Effect of novel surface treatment on corrosion behavior and mechanical properties of a titanium alloy
2021-07-20,U
, U
Research Institute,Baoshan Iron & Steel Co.,Ltd.,Shanghai 201999,China
Abstract: A new surface treatment process is presented that improves the properties of Ti-6Al-4V alloy.This process is based on thermal oxidation,in which the surface properties of the titanium alloy are improved via simultaneous nitriding and oxidation.The test results indicate that the resulting surface layer consists of TiO2 and TiN.The sample with the best mechanical properties and corrosion resistance was sintered at a temperature of 800 ℃.
Key words: Ti-6Al-4V alloys; surface treatment; corrosion resistance
1 Introduction
Due to its outstanding specific strength and good corrosion resistance,the Ti-6Al-4V alloy has been widely used in the aerospace,aviation,and maritime industries[1].Ti-6Al-4V is a dual-phase alloy com-prising α and β phases that exhibit different electro-chemical activities in service environments.A gal-vanic coupling effect occurs between these phases,which results in galvanic corrosion[2].
Surface engineering techniques have generally become popular for enhancing the performance and service reliability of metals,including titanium (Ti) alloys in aqueous environments[3].For example,laser deposition and plasma spray technologies have been developed to improve the surface properties of Ti-6Al-4V alloy to enable it to meet actual needs[4].However,these techniques have limitations that hinder their application,such as their high cost.
Chemical heat treatment,in which metal structures are heated in a specific atmosphere,provides a flexible,easy-to-operate,and inexpensive alternative method for improving the surface pro-perties of a structure.The most common atmo-spheres used in chemical heat treatment include nitrogen and hydrogen.The titanium nitride (TiN) produced by nitride treatment features high hard-ness,good chemical stability,and excellent corro-sion resistance[5].However,researchers have report-ed[6-7]that it is difficult for nitrogen to penetrate titanium alloys at low temperatures.When the first layer of TiN forms on the Ti surface,the thermal diffusion coefficient for N to Ti is significantly reduced as the TiN layer serves as an effective barrier.It is only when the service temperature becomes sufficiently high,e.g.,over 900 ℃,can N permeate Ti alloys[6].Titanium dioxide (TiO2) has also been found to improve the surface performance of Ti alloys[8].Although spontaneously formed titanium oxides offer good corrosion resistance,they can be damaged in practice.Alternatively,oxide films with excellent corrosion resistance and mech-anical properties can be obtained by thermal oxida-tion[8],with the temperature ranging from 300 ℃ to 900 ℃[9].In this work,a novel low-temperature surface heat-treatment technique is presented that uses a mixed atmosphere of nitrogen (N) and oxygen (O) to form a surface oxide layer of TiN and TiO2.
A variety of traditional electrochemical methods were used in this work to investigate the corrosion regularity of a titanium alloy.A three-electrode system was established,with the electrode prepared by the Ti-6Al-4V alloy as the working electrode,a saturated calomel electrode as the reference electrode,and a platinum electrode as the counter electrode[10].The corrosive medium used was 3.5% sodium chloride solution.A potentiodynamic polar-ization curve measurement was performed to accu-rately determine the corrosion resistance of different samples and obtain the significant parameterRct.Scanning electron microscopy (SEM) and X-ray diffraction (XRD) were also performed to observe the microscopic appearance and characterize the corrosion performance.
2 Experimental methods
2.1 Materials and specimens
The Ti-6Al-4V alloy was supplied by Baosteel.Its chemical composition (mass fraction) comprised 90%Ti,6%aluminum (Al),and 4%vanadium (V).Alloy coupons with the dimensions 10 mm×10 mm×5 mm were machined from titanium bars.The surfaces of the coupons were ground using 1 000-grade abrasive paper,polished using a diamond polishing paste,and then dried in air.
2.2 Chemical heat treatment
The prepared specimens were heated in a tube furnace in a mixture of 90% nitrogen and 10% oxygen at standard atmospheric pressure.All specimens were heated to their target temperatures (300,500,700,800,and 850 ℃) at a heating rate of 5 K/min.The specimens were then held at the target temperatures for 1 h,and cooled in the furnace to a room temperature of 25 ℃.
