分光光度计(上海元析UV-6100型)测定;溶液pH值采用精密酸度计(上海雷磁PHS-3C型)测定;溶液金属离子浓度采用电感耦合等离子体发射光谱分析仪(ICP-OES,DV 2000, PerkinElmer公司,美国)测定.
1.4 数据处理
实验数据用Excel 2016进行数据处理、分析和统计,并采用Origin进行图表绘制.
2 结果与讨论
2.1 Mg@C3A物相表征
如图1所示, Mg@C3A在2=33.30°、47.62°和59.27°处出现强且尖锐的衍射峰,分别对应于C3A标准卡片(JCPDS No. 38-1429)衍射峰的(440)、(800)和(844)晶面[14-15,18],表明镁的引入未改变材料的立方相晶体结构[21].将样品的(440)晶面特征衍射峰放大,镁引入后,(440)晶面的衍射峰峰位向高衍射角度偏移,说明Mg以同晶取代方式替换C3A的晶格中的Ca2+[17].

同时,镁引入后的(440)特征峰峰形明显宽化,且晶面间距((440))从0.2705nm降至0.2701nm,这可能是由于Mg2+的离子半径(0.066nm)小于Ca2+的离子半径(0.099nm)[20].此外,在Mg@C3A样品的图谱中还观测到了位于42.92°和62.29°的衍射峰,对应于四方相MgO的(200)和(220)晶面,表明Mg2+不仅以同晶取代方式掺杂进C3A晶体,还以MgO的形式存在.
如图2所示,在506和752cm-1处的特征峰分别为n1[AlO45-]弯曲振动峰和n3[AlO45-]非对称伸缩振动峰[21],表明样品中存在构成C3A的AlO45-四面体[22].镁引入后,AlO45-基团特征峰峰形明显宽化,说明镁的引入增加了C3A晶格结构的无序度[27].同时,353cm-1处出现Ca-O键发生蓝移,进一步表明Mg以同晶取代的形式替换Ca掺杂进C3A的晶格中,与XRD分析的结果一致[24].此外,位于271和581cm-1处的特征峰为Mg-O键的振动峰,证实了样品中存在MgO[25-26].

图2 Mg@C3A和C3A样品的拉曼图谱

图3 纯C3A和Mg@C3A样品的SEM照片
从图3a可以看出,纯C3A呈块状结构,颗粒尺寸为12.3mm.由图3b可知,镁引入后,Mg@C3A保持块状结构,粒径增至21.4mm,其表面凸显出圆球形小颗粒,表明材料表面可能存在MgO颗粒.综上所述,Mg@C3A是由Mg掺杂C3A同构体和表面MgO组成,其中Mg的引入不改变C3A晶体结构和基本形貌.
2.2 Mg@C3A对NH4+与PO43-的共去除性能
2.2.1 Mg@C3A投加量的影响 在氨氮浓度为160mg/L,磷酸根浓度为160mg/L的条件下反应12h,考察不同投加量(1~5g/L)对Mg@C3A去除氨氮与磷酸根的效果影响.结果如图4所示,当Mg@C3A的投加量从1g/L增至3g/L时,NH4+的去除量由20.0mg/g左右上升到26.3mg/g.而当投加量增至4和5g/L,去除量呈下降趋势,这可能是由于高剂量引起Mg@C3A释放大量的离子不能被有效利用所致[27].随着投加量增加,NH4+的去除率由10.6%增至64.0%.而PO43−的去除趋势不同,随着投加量增加,PO43−的去除量显著降低,从128.0mg/g降至31.1mg/g. Mg@C3A对PO43−的去除率随着投加量增加由80.0%升至97.2%.结果表明,Mg@C3A对NH4+和PO43−的去除行为不同.从水处理成本和效率角度考虑,Mg@C3A的投加量设为3g/L.
2.2.2 初始NH4+浓度的影响 在Mg@C3A投加量为3g/L,磷酸根浓度为160mg/L的条件下反应12h,考察氨氮初始浓度(10~1000mg/L)对Mg@C3A去除氨氮与磷酸根的效果影响,结果如图5所示.当初始NH4+浓度从10mg/L增至200mg/L时,Mg@C3A对NH4+的去除量由2.1mg/g升至28.3mg/g,而当浓度继续增大,NH4+的去除率增速变缓,在初始NH4+浓度为1000mg/L时达38.4mg/g.而PO43−的去除量呈现出缓慢的增长趋势,其在初始NH4+浓度为10~1000mg/L的范围内e由46.8mg/g增至54.0mg/g,表明高NH4+浓度有利于PO43−的去除.这可能是由于高NH4+浓度提高了溶液的过饱和度,有利于鸟粪石生成[28].


