Disease modifying treatment of spinal cord injury with directly reprogrammed neural precursor cells in non-human primates
2021-07-02VladimirBaklaushevOlegDurovVladimirKalsinEugeneGulaevSergeyKimIlyaGubskiyVeronikaRevkovaEkaterinaSamoilovaPavelMelnikovDzhinaKaralOglySergeyOrlovAlexanderTroitskiyVladimirChekhoninAlexanderAveryanovJanEricAhlfors
Vladimir P Baklaushev,Oleg V Durov,Vladimir A Kalsin,Eugene V Gulaev,Sergey V Kim,Ilya L Gubskiy,Veronika A Revkova,Ekaterina M Samoilova,Pavel A Melnikov,Dzhina D Karal-Ogly,Sergey V Orlov,Alexander V Troitskiy,Vladimir P Chekhonin,Alexander V Averyanov,Jan-Eric Ahlfors
Vladimir P Baklaushev,Vladimir A Kalsin,Veronika A Revkova,Ekaterina M Samoilova,Alexander V Averyanov,Biomedical Research,Federal Research and Clinical Center of Specialized Medical Care and Medical Technologies,FMBA of Russia,Moscow 115682,Moskva,Russia
Oleg V Durov,Eugene V Gulaev,Department of Neurosurgery,Federal Research and Clinical Center of Specialized Medical Care and Medical Technologies,FMBA,Moscow 115682,Moskva,Russia
Sergey V Kim,Department of Anesthesiology,N.N.Blokhin Russian Cancer Research Centre,Moscow 115478,Moskva,Russia
Ilya L Gubskiy,Ilya L Gubskiy,Radiology and Clinical Physiology Scientific Research Center,Federal center of brain research and neurotechnologies of the Federal Medical Biological Agency,Moscow 117997,Russia
Pavel A Melnikov,Department of Neurobiology,Serbsky National Medical Research Center for Psychiatry and Narcology,Moscow 119992,Moskva,Russia
Dzhina D Karal-Ogly,Sergey V Orlov,Department of Primatology,Russian Acad Med Sci,Research Institute of Medical Primatology,Sochi 119992,Sochi,Russia
Alexander V Troitskiy,Department of Vascular Surgery,Federal Research and Clinical Center of Specialized Medical Care and Medical Technologies,FMBA of Russia,Moscow 115682,Moskva,Russia
Vladimir P Chekhonin,Department of Basic and Applied Neurobiology,V.P.Serbsky Federal Medical Research Center for Psychiatry and Narcology,Russia
Vladimir P Chekhonin,Department of Medical Nanobiotechnology,Pirogov Russian National Research Medical University (RNRMU),Moscow 115682,Moskva,Russia
Jan-Eric Ahlfors,New World Laboratories,Laval H7V 4A7,Quebec,Canada
Abstract BACKGROUND The development of regenerative therapy for human spinal cord injury (SCI) is dramatically restricted by two main challenges:the need for a safe source of functionally active and reproducible neural stem cells and the need of adequate animal models for preclinical testing.Direct reprogramming of somatic cells into neuronal and glial precursors might be a promising solution to the first challenge.The use of non-human primates for preclinical studies exploring new treatment paradigms in SCI results in data with more translational relevance to human SCI.AIM To investigate the safety and efficacy of intraspinal transplantation of directly reprogrammed neural precursor cells (drNPCs).METHODS Seven non-human primates with verified complete thoracic SCI were divided into two groups:drNPC group (n=4) was subjected to intraspinal transplantation of 5 million drNPCs rostral and caudal to the lesion site 2 wk post injury,and lesion control (n=3) was injected identically with the equivalent volume of vehicle.RESULTS Follow-up for 12 wk revealed that animals in the drNPC group demonstrated a significant recovery of the paralyzed hindlimb as well as recovery of somatosensory evoked potential and motor evoked potential of injured pathways.Magnetic resonance diffusion tensor imaging data confirmed the intraspinal transplantation of drNPCs did not adversely affect the morphology of the central nervous system or cerebrospinal fluid circulation.Subsequent immunohistochemical analysis showed that drNPCs maintained SOX2 expression characteristic of multipotency in the transplanted spinal cord for at least 12 wk,migrating to areas of axon growth cones.CONCLUSION Our data demonstrated that drNPC transplantation was safe and contributed to improvement of spinal cord function after acute SCI,based on neurological status assessment and neurophysiological recovery within 12 wk after transplantation.The functional improvement described was not associated with neuronal differentiation of the allogeneic drNPCs.Instead,directed drNPCs migration to the areas of active growth cone formation may provide exosome and paracrine trophic support,thereby further supporting the regeneration processes.