2.3 Surface characterization of the prepared specimens
The surface and cross-sectional morphologies of the heat-treated specimens were characterized using a HITACHI SU-1500 SEM and coupled energy dispersive X-ray spectroscopy (EDS).The struc-tural analysis was conducted using an XRD.
2.4 Corrosion testing
The heat-treated specimens used in the corrosion tests had dimensions of 10 mm×10 mm.In this test,a three-electrode cell was used,with the specimen as the working electrode,and a platinum plate and a saturated calomel electrode as the counter and reference electrodes,respectively.An electro-chemistry work station (CHI660C) was connected to the electrodes to measure the corrosion.The test solution was 3.5%NaCl solution.
2.5 Microhardness analysis and surface rough-ness measurements
Microhardness tests of the heat-treated specimens were performed using a Vickers microhardness tester under different loading conditions (i.e.,HV0.1,HV0.2,HV0.3,HV0.5,and HV1).The amount of indentation was calculated to determine the mechanical properties of the specimens that had been heat-treated at different temperatures.The dwell time of the hardness indent action was 10 s.To ensure reproducibility of the results,each specimen was tested for 5-8 times to obtain an average microhardness value.
3 Results and discussion
3.1 Surface morphology and compositional analysis
The surface microstructures of the samples heated at five different temperatures are presented in Fig.1.Nonuniform coating (labeled as A) and matrix organization (labeled as B) are evident in Fig.1(a),which shows that the coating was not sufficiently uniform after preparation at low temperatures.As the temperature increased to 500 ℃,the substrate became nearly invisible and the nonuniformity of the coating obviously improved.Fig.1(c) clearly shows that many clusters had appeared in the sur-face layer.The distribution of the surface elements is presented in Fig.2.The EDS results show that these clusters were mainly vanadium oxide and aluminum oxide.The O enrichment in these loca-tions corresponds to a decrease in the Ti content.The surface of the sample treated at 800 ℃ was smooth and contained almost no clusters.The O enrichment decreased as the temperature increased,which improved the uniformity of the coating.As the temperature continued to increase to 850 ℃,a number of small raised particles were observed in a dispersive distribution on the coating surface,which were mainly composed of Ti and aluminum oxide with particle grain sizes of approximately 1 μm.
A comparison of the coating morphologies of five samples treated at different temperatures reveals that the coating treated at 800 ℃ had the smoothest surface,most compact structure,and a uniform distribution of elements,all of which contributed to its having the best corrosion resistance and mech-anical properties.
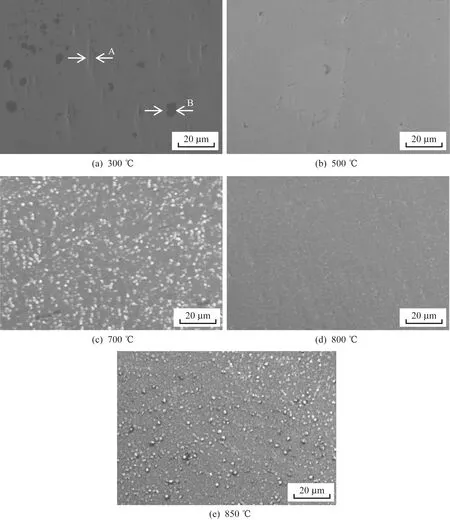
Fig.1 Images of coatings treated at five different temperatures

Fig.2 FESEM-BSE area scan microanalysis of the sample and the distribution of the surface elements
The corrosion morphologies of the untreated sample and the sample treated at 800 ℃ after a Tafel test are shown in Fig.3,in which it can be observed that the surface of the untreated sample had been severely damaged in the 3.5%NaCl solution,whereas the coating of the treated sample showed no obvious perforation or other corrosion phenomena.As such,the coating provided good corrosion resistance for the substrate.
