Corrosion behavior of super 13Cr stainless steel in a H2S and CO2 environment
2021-07-20,
,
Research Institute,Baoshan Iron & Steel Co.,Ltd.,Shanghai 201999,China
Abstract: The corrosion behavior of 95 ksi grade super 13Cr stainless steel in an environment consisting of H2S and CO2 was investigated.The results reveal that for both loading methods (constant load and four-point bending),local corrosion occurred on the surface of the samples tested at ambient temperature but was absent from the samples tested at high temperatures.The local corrosion was caused by the formation of pits at nonmetal inclusions;the pits were formed under the action of stress in an acidic environment,which was due to an acid solution.Subsequently,the corrosion extended along the direction of stress.The sensitivity of stress corrosion cracking increased because of the local corrosion.
Key words: martensitic stainless steel; corrosion; inclusion; H2S; CO2
1 Introduction
13Cr martensitic stainless steel is a corrosion-resistant alloy commonly used in the field of oil and gas exploitation.The super 13Cr steel that has an ultra-low carbon content and contains Ni,Mo,and other elements can achieve high strength of over 110 ksi (1 ksi=6.895 MPa) and has good corro-sion resistance in an oilfield environment that con-tains CO2and chloride ions (Cl-).Therefore,in recent years,super 13Cr steel has been widely used in the exploitation of oil and gas resources that occurs in environments having high concentrations of CO2.However,this steel is unsuitable for corro-sive environments that have high concentrations of H2S.Corrosion failures have occurred in some oil and gas fields in recent years.Therefore,research on the corrosion behavior and application limit of super 13Cr steel is essential from the perspective of material development and essential for its end users[1-13].
In recent years,several studies have focused on the corrosion behavior of super 13Cr steel in various environments,especially environments containing CO2and Cl-at high temperatures[1-3].For example,SUNABA et al.[1]studied the corrosion behavior of different series of 13Cr and 15Cr steels in an environment in which Cl-and CO2coexisted at high temperatures.The results revealed that chloride ion concentration had a significant effect on the cor-rosion rate of common 13Cr and 13Cr- 4Ni-1Mo,but the corrosion rates of 5Ni2Mo of super 13Cr and 6Ni2Mo of super 15Cr were not sensitive to chloride ion concentration.Other studies have focused on the corrosion behavior of super 13Cr martensitic stainless steel in corrosive environments during well completion[4-6].The pitting corrosion behavior of 5Ni2Mo of super 13Cr in a solution of 15%HCl+1.5%HF+3%HAc+4.5% inhibitor was studied by LEI et al..The results showed that the pitting corrosion of this material was induced mainly in the residual acid flowback stage,and residual acid was the main cause of pitting corrosion.
Although the application limit of 95 ksi is specified in the standard ISO 15156-3 for super 13Cr steel,studies on the corrosion behavior and mechanism of super 13Cr steel in environments containing H2S and CO2are relatively rare.The existing research mainly focuses on the sulfide stress corrosion cracking behavior and application limit of super 13Cr steel in H2S-containing environ-ments[7-13].However,tubing and casing made up of 95 ksi super 13Cr are widely used in oilfields with environments containing high proportions of CO2and low proportions of H2S.In this work,the corrosion behavior of 95 ksi super 13Cr in an environment containing H2S,CO2,and Cl-is studied using the constant load and four-point bending methods and the mechanism of its corrosion behavior is discussed.
2 Experimental procedures
The specimens were taken from aφ177.8 mm×10.36 mm pipe.The composition and inclusion rating are shown in Tables 1 and 2,respectively,and the mechanical properties are shown in Table 3.