Key Words:Direct cell reprogramming;Neural precursor cells;Directly reprogrammed neural precursor cells;Spinal cord injury;Nonhuman primates;Regenerative therapy,Evoked potentials
INTRODUCTION
Traumatic spinal cord injury (SCI) is a severe and often incurable disease of the central nervous system,with an annual age-standardized incidence rate of 13 (11-16) per 100000[1].Every year nearly 500000 or even more new people suffer an SCI[1,2].Due to the extremely high degree of disability it causes,SCI is considered to be the highest priority candidate for the development of regenerative approaches for clinically unmet needs[3].All current approaches to medical rehabilitation (physical neurorehabilitation,neurostimulation,kinesiotherapy,etc.) after severe complete SCI (severe ASIA A and B) have poor efficacy,providing no noticeable restoration of lost function[4].
Neural stem cell (NSC)/neural progenitor cell (NPC) transplantation is generally considered one of the most promising future therapies for SCI[5-10].Development of methods for generating autologous human NSCs/NPCs by direct reprogramming of somatic cells[11-15] has offered fundamentally new possibilities for this approach.Several studies on animal models of SCI (including primates) have established proof of efficacy for allogeneic or even xenogeneic NSC/NPC transplantation[9,12,16,17].However,there are still very few clinical trials using these cells,and therefore the possibility of functional restoration after spinal cord injury remains an open question[7,10].
Autologous NSCs obtained by directly reprogramming cells from various starting cell types are the most promising for clinical use because of better genome stability and lower risk of tumor transformation compared to induced pluripotent stem derived NPCs.Recently it has been shown that directly reprogrammed neural precursor cells(drNPCs) obtained through transient transfection of bone marrow mononuclear cells with non-integrating synthetic plasmids expressing musashi-1,neurogenin-2,and methyl-CpG binding domain protein 2 demonstrated normal karyotype and all fundamental features of neural stem cells[11].Transplantation studies in small animal models have provided very promising preliminary results for the use of human drNPCs in the treatment of experimental SCI and stroke[12,13] prompting us to initiate a study in non-human primates (NHP).
Previously we described the novel model of controlled complete SCI on NHP[18].The goal of the current study was to investigate the safety and efficacy of intraspinal transplantation of allogeneic drNPCs in this NHP (Macaca mulatta) model of complete subacute SCI.
MATERIALS AND METHODS
Directly reprogrammed neural precursor cells
Allogeneic drNPCs were created from the bone marrow mononuclear cells of one femaleMacaca mulattaaccording to methods previously described[11].The bone marrow (5 mL) was collected from the head of the humerus under ketamine anesthesia(10 mg/kg).Briefly,direct reprogramming was made by means of transient transfection of a cocktail of three transcription factors:musashi-1,neurogenin-2,and methyl-CpG binding domain protein 2.
Two weeks before transplantation,the cryopreserved drNPC cells were thawed and seeded onto laminin-coated plates and expanded in neuro Cult-XF basal medium(Stem Cell Technologies) with 1% Pen-Strep (Gibco),1 × B-27 Supplement (50 ×)(Gibco),20 ng/mL of bFGF (Peprotech),and 20 ng/mL of epidermal growth factor(Peprotech) (complete growth medium).The cells were incubated at 37 °С in 5% СО2and 5% О2.Shortly before intraspinal injection,the cells were detached from the plates with Stem Pro Accutase (Thermo Fisher Scientific).A sample was taken for flow cytometry analysis,and the rest were divided into two vials.Cells from one vial were seeded onto laminin-coated cover glass Petri dishes for immunocytochemistry (ICC)analysis,as described below.The cells from the other vial were tested for viability using a Luna 2 cell counter and injected into animals.Viability was no less than 98% in all cell preparations,and lapsed time between formulation and injection did not exceed 20 min.