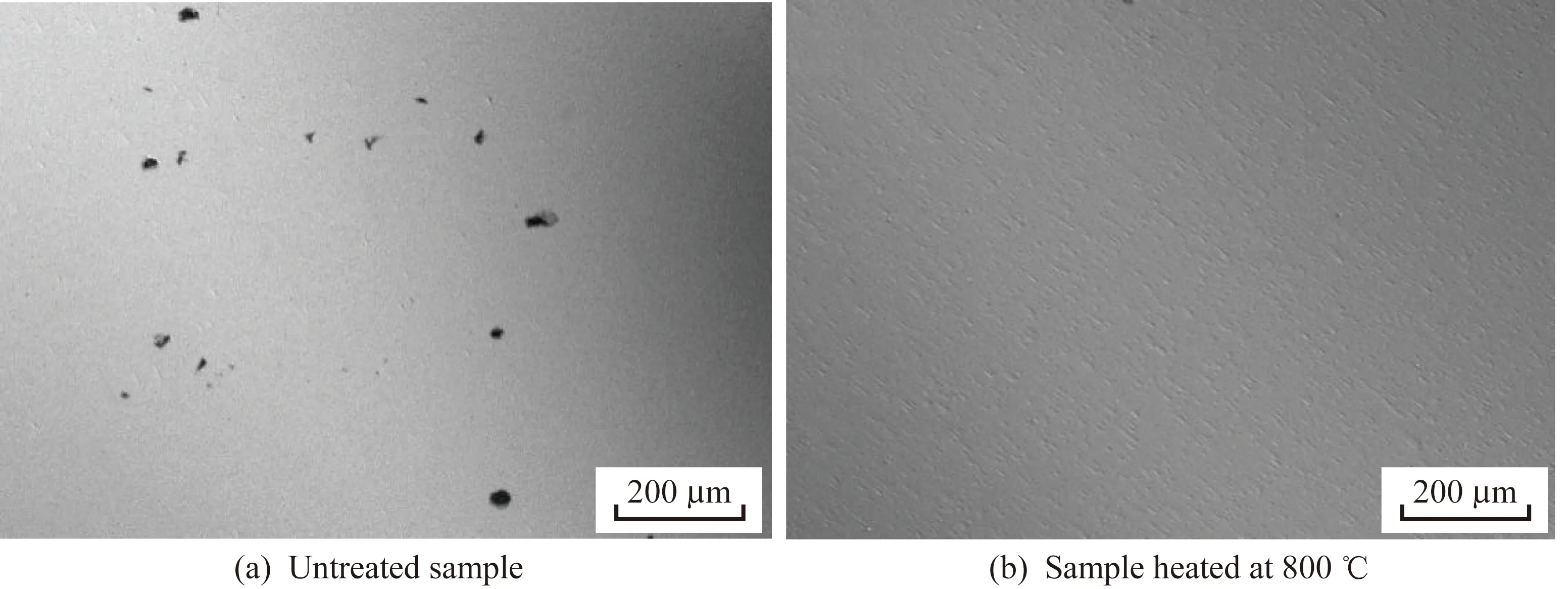
Fig.3 Surface morphologies after the Tafel test
3.2 Cross-sectional analysis
The results of the electrochemical and micro Vickers hardness tests indicate that the sample heated to 800 ℃ had the best corrosion resistance and mechanical properties.The cross-sectional morphology of this sample is shown in Fig.4.The thickness of the coating at this temperature was about 10 μm,with some discrete holes distributed inside the coating,which were sufficiently small to allow the coating to maintain good sealing ability.The line-scanning results show that the surface layer was composed of the elements Ti,V,Al,O,and N,of which Ti,V,Al,and N were distributed in both the substrate and coating,whereas the O was mainly distributed in the coating.The O content first increased and then decreased with depth from the surface,reaching a maximum of 7 μm from the surface,whereas the contents of N,Ti,and V reached a minimum.An Al-rich zone of approxi-mately 5-8 μm was observed in the coating[11],with a super-low Al content elsewhere on the coating.This uneven distribution is due to the segregation of Al,which is mainly concentrated in this area in the form of alumina.The N content in the Al-enriched layer basically remains unchanged,but the N content at the boundary between the substrate and coating first increases and then decreases.In addition,elements Ti and V are evenly distributed in the coating,with no significant enrichment in any location.The cross-section of the EDS line scan results shows that the O in the mixed-atmosphere heat treatment is mainly in the form of an oxide layer,whereas N is present in both the nitride and diffusion layers.