图5 初始NH4+浓度对Mg@C3A去除NH4+与PO43-的影响
2.2.3 初始PO43-浓度的影响 在Mg@C3A投加量为3g/L,氨氮浓度为160mg/L的条件下反应12h,考察磷酸根初始浓度(10~1000mg/L)对Mg@C3A去除氨氮与磷酸根的效果影响,结果如图6所示.当PO43−初始浓度从10mg/L增至200mg/L时, Mg@C3A对PO43−的去除量由3.3mg/g升至62.5mg/g,而当浓度继续增大,PO43−的去除量增加缓慢,在浓度为1200mg/L时达78.9mg/g.而NH4+的去除量保持在26.9mg/g左右,表明PO43−对NH4+的去除无明显的影响.

图6 初始PO43-浓度对Mg@C3A去除NH4+与PO43-的影响
2.2.4 反应温度的影响 图7为NH4+和PO43−初始浓度160mg/L,反应时间12h时,Mg@C3A在298~ 318K下与NH4+和PO43−的反应结果.由图可见,温度对NH4+的去除影响较大.温度从298K升高至308K时,Mg@C3A对NH4+的去除量迅速增加,由26.3mg/g提高至48.2mg/g.随后,温度升至318K,其去除量缓慢增至51.6mg/g.PO43−的去除量随温度升高由51.9mg/g缓慢升至53.3mg/g.结果表明,Mg@C3A与NH4+和PO43−的反应为吸热反应,温度升高有利于NH4+和PO43−的共去除.此外,这一现象与文献中高温会抑制鸟粪石法对NH4+和PO43−去除的现象[13]不一致,说明除鸟粪石沉淀外,部分NH4+和PO43−还可通过另一种方式被去除,将在机理部分进行深入分析.
2.2.5 溶液pH值的影响 图8为NH4+和PO43−初始浓度为160mg/L,反应时间12h时,Mg@C3A在溶液pH值为4~10的条件下与NH4+和PO43−的反应结果,可以看出,随着pH值的升高,Mg@C3A对NH4+的去除量呈现缓慢的增长趋势,说明pH值的升高有利于Mg@C3A对NH4+的去除.而PO43−的去除量受pH值影响较为微弱,其随pH值的增大由51.9mg/g降至48.2mg/g.由此可见,Mg@C3A对NH4+和PO43−的去除行为及机制不同.