Immunophenotyping of drNPCs
Cells were cultured for 14 d in complete growth medium followed by fixation with icecold buffered 4% paraformaldehyde for 30 min.For flow cytometry cells were detached with Stem Pro Accutase followed by fixation with ice-cold buffered 4%paraformaldehyde for 10 min.The following antibodies were used:nestin (R and D and Abcam),SOX2 (BD Biosciences),βIII-tubulin (R&D),microtubule associated protein 2 (MAP2) (Sigma-Aldrich),glial fibrillary acidic protein (GFAP) (DAKO),NF-200 (Sigma-Aldrich),macro H2A.1 (Abcam),human leukocyte antigen (HLA)-ABC(BD Pharmingen),and HLA-DR (Miltenyi Biotec).For flow cytometry,directly labeled primary antibodies were used at a concentration of 10 μg/mL.For ICC,all primary antibodies were diluted in PBS-TT (PBS with 0.2% Tween 20,0.3% Triton X-100,and 1.0% normal goat serum) at a concentration of 1-5 μg/mL.Goat anti-mouse IgG (H +L) labeled with Alexa Fluor 488 and goat anti-rabbit IgG (H + L) labeled with Alexa Fluor 633 (Life Tech,United States),all diluted at 1:400 in PBS-TT,served as secondary antibodies.Cell nuclei were counterstained with Hoechst (1 μg/mL;Invitrogen,United States).A Nikon A1 scanning laser confocal microscope (Nikon Company,Japan) was used to evaluate all ICC,while an S3e cytometer (Bio-Rad) was used for flow cytometry.
Animals
Seven matureMacaca mulattamale NHPs were enrolled in this study.Animals were kept under natural light conditions with free access to water and fed two times a day.All procedures were performed in accordance with the European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes(European Treaty Series No.123,Strasbourg,March 18,1986;Directive 2010/63/EU of the European Parliament and Council of 22 September 2010 On the Protection of Animals Used for Scientific Purposes).The protocol of the study was approved by the Bioethics Committee of Research Institute of Medical Primatology (Statement from July 13,2016),and the Local Ethical Committee of the FRCC of FMBA of Russia(Statement No.10b of September 12,2016).
Prior to surgery,NHPs were housed in an open-air cage (20 m2enclosure with enriched environment such as climbing/hanging gear and toys providing an opportunity for games and socialization) at the Sochi Institute of Medical Primatology.After surgery,NHPs were housed in large individual cages with toys and climbing/hanging gear,adopted for the animals with distal paralysis in one limb.The cages were near each other,which allowed physical contacts between NHPs and their socialization.The length of the study was minimized.Subjects were kept under natural light conditions with free access to water and fed two times a day.To avoid distress,NHPs were contacted only with the staff they knew well,whom they allowed to do intramuscular injections.All manipulations out of the cage [preparation for the surgery,detection of the evoked potentials,neurological examination of the paralyzed left limb,magnetic resonance diffusion tensor imaging (MRI),etc.] were conducted under sedation by ketamine (10 mg/kg,intramuscular).Health and well-being of the animals were monitored daily by vet staff using exterior,activity,and appetite as criteria and were followed by medical examination,if necessary.
The animals were randomly assigned to two groups:the lesion control group [(LC),n=3] and drNPC transplantation group (NPC,n=4) (Table 1).
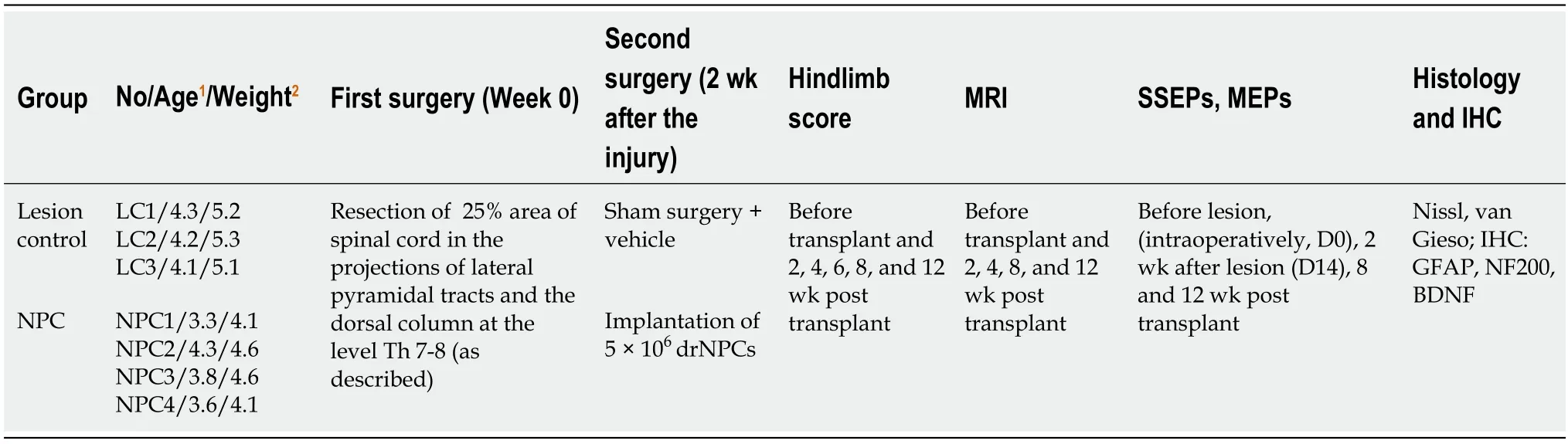
Table 1 Characteristics of experimental groups
SCI induction
SCI induction was performed as described before[18].Briefly,animals were anesthetized by endotracheal inhalation with isoflurane (1.2-2.0 vol%).After skin incision and paraspinal muscle separation,Th 8 interlaminectomy was performed.The dura mater was dissected,and the spinal cord exposed.Guided by intraoperative recording of somatosensory evoked potentials (SSEPs) and motor evoked potentials(MEPs),25% of the spinal cord cross-section in the projection of the left dorsal funiculus and left lateral corticospinal tract was excised with a length of about 5 mm.Further excision was continued until the SSEPs and MEPs from the corresponding segments of the left hindlimb disappeared.Before closing the wound,duraplasty was performed using the autofascia,followed by sealing with neurosurgical fibrin glue.In the postoperative period,all animals received antibiotic therapy (ceftriaxone,50 mg/kg,intramuscular,once a day).Pain was managed by the administration of ketonal (15 mg/kg).