3.3 XRD analysis
The XRD test results are shown in Fig.5,in which it is evident that only a Ti diffraction peak is present in the XRD patterns at 300 ℃ and 500 ℃.This result indicates that the coating prepared at these two temperatures had such a low surface oxide content that no corresponding diffraction peak could be detected in the XRD patterns.When the temperature was increased to 700 ℃,the XRD results show that the diffraction peaks were those of rutile[12],TiO2,and Ti,and as the temperature did not reach the reaction temperature of Ti and N,no TiN peak was detected.When the temperature was increased to 800 ℃,the Ti diffraction peak disappeared,leaving a surface layer of TiO2and TiN.As the chemical heat-treatment temperature increased,the content of TiN continuously increased,and when TiN ex-ceeded its solid solubility in TiO2,TiN existed in a single-phase form.As the temperature continued to increase to 850 ℃,it can be seen that the TiN peak was not significantly strengthened.After forming the first layer of TiN,the TiN served as a barrier,resulting in a sharp decrease in the thermal diffusivity of N to Ti.The oxidation process was further aggravated and the Ti peak was further enhanced.
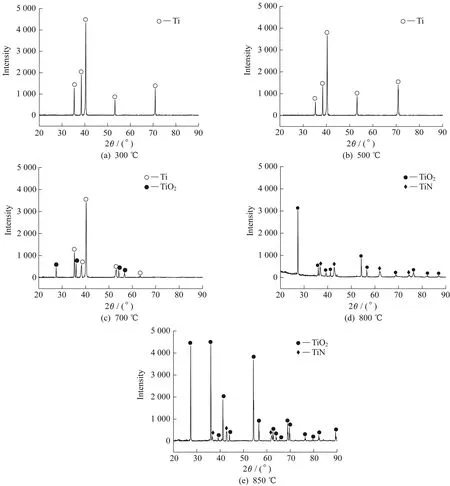
Fig.5 XRD patterns of samples treated at different temperatures
3.4 Corrosion behavior analysis
Table 1 shows the polarization data of different samples tested in the 3.5%NaCl solution.The corrosion potentials (φcorr),corrosion current densities (icorr) and polarization resistance (Rp) were obtained from the curves using the Tafel extrapolation method.

Table 1 Polarization data for treated coatings with substrate
The cathodic reaction is controlled by chloridion (Cl-) and hydronium ions (H3O+),whereas the anodic reaction is controlled by the composition of the coating on the metal surface.Thus,the reaction is both anodically and cathodically controlled,which generally results in a synergistic effect[10].
From the polarization data,it was determined that the coatings induced better corrosion resistance,which kept the current densities low throughout the test.From the polarization graphs presented in Fig.6,it can be observed that all the curves of the treated samples experienced a decrease in the corrosion current density,an increase in the polarization resis-tance,and a positive shift in the corrosion potential,as compared with the curve of the untreated sample.These results indicate that a corrosion attack will preferably occur in the substrate than in the coating.A large fluctuation in the passivation cur-rent of the untreated sample occurs in the anode polarization curve,which indicates damage to and re-passivation of the TiO2on the surface.This phenomenon did not occur in the treated sample,which shows that the sample surface after treatment has better stability.
With an increase in the chemical heat-treatment temperature,the corrosion potential first underwent a positive shift,followed by a negative shift.At a temperature of 500 ℃,the corrosion potential reached its maximum of -0.17 V.The corrosion potential stayed at approximately -0.30 V while the temperature continues to increase.With respect to the corrosion current density,the sample treated at 800 ℃ had the best corrosion resistance,with a corrosion current density of 2.8 nA/cm2,which dropped two orders of magnitude compared to that of the untreated sample.The corrosion current den-sity and polarization resistance remained substan-tially the same at 300 ℃,500 ℃,700 ℃,and 850 ℃.Thus,it is clear that the sample treated at 800 ℃ had the highest corrosion resistance.