图7 反应温度对Mg@C3A去除NH4+与PO43-的影响

图8 pH值对Mg@C3A去除NH4+与PO43-的影响
2.3 机理分析
为进一步探究Mg@C3A共去除NH4+和PO43-的机理,通过XRD对Mg@C3A与NH4+和PO43-反应后产物进行分析.图9为Mg@C3A与NH4+和PO43-反应前后的XRD图谱.反应后,图谱中未观察到C3A的衍射峰,说明Mg掺杂C3A同构体与NH4+和PO43-的反应完全;钙铝双金属氢氧化物(钙铝LDH)和AlPO4的特征衍射峰出现,分别与各自的标准图谱(JCPDS No.78-2051和No.47-0608)相对应,其匹配度值(FOM)分别为19.2和31.7.此外,反应产物中也存在少量鸟粪石(MAP,No.15-0762)的特征峰,结合Mg@C3A在纯水中钙、铝、镁离子的释放情况(表1),可见,Mg@C3A投加至水体后发生水化,其释放的一部分钙、铝、镁离子分别与NH4+和PO43-结合生成鸟粪石(MAP)和AlPO4沉淀,而另一部分与溶液中的Cl-组装形成了钙铝LDH.
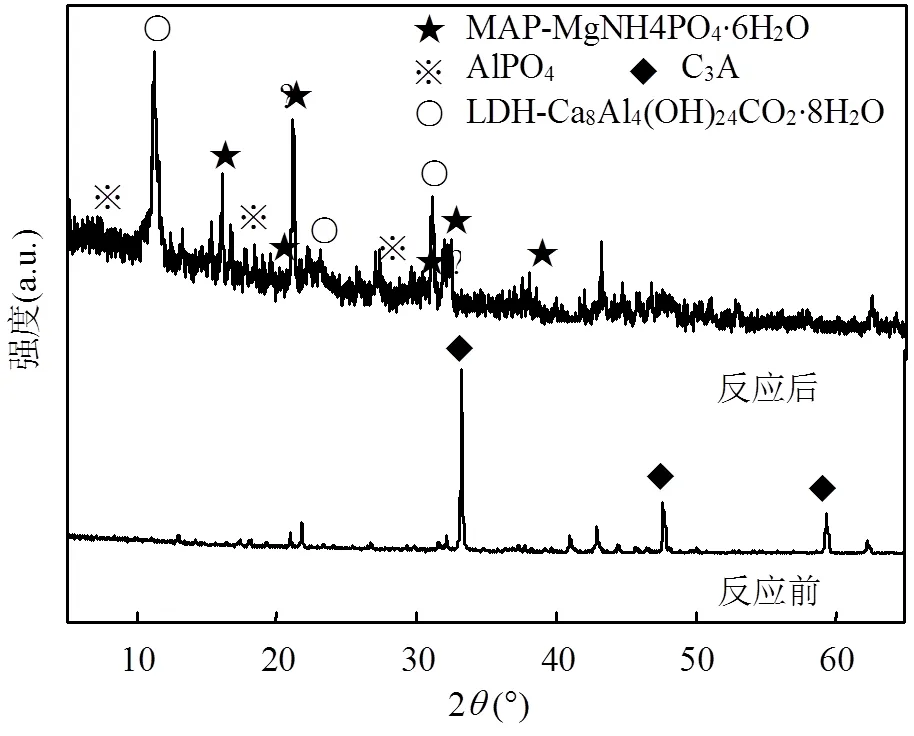
图9 Mg@C3A去除NH4+与PO43-前后的XRD图谱

表1 Mg@C3A在纯水中的金属离子释放量及pH值

表2 Mg@C3A与NH4+和PO43-反应前后的pH值以及在纯水水化后的pH值

表3 反应后固相中NH4+和PO43-
为进一步研究NH4+与PO43-的去除机制, Mg@C3A反应前后的溶液pH值变化如表2所示.由表2明显看出,反应前后溶液pH值高于反应前,但较水化后低,表明反应过程中Mg@C3A消耗了溶液中OH-.此外,将生成的产物进行溶解,以深入探究反应过程,结果如表3所示,通过生成MAP去除NH4+的量为0.15mmol/g.而NH4+的总去除量为1.92mmol/g(图5),可以推测大部分NH4+(1.77mmol/g)通过与OH-结合生成游离NH3被去除.PO43-去除则主要是AlPO4(0.42mmol/g)> MAP(0.15mmol/g).
基于上述讨论,Mg@C3A与NH4+和PO43-的反应机制如图10所示.Mg@C3A去除NH4+的机制主要是OH-的中和作用和鸟粪石的沉淀作用;PO43-主要通过与Mg或Al结合形成鸟粪石或磷酸铝沉淀被去除.