Implantation of drNPCs
All NHPs underwent a second surgery 2 wk after the SCI induction.In the experimental (drNPC) group,the spinal cord around the injury was exposed again,and a drNPC suspension was injected into the perifocal zone in the projection of the dorsal funiculus and lateral corticospinal tract above and below the lesion (Supplementary Figure 1).A dose of 5 × 106cells,resuspended in a total volume of 100 mL in Hanks’solution,was injected at four sites at a rate of 5 mL/min (25 mL per injection) by means of a sterile system consisting of a silicone tube and a 28G needle attached to a Hamilton 500 microsyringe,which was connected to a nanoinjector (Leica Microsystems).After each injection,the needle was left in the spinal cord tissue for 3 min and then slowly withdrawn.To prevent spinal cord compression by the scar tissue of the dura mater,duraplasty with the autofascia was repeated.In the control(LC) group,the same surgery was performed,with the equivalent number of injections and volume of Hanks’ solution.No immunosuppression therapy was administered for either group.
Neurophysiological and imaging assessment
Hindlimb function:The degree of neurological deficit was determined using the NHP hindlimb score system suggested with our modifications[18] to assess the severity of lower monoplegia.The scores were assessed for the ipsilateral (left) limb.The score included an assessment of active flexion in large joints,reliance on the limb,tendon and periosteal reflexes,muscle tones,toe gripping,activity,and movement coordination.
MRI morphometry:MRI morphometry was conducted as described previously[18].Briefly,T2-weighted images were obtained in two orthogonal planes at the thoracic and cervical level as well as at the level of the head.The structures were measured using the RadiAnt software (Medixant).The area was calculated using the ImageJ freeware package (National Institutes of Health,Bethesda,MD,United States).
Neurophysiological examination:Intraoperative monitoring of transcranial myogenic electrical potentials,MEPs and SSEPs,was performed during all the surgical interventions using the Neuro-IOM system (Neurosoft,Russia),as described previously[18].Briefly,the registration of the latency and amplitude of the muscle response for abductor hallucis (AH),musculus tibialis anterior (TA),and musculus quadriceps femoris (QF) was performed with the active electrode placed in the region of the motor point.The amplitude and latency parameters of the cortical SSEP response of the hindlimbs in the form of the first positive (P1) and negative (N1) peaks were evaluated by sequential stimulation of nervus tibialis.The absolute values of the SSEP and MEP parameters varied in different animals and different muscle groups;therefore,we used a scoring system from the study[19] modified by us[18] from 0 (no evoked potentials) to 5 [the amplitude is 50%-100% of the baseline (before the injury)and the latency is no higher than 110% of the baseline] for SSEP-nervus tibialis as well as MEP-AH,MEP-TA,and MEP-QF.
Animal termination
Twelve weeks post transplantation,all animals were deeply anesthetized with an intravenous administration of ketamine (20 mg/kg) followed by infusion of a single extra-high dose of propofol (5 mL of a 1% solution).Transcardial perfusion was performed with a buffered,cooled 10% formalin solution as described previously[18].The vertebral columns were post-fixed for 24 h in the same solution at 4 °C.The spinals cords were isolated from the fixed preparations and sagittal sections(vibratome,100 μm and paraffin 5-7 μm) were prepared from the region of injury and transplantation.