Fig.6 Comparative polarization curves of treated sample in 3.5% NaCl solution
The micrograph also shows that a dense oxide film formed at 800 ℃.This dense membrane struc-ture can effectively prevent harmful ions from invading the surface of the metal substrate and thereby prevent corrosion.Compared to other tem-peratures,a layer consisting mainly of TiO2and TiN formed at 800 ℃.Fig.7 shows that the microhard-ness of the film is best after treatment at 800 ℃.The hardness of the film layer indicates good binding properties between the film layer and the metal matrix,which provides the film with better corrosion resistance.
3.5 Microhardness and surface roughness
The microhardness values of the samples as a function of the applied load are presented in Fig.7,in which the curves can be divided into three cate-gories.Samples e and f show high hardness even under a large load (9.8 N),with the microhard-ness values of samples b and c are lower than those of the untreated sample,which stabilized beyond 3-5 N at low values of approximately 350(HV).The microhardness of sample d increased slightly at lower indentation loads and then obviously decreased when the loads were increased,which indicates that the presence of the coating had only a limited effect under low indentation loads.
Under the same load,the hardness of the coating first increased and then decreased with increases in the temperature.The hardness of the coating was higher than that of the substrate when the tempera-ture was higher than 700 ℃ and reached its maxi-mum of approximately 1 243(HV0.1) at 800 ℃,which is an increase of 210%.When the temper-ature continued to increase to 850 ℃,the hardness of the coating slightly decreased.
The single curve in Fig.7 shows that the coating hardness decreased with the increase of the load.A comparison of samples e and f shows that the hardness drop speed of the coating treated at 800 ℃ was significantly lower than that treated at 850 ℃,which indicates that the coating treated at 800 ℃ had good mechanical properties under high loads.
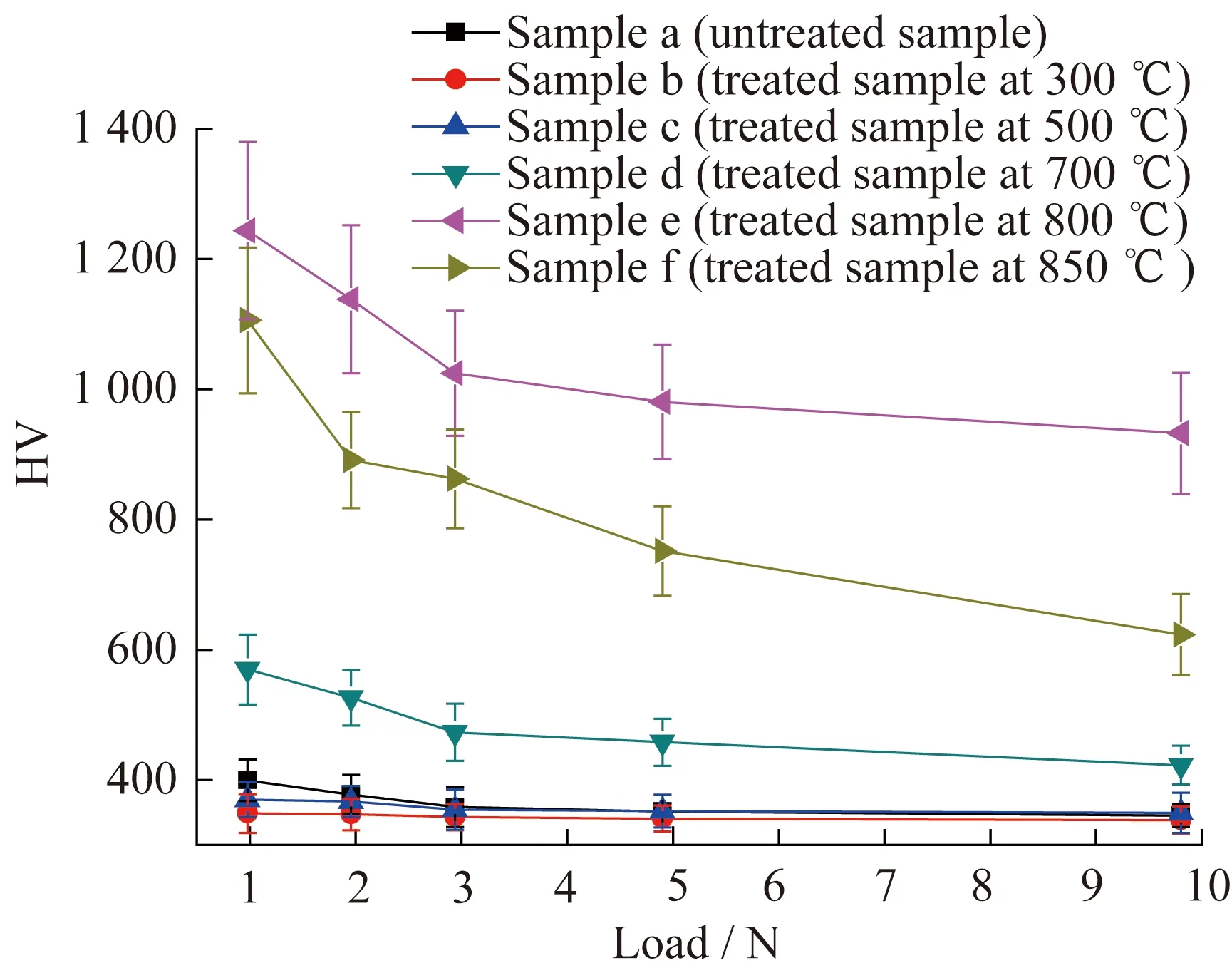
Fig.7 Microhardness results under different loading con-ditions from HV0.1 (0.98 N) to HV1.0 (9.8 N) of untreated sample and treated samples at different temperatures