Table 1 Chemical composition of the specimens %

Table 2 Cleanness of the specimens
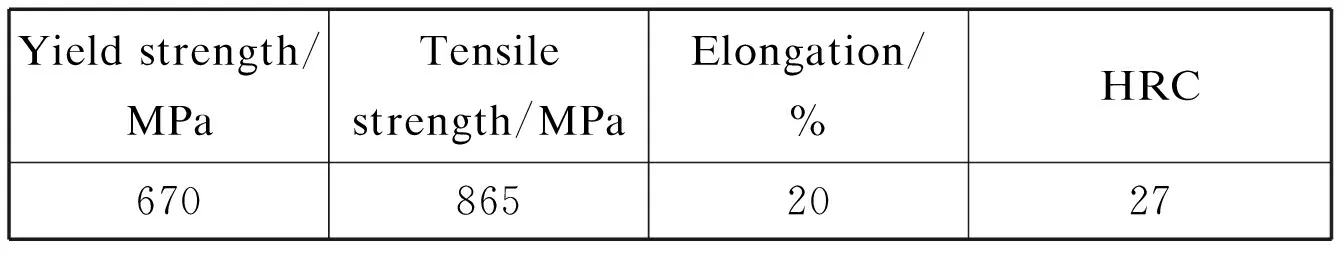
Table 3 Mechanical properties of the specimens
Two test methods were used,namely the constant load method and the four-point bending method.In the former,the diameter of the gauge section was 6.4 mm,and the surface of the sample was polished until no scratch was observed after 8×evaluation using a stereomicroscope;in the latter,the sample (size:15 mm×5 mm×115 mm) was ground and then polished to 600 μm with water sandpaper.After degreasing and drying,the above samples were used for standby.
Combining the standard and the actual application environment of the oilfields,a series of stress corrosion test conditions were selected.Four sets of tests for simulating the corrosion of super 13Cr steel under the conditions of trace H2S,low CO2partial pressure,and trace H2S and high CO2partial pres-sure were conducted.The test conditions are listed in Table 4.Among these conditions,the constant load test and the four-point bending test were performed with stress ring equipment and autoclave equipment,respectively,from CORRTEST.The test solution was configured by dissolving a chemical solvent in deionized water.The solution was purged with high-purity N2at 1 L/h before being transferred to the reaction vessel,and then purged for 1 h after being transferred to a closed vessel to eliminate the influence of O2.
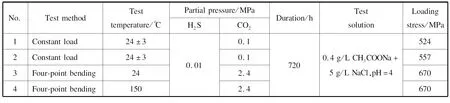
Table 4 Test conditions of sulfide stress corrosion cracking (SSC)
After the tests,the samples were cleaned and dried.Metallographic analysis via optical micro-scopy (OM) of the sample was performed with a ZEISS AXIO Imager M2m optical metallurgical microscope.Similarly,the macroscopic features of the sample were analyzed with a digital camera and a Leica M205A stereomicroscope.The corrosion products and fracture surfaces were analyzed via scanning electron microscopy (SEM;ZEISS EVO MA25) coupled with energy dispersive spectroscopy (EDS;OXFORD energy spectrum analyzer).
3 Results and discussion
3.1 Results
Fig.1 shows the metallographic structure of the sample,where a tempered structure (i.e.,tempered martensite) of a typical super 13Cr martensitic stainless steel is observed.

Fig.1 Metallurgical photograph of the specimen
Fig.2 shows the surface morphologies of the samples after the stress corrosion tests (performed under the test conditions listed in Table 4) and the cor-rosion products on the surface are cleaned.The first and second groups of specimens undergo constant load tests,where loading to 80% and 85% of the specific minimum yield strength,respectively,are per-formed.The third and fourth groups of specimens undergo four-point bending tests where loading to 100% of the specific minimum yield strength is performed.After the tests,the fracture occurring in the four groups of specimens is insignificant.How-ever,after cleaning the surface corrosion products of the first and second groups of constant load speci-mens,substantial localized circumferential corrosion is observed in the gauge length section.Moreover,pitting corrosion occurs on the edge of one speci-men in the third group of tests (the position circled in Fig.2(c)).
The corroded area of the sample was observed with a stereomicroscope.The results revealed that the pitting corrosion observed in Fig.2(c) origi-nated from the sample edge,extending more than 2 mm along the vertical direction of the tensile stress load on the sample (as shown in Fig.3).
The section of the corroded sample was cut longi-tudinally by means of wire cutting,and then the corroded part was observed via SEM.Fig.4(a) shows an SEM photo of the pitting corrosion depicted in Fig.2(c).Furthermore,the results shown in Figs.4(b)-(d) reveal the typical morphologies of the corrosion areas occurring in the first and second groups of specimens.The corrosion product film at the corrosion position of the sample is thicker than that forms in other regions.The morphology of the corrosion area varies among the different samples,but in each case the typical pitting morphologies extend from the surface to the interior.In addition,significant extensional microcracks occur at the bottom of the pitting area.