图10 Mg@C3A共去除NH4+与PO43-反应机制示意
3 结论
3.1 Mg@C3A呈块状结构,表面粗糙,且有球形小颗粒均匀分布在材料表面.Mg@C3A是由Mg掺杂C3A同构体和表面MgO组成,其中Mg的引入不改变C3A晶体结构和基本形貌.
3.2 当Mg@C3A的投加量为3g/L,NH4+和PO43−的最大去除量分别为38.4mg/g和78.9mg/g.Mg@C3A对NH4+和PO43−的去除均是吸热反应;溶液pH值的升高有利于NH4+的去除.
3.3 Mg@C3A对NH4+的去除主要是以OH-的中和作用为主导及鸟粪石的沉淀作用主导,PO43-主要是与Mg2+或Al3+结合形成鸟粪石或磷酸铝被去除.
[1] Lu X, Shih K, Li X Y, et al. Accuracy and application of quantitative X-ray diffraction on the precipitation of struvite product [J]. Water Research, 2016,90:9-14.
[2] Hovelmann J,Putnis C V. In Situ Nanoscale Imaging of struvite formation during the dissolution of natural brucite: implications for phosphorus recovery from wastewaters [J]. Environmental Science & Technology, 2016,50(23):13032-13041.
[3] 吴 健,平 倩,李咏梅.鸟粪石结晶成粒技术回收污泥液中磷的中试研究[J]. 中国环境科学, 2017,37(3):941-947.
Wu J, Ping Q, Li Y M. A pilot-scale study on struvite pellet crystallization for phosphorus recovery from sludge liquor [J]. China Environmental Science, 2017,37(3):941-947.
[4] Huang H, Liu J, Xiao J, et al. Highly efficient recovery of ammonium nitrogen from coking wastewater by coupling struvite precipitation and microwave radiation technology [J]. ACS Sustainable Chemistry & Engineering, 2016,4(7):3688-3696.
[5] Zhao T-L, Li H, Huang Y-R, et al. Microbial mineralization of struvite: Salinity effect and its implication for phosphorus removal and recovery [J]. Chemical Engineering Journal, 2019,358:1324-1331.
[6] 胡德秀,张 艳,张 聪.EDTA对剩余污泥磷释放及MAP法磷回收影响[J]. 中国环境科学, 2019,39(4):1611-1618.
Hu D X, Zhang Y, Zhang C. Effects of EDTA on phosphorus release in excess sludge and phosphorus recovery by MAP [J]. China Environmental Science, 2019,39(4):1611-1618.
[7] Yan H, Shih K. Effects of calcium and ferric ions on struvite precipitation: a new assessment based on quantitative X-ray diffraction analysis [J]. Water Research, 2016,95:310-318.
[8] 汤 琪,罗固源.磷酸铵镁沉淀法处理磷酸盐工业废水[J]. 化工进展, 2008,27(4).
Tang Q, Luo Guyuan. Treatment of phosphate wastewater by magnesium ammonium phosphate precipitation process [J]. Chemical Industry and Engineering Progress, 2008,27(4).
[9] Uysal A, Yilmazel Y D, Demirer G N. The determination of fertilizer quality of the formed struvite from effluent of a sewage sludge anaerobic digester [J]. J Hazard Mater, 2010,181(1-3):248-254.
[10] Myers R J, Geng G, Rodriguez E D, et al. Solution chemistry of cubic and orthorhombic tricalcium aluminate hydration [J]. Cement and Concrete Research, 2017,100:176-185.
[11] Myers R J, Geng G, Li J, et al. Role of adsorption phenomena in cubic tricalcium aluminate dissolution [J]. Langmuir, 2017,33(1):45-55.
[12] Pommersheim J, Chang J. Kinetics of hydration of tricalcium aluminate [J]. Cement and Concrete Research, 1986,16(3):440-450.
[13] Sun M, Zhang P, Wu D, et al. Novel approach to fabricate organo-LDH hybrid by the intercalation of sodium hexadecyl sulfate into tricalcium aluminate [J]. Applied Clay Science, 2017,140:25-30.
[14] Zhang P, Ouyang S, Li P, et al. Ultrahigh removal performance of lead from wastewater by tricalcium aluminate via precipitation combining flocculation with amorphous aluminum [J]. Journal of Cleaner Production, 2020,246:118728.
[15] Zhang P, Zeng X, Wen X, et al. Insights into efficient removal and mechanism for ammonium from aqueous solution on tricalcium aluminate [J]. Chemical Engineering Journal, 2019,366:11-20.
[16] Fukuda K, Inoue S, Yoshida H. Cationic substitution in tricalcium aluminate [J]. Cement and Concrete Research, 2003,33(11):1771- 1775.
[17] Stephan D, Wistuba S. Crystal structure refinement and hydration behaviour of doped tricalcium aluminate [J]. Cement and Concrete Research, 2006,36(11):2011-2020.