Histological and immunohistochemical analysis
Morphological studies were carried out on cresyl violet (Nissl),and hematoxylin and eosin stained paraffin sections,as described previously[18].Immunohistochemical staining was performed on 3-5 μm paraffin sections as well as on 50-100 μm vibratome sections,both using fluorescence detection.Sections of the spinal cord were stained with antibodies to β-tubulin III (2 μg/mL),nestin (2 μg/mL;R&D),SOX2 (5 μg/mL;BD Biosciences),MAP2 (5 μg/mL;Sigma-Aldrich),NF200 (5 μg/mL;Sigma-Aldrich),GFAP (2 μg/mL;DAKO),brain derived neurotrophic factor (5 μg/mL;Abcam),and macro H2A.1 (Abcam).Secondary antibodies used were Alexa Fluor 488 goat anti-mouse IgG (H + L) and Alexa Fluor 633 goat anti-rabbit IgG (H + L) (highly cross-absorbed,all dilutions 1:400;Invitrogen,United States);counterstaining was done with Hoechst.Fluorescence was detected by a confocal microscope Nikon A1(Nikon,Japan).For the quantification of positive cells,we used NIS Elements software(Nikon).
Statistical analysis
Statistical analysis of the hindlimb score,SSEP,MEP,and MRI was carried out on the three NHPs of the LC group and the four NHPs of the NPC group at each time point.The data were summarized as the median and the first and third quartiles or as the mean ± SD.
To compare baseline data in the groups,the nonparametric Mann-Whitney test for quantitative data and theχ2or Fisher exact test for qualitative data were used.The correlation between quantitative variables was estimated by Spearman’s method.The hindlimb score and MEP/SSEP score data were analyzed by calculating Pearson’s linear correlation coefficient.
For the main analyses (hindlimb score,MEPs,SSEPs),we used a mixed linear model with time points as nested data,the group and timeline being fixed factors;their interaction was also estimated.A two-sided probability threshold of 0.05 was used to determine statistical significance.The analyses were performed using IBM® SPSS®Statistics Version 23.0.
RESULTS
Immunophenotyping of drNPCs
Flow cytometry and immunocytochemical characterization of cultured allogeneicMacaca mulattadrNPCs was performed prior to transplantation.Expression of the immature neural stem/progenitor markers SOX2 and nestin was detected in 85.6%and 90.5% of the cells,respectively,as determined by flow cytometry,while GFAP expression was ubiquitous with up to 99.4% of the cells positive for this marker.The neuronal markers βIII-tubulin and MAP2 were detected in 85.8% and 58.6% of the cells,respectively (Figure 1A-C).Human leukocyte antigen DR expression was detected in a very small subpopulation,not more than 4.4% (Figure 1A),while HLAABC was not detected in a noninflammatory environment at all (Supplementary Figure 2).When drNPC were cultured on laminin coated plastic in complete growth media most cells coexpressed nestin and GFAP (Figure 1B).A smaller GFAP positive population appeared to have decreased nestin expression,indicating glial fate differentiation.drNPC cultures exhibited spontaneous early differentiation with formation of βIII-tubulin and MAP2 positive networks (Figure 1C-E) with most cells maintaining SOX2 expression,confirming their immature status.Taken together,flow cytometry and ICC demonstrated that drNPC cells are a relatively homogeneous neural stem/progenitor population that can initiate neuronal and glial differentiation in culture.
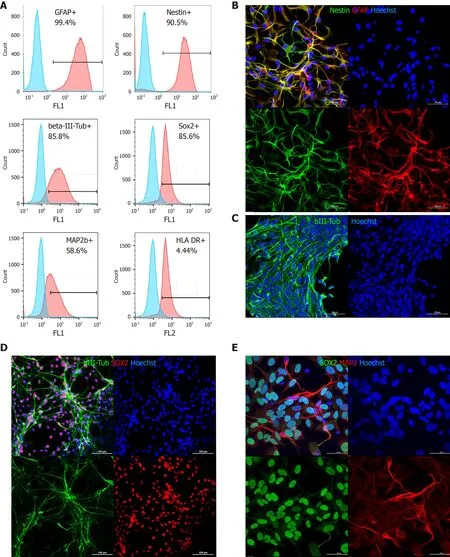
Figure 1 Phenotypic characterization of directly reprogrammed neural precursor cells by flow cytometry and immunocytochemistry.