The energy spectrum analysis was performed on the corrosion product area of the sample,as shown in Fig.5.

Fig.5 SEM and EDS results of the corrosion areas
As shown in Fig.5,elemental sulfur occurs along the entire corrosion scale.This indicates that,although the partial pressure of CO2is higher than that of H2S,the scale has transformed from oxide-film dominated (usu-ally produced in CO2environments) into sulfide-film dominated (usually produced in H2S environments).
Furthermore,EDS analysis revealed that calcium (Ca),magnesium (Mg),aluminum (Al),and silicon (Si) elements were present in the corrosion area.Al and Si are usually added as deoxidizers in the steel-making process,and Ca and Mg are the main metal elements occurring in the slag system during the pro-cess.Except for MnS inclusions,nonmetallic inclusions usually contain Al,Mg,Ca,and Si.Sodium and potassium may have resulted from the attachment of metal ions in the solution under corrosion condi-tions.
3.2 Discussion
SSC,where hydrogen atoms enter the hydrogen traps of steel materials,is generally considered the main cracking mechanism of martensitic stainless steel exposed to a H2S environment.With increasing hydrogen concentration,the volume of hydrogen mole-cules expands rapidly,resulting in material cracking.However,the results of the present study indicate that the corrosion of martensitic stainless steel occurs under the coaction of stress and the corro-sion medium.Pitting corrosion,typically involving inclusions,is the main form of corrosion observed.In contrast to the observations reported in other studies,stress corrosion cracking occurs at the bottom of the pitting pit.
The EDS results show that elements of non-metallic inclusions occur in the pitting areas.This is confirmed via energy spectrum analysis of the corrosion areas.The assumption is that the existence of inclusions leads to a local potential difference,and hence anodic dissolution corrosion in an acidic environment,which results in corrosion.
Energy spectrum analysis of the corrosion region reveals that the elements exist as nonmetal inclu-sions.The assumption is that,owing to the existence of inclusions,a local potential difference is gener-ated and leads to anodic dissolution corrosion in a sour environment,thereby resulting in corrosion.
Several groups of tests conducted at room temper-ature reveal varying degrees of corrosion under different test conditions.No pitting corrosion is observed at 150 ℃.This may have resulted from the fact that the high-temperature diffusion and migra-tion of hydrogen are intensified,and the occurrence of hydrogen embrittlement caused by the low-temperature aggregation of hydrogen is hindered.However,the temperature has a significant impact on the pH value of the solution (as shown in Fig.6),i.e.,the pH values are low at low temperatures,which is conducive to the occurrence of anodic dissolution corrosion.
Analysis of the corrosion product film reveals that in the low H2S partial pressure environment,the oxide passivation film usually generated by 13Cr steel in a CO2environment has partially transformed into the sulfide corrosion product film.This film is relative-ly loose and has weak adhesion with the substrate,thereby contributing to the growth of pitting cor-rosion.
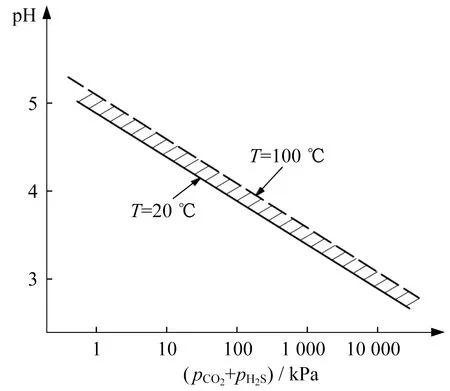
Fig.6 The pH values of condensed water under CO2 and H2S pressure
The morphologies of cracks at the bottom of the pits are shown in Fig.4.These cracks all originate in this region,and may have resulted from the con-centration of stress in the pitting areas.As shown in Fig.7,an imperfection with a depth of 100 μm can lead to significant stress concentration at the surface of the specimen.The four-point bending test is mainly concentrated in the support point position,and the above effect is insignificant.