[18] Wang T, Zhang P, Wu D, et al. Effective removal of zinc (II) from aqueous solutions by tricalcium aluminate (C(3)A) [J]. Journal of Colloid and Interface Science, 2015,443:65-71.
[19] Taylor H, Taylor H, Taylor H, et al. Cement chemistry [Z]. 1998:134.
[20] Meng A, Xing J, Li Z, et al. Cr-Doped ZnO Nanoparticles: synthesis, characterization, adsorption property, and recyclability [J]. ACS Applied Materials & Interfaces, 2015,7(49):27449-57.
[21] Gastaldi D, Boccaleri E,Canonico F. In situRaman study of mineral phases formed as by‐products in a rotary kiln for clinker production [J]. Journal of Raman Spectroscopy, 2008,39(7):806-812.
[22] Torréns-Martín D, Fernández-Carrasco L, Martínez-Ramírez S, et al. Raman spectroscopy of anhydrous and hydrated calcium aluminates and sulfoaluminates [J]. Journal of the American Ceramic Society, 2013,96(11):3589-3595.
[23] Li X, Ma J, Yang L, et al. Oxygen vacancies induced by transition metal doping in gamma-MnO2for highly efficient ozone decomposition [J]. Environmental Science & Technology, 2018, 52(21):12685-12696.
[24] Panagopoulou M, Vernardou D, Koudoumas E, et al. Tunable properties of Mg-doped V2O5thin films for energy applications: Li-ion batteries and electrochromics [J]. The Journal of Physical Chemistry C, 2016,121(1):70-79.
[25] De La Pierre M, Carteret C, Maschio L, et al. The Raman spectrum of CaCO3polymorphs calcite and aragonite: a combined experimental and computational study [J]. Journal of Chemical Physics, 2014, 140(16):164509.
[26] Böckelmann H K, Schlecht R G. Raman scattering from microcrystals of MgO [J]. Physical Review B, 1974,10(12):5225-5231.
[27] Ren Y, Abbood H A, He F, et al. Magnetic EDTA-modified chitosan/ SiO2/Fe3O4adsorbent: Preparation, characterization, and application in heavy metal adsorption [J]. Chemical Engineering Journal, 2013,226: 300-311.
[28] Corre K S L, Valsami-Jones E B, Hobbs P C, et al. Phosphorus recovery from wastewater by struvite crystallization: a review [J]. Critical Reviews in Environmental Science & Technology, 2009, 39(6):433-477.
The co-removal of ammonium and phosphate by novel cement-based material magnesium-rich tricalcium aluminate and its mechanism investigation.
ZOU You-qin, LI Yong-li, HAO Peng-fei, OUYANG Si-da, ZHU Zhong-bang, ZHANG Ping*
(School of Environmental and Chemical Engineering, Nanchang University, Nanchang 330031, China)., 2021,41(6):2639~2645
Magnesium-rich C3A (Mg@C3A) was successfully prepared via doping magnesium into cement-based material tricalcium aluminate (C3A) for the co-removal of ammonium (NH4+-N) and phosphorus (PO43-). The effects of Mg@C3A dosage, contaminants concentration, solution pH value, and temperature as well as the mechanism were investigated. Mg@C3A was composed of the C3A isomorphism formed by the replacement of Ca with Mg and the surface MgO, in which the Mg doping does not affect the cubic symmetric structure and morphology of C3A. The maximum removal capacity of NH4+and PO43−were 38.4, 78.9mg/g at the Mg@C3A dosage of 3g/L, respectively. The increase of temperature was conducive to the co-removal of NH4+and PO43−by Mg@C3A. High pH value enhanced the NH4+removal. The removal mechanism of NH4+was mainly neutralization and the precipitation; PO43−was removed via the precipitation combining with Mg2+or Al3+to form struvite or aluminum phosphate.
magnesium-rich tricalcium aluminate (Mg@C3A);ammonium (NH4+);phosphate(PO43-);co-removal
X703.1
A
1000-6923(2021)06-2639-07
邹友琴(1973-),女,江西奉新人,副教授,博士,主要从事水资源与环境研究.发表论文20余篇.
2020-11-02
国家自然科学基金资助项目(21767018);江西省重点研发计划项目(20192BBG70054)
* 责任作者, 副研究员, zhangping@ncu.edu.cn