Time course of neurological deficit
Analysis of hindlimb score changes with time showed no noticeable improvement of the neurological state of control animals (LC group) that underwent SCI induction surgery and were administered vehicle.In contrast,the animals with implanted drNPCs (NPC group) exhibited recovery of motor function beginning on the fourth week after transplantation.Statistical analysis showed that the time course of the hindlimb score recovery was significantly different in the NPC group (P<0.01,as estimated using the mixed linear model) as compared to the LC group (Figure 2).
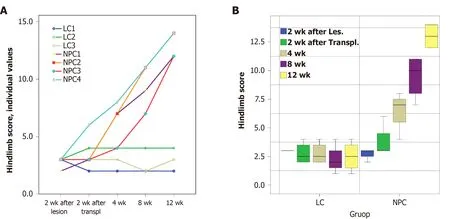
Figure 2 Changes in the hindlimb score in the experimental groups up to 12 wk after directly reprogrammed neural precursor cell transplantation or vehicle injection.
Analysis of individual parameters of the hindlimb score in drNPC treated animals showed that the muscle tone and the tendon and periosteal reflexes were normalized by 12 wk after transplantation (Supplementary Table 1).By that time,all NHPs were able to flex the hindlimb at large joints,use the hindlimb when walking,and use its digits for grip when climbing.Two of the four animals (NPC1 and NPC4) exhibited the most significant functional recovery as they were able to jump using the ipsilateral hindlimb for support and actively use this limb when climbing.In general,their behavior differed little,if at all,from that of healthy NHPs,indicating near complete recovery of function.
Analysis of SSEPs and MEPs
Estimation of MEPs and SSEPs both immediately after the injury and 2 wk post injury showed that the SSEPs from nervus tibialis of the left hindlimb and MEPs from the left AH and TA were either absent (NPC3,NPC4) or had amplitudes decreased by more than 80% (LC1-3,NPC1,NPC2) in all of the seven animals shortly before the second surgery and implantation,indicating a complete SCI (Supplementary Table 2 and Figure 3A).The absolute values of amplitude and latency varied substantially in different animals and different groups of muscles (Supplementary Tables 2-4);therefore,when performing comparative analysis,we took the values of these parameters in each animal before the injury to be 100%.We also used the point scale for estimation of SSEPs and MEPs suggested by Yeet al[19],which we adapted for our model[18] (Supplementary Table 5).In both groups the amplitude of SSEP,MEP-AH,and MEP-TA at day 14 (before the second surgery) varied from 0 points (absence of EP) to 1 point in the EP score (the maximal value was 17.4% in NHP LC2) (Supplementary Table 2).Due to this heterogeneity,we decided to define,as criteria for recovery at week 12,an increase in amplitude of 1 point or more.In the LC group,neither MEP nor SSEP parameters changed considerably at 12 wk after the vehicle injection.Even though NHPs in the LC group had residual MEP-AH (from 10% to 17%,1 point) at day 14,we did not find MEP-AH at week 12 in two of the three NHPs(LC1,LC3),indicating deterioration over time.In LC2,which had 17.4% of residual MEP-AH amplitude at day 14,we detected MEPs from the left AH;however,their amplitude was as low as 4% of the baseline value,i.e.lower than the residual MEP-AH(Figure 3A and Supplementary Table 2).In contrast,the amplitude of the MEPs of the ipsilateral AH recovered over 50% of the baseline value in two of the four NHPs with implanted drNPCs (NPC1,NPC2) and to 14% and 17%,respectively,in the other two NHPs (NPC3,NPC4) (Figure 3A and Supplementary Table 2).Thus,all NHPs in the NPC group demonstrated recovery of MEP-AH by our criteria:from 1 to 3 points for NPC1 and NPC2 [NPC1:9% (day 14)-56% (week 12);NPC2:11% (day 14)-55% (week 12)];and from 0 to 1 point for NPC3 and NPC4 (from 0 at day 14 to 14% and 17% at week 12 for NPC3 and NPC4,respectively) (Supplementary Table 5).