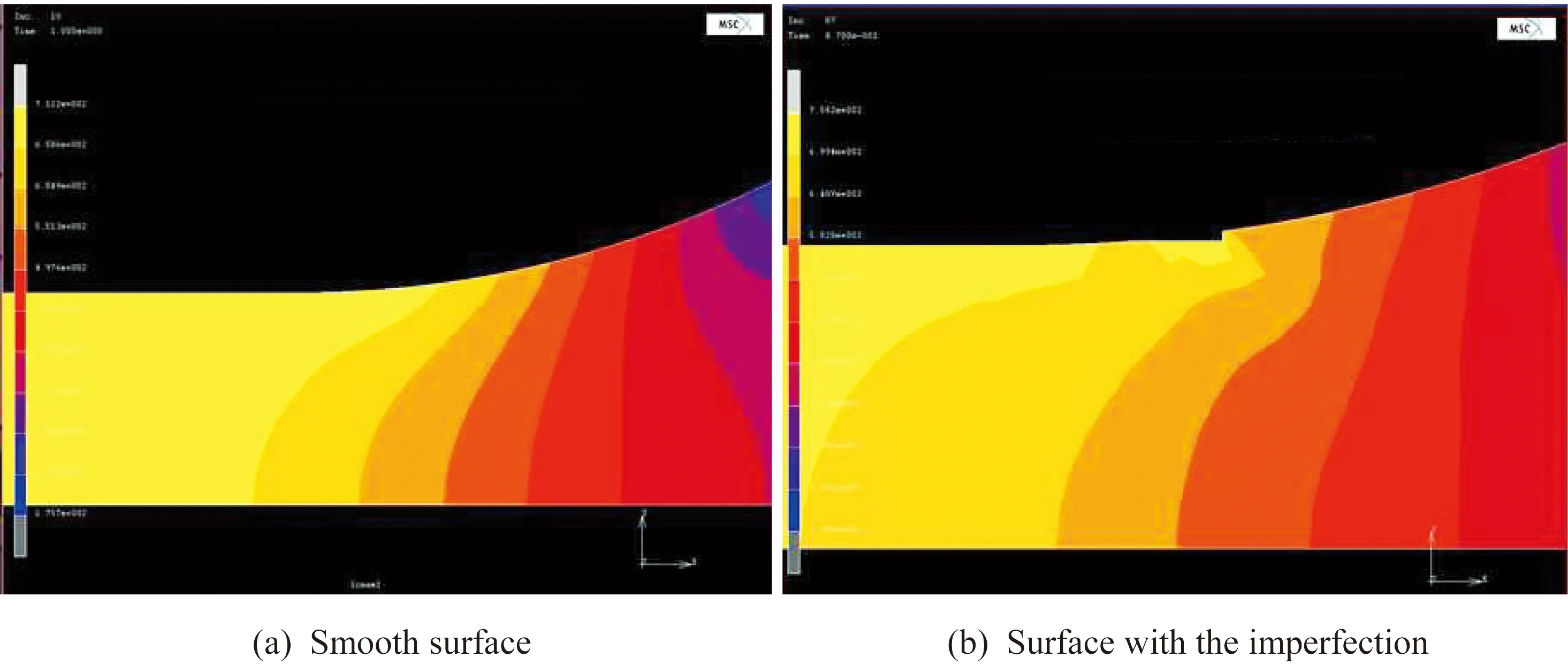
Fig.7 Influence of the surface imperfection in the constant load test
4 Conclusions
(1) Pitting corrosion of super 13Cr in an environment consisting of H2S and CO2at room tem-perature results mainly from the anodic dissolution corrosion caused by inclusions in the sour environ-ment.Moreover,the sulfide corrosion product film forms at the same time offers only weak protection to the matrix,and hence the corrosion extends further into the matrix.
(2) The stress concentration effect at the pitting areas promotes the initiation and development of hydrogen embrittlement stress corrosion cracks and leads to crack propagation.
(3) The use of super 13Cr in environments con-taining H2S at low temperatures should be avoided,and the boundary conditions such as the partial pressure of H2S,temperature,and pH value should be strictly controlled.
杂志排行
Baosteel Technical Research的其它文章
- Effect of vanadium on the microstructure and properties of metastable austenitic stainless steel AISI 301LN
- Property uniformity of thick nickel-based alloy plate for nuclear power steam-generator divider plate
- Improving emulsion odor in cold rolling production
- Evaluation of automatic girth weldability of pipeline in special conditions
- Contributions to Baosteel Technical Research wanted
- Effect of novel surface treatment on corrosion behavior and mechanical properties of a titanium alloy