Generally,the roughness of coatings prepared by thermal oxidation should increase with increased tem-perature.However,in this experiment,as shown in Fig.8,the roughness of the coating did not increase significantly with increased temperature.The sample treated at 800 ℃ was less rough than other sam-ples,except for the sample treated at 500 ℃.The roughness of the coating obviously increased at 850 ℃ due to the increase in the oxidation reaction,and the roughness was well matched with the micro-morphology of the surface.Thus,nitriding at 800 ℃ effectively increased the flatness of the surface coating.
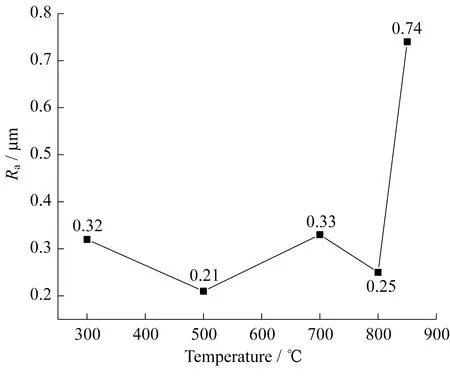
Fig.8 Coating roughness at different temperatures
4 Conclusions
(1) Chemical heat treatment in a mixed atmos-phere at low temperatures enables good surface coat-ings to be obtained.
(2) The sample treated at 800 ℃ showed the highest corrosion resistance,with a corrosion current density dropped two orders of magnitude compared with that of the substrate.An enormous increase in corrosion resistance was attained at 800 ℃.
(3) The hardness of a sample was improved rela-tive to that of the substrate when the temperature of the chemical heat treatment was higher than 700 ℃,with a maximum hardness value of 1 243 (HV0.1) obtained at a temperature of 800 ℃.In addition,the roughness of the coating prepared at 800 ℃ was low,and its wear resistance was improved.
(4) Titanium dioxide was present in the form of rutile in the surface layer.When the temperature was greater than or equal to 800 ℃,Ti and N reacted to form TiN.Some TiNs were present in the coating and the rest was in the diffusion layer.The coating prepared at 800 ℃ had a compact structure with a thickness of 10 μm.
杂志排行
Baosteel Technical Research的其它文章
- Effect of vanadium on the microstructure and properties of metastable austenitic stainless steel AISI 301LN
- Corrosion behavior of super 13Cr stainless steel in a H2S and CO2 environment
- Property uniformity of thick nickel-based alloy plate for nuclear power steam-generator divider plate
- Improving emulsion odor in cold rolling production
- Evaluation of automatic girth weldability of pipeline in special conditions
- Contributions to Baosteel Technical Research wanted