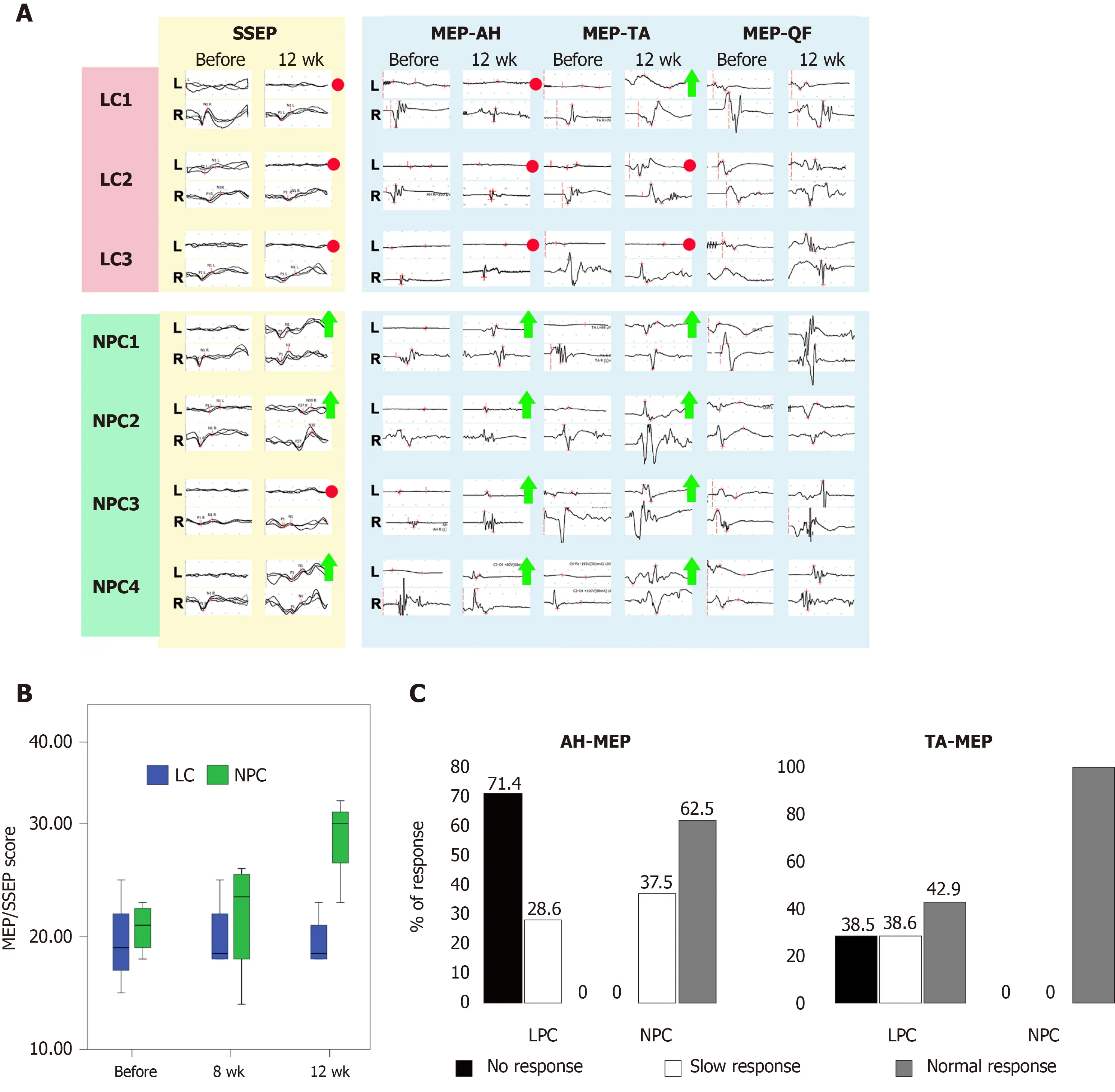
Figure 3 Functional assessment.
In the LC group only one NHP (LC1) demonstrated some spontaneous recovery of MEP-TA from 0 at day 14 to 1 point (6%) at week 12 (Figure 3A and Supplementary Table 3).MEP-TA amplitude in the other two NHPs did not change from day 14 to week 12.In contrast,all NHPs of the NPC group demonstrated recovery from 1 point at day 14 to 3 points at week 12 (Supplementary Table 3).
The differences between the NPC and LC groups in the degree of recovery of the MEPs from AH became significant at 12 wk (P<0.05,as estimated using the mixed linear model,Figure 3B).There were no significant differences between groups in the MEPs from the ipsilateral TA.In both groups the MEPs from musculus quadriceps spontaneously recovered up to 3-5 points at day 14 indicating that the intersection of the medial corticospinal tract fibers was not complete (Figure 3A).We decided to analyze MEP-QF at all timepoints as an internal control.The amplitude and the latency of MEP-QF did not differ significantly between timepoints or treatment groups,demonstrating safety of the drNPC injection.
None of the animals in the LC group exhibited SSEPs at 12 wk after vehicle injection;SSEPs were absent even in the animal LC2 that retained residual (approximately 10%) evoked potentials 2 wk after the injury.In three of the NHPs with implanted drNPCs (NPC1,NPC2,NPC4),the SSEP amplitude recovered to 63.1%,27.7%,and 21.8% of the baseline level,respectively.NPC3 did not show any recovery of SSEP.
Statistical analysis showed significant differences between the LC and NPC groups in the ipsilateral nervus tibialis SSEP amplitude 12 wk post drNPC transplantation (P<0.05,as estimated using the mixed linear model).Qualitative comparison of the MEP-AH and MEP-TA latencies at 4 and 12 wk post transplantation showed that the groups significantly differed in these parameters as well (P<0.01 andP<0.05 for the MEP-AH and MEP-TA latencies,respectively,as estimated by theχ2test) (Figure 3C).Statistical analysis of the SSEP/MEP scale showed significant differences between the treatment groups in the total score 12 wk post transplantation (P<0.01,as estimated using the mixed linear model) (Figure 3B).Taken together,our data showed that 12 wk after drNPCs intraspinal transplantation of three of the four animals exhibited SSEP recovery,and all animals had recovery of MEP-AH and MEP-TA by at least 1 point.We detected no impairment of residual EP from TA or QF or any other negative adverse effects to innervation both in the ipsilateral and contralateral sides of all animals in the study.
MRI morphometry
To examine the potential tumorigenic effect of drNPCs and any impact on cerebral liquid flow after intraspinal drNPCs transplantation,the brain and spinal cord were evaluated by MRI followed by morphometry of the cerebral ventricles and aqueduct,the anterior and posterior subarachnoid spaces,and central canal at 2 wk after the SCI induction and 4,8,and 12 wk post intervention (drNPC transplantation or vehicle injection) (Figure 4A).Additionally,we used postmortem high-resolution MRI for morphological investigation of SCI volume after drNPC transplantation in comparison with the vehicle control group (Figure 4B).None of the animals displayed signs of hydrocephalus syndrome or morphological signs of syringomyelia;there were no noted alterations in the cerebral ventricles or subarachnoid spaces at 12 wk in all four animals (Figure 4A,4C and 4D).Dilation of the central canal was observed in one NHP in the vehicle control group (Supplementary Figure 3) and appeared dependent on the degree of postoperative epidural fibrosis.
To investigate possible structural alterations of the spinal cord after intraspinal drNPC transplantation,we determined the spinal cord area at the site of the injury as well as below and above it before the transplantation and for the 12 wk post intervention (Figure 4F).Statistical analysis of these data did not show significant differences in morphometric parameters between the LC and NPC groups,indicating that all animals tolerated the injection procedure and the drNPC transplantation.
At week 12 post intervention,the volume of injury (the sum of all areas with pathological hyperintense signals in T2 weighted coronal sections,Figure 4B) was in the range of 28.0-36.5 mL and did not differ significantly between groups (Figure 4G).This suggests that drNPCs transplantation did not add measurable volume to the spinal cord nor contribute significantly to a gliomesodermal scar at the macroscopic level.
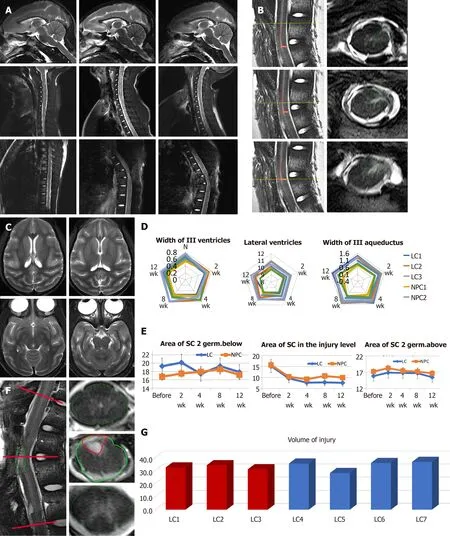
Figure 4 T2 weighted magnetic resonance imaging and the results of the brain and spinal cord morphometry.
Taken together,our MRI data confirmed the relative consistency of the SCI lesion in the LC and NPC groups and demonstrated that the intraspinal transplantation of drNPCs did not adversely affect the morphology of the central nervous system or cerebrospinal fluid circulation.
Immunohistochemical analysis
We next sought to detect transplanted cells and to investigate their fate post transplantation using IHC.The allogeneic drNPCs were derived from the bone marrow of a donor female,whereas the recipient NHPs were males.By using an antibody against macro-H2A.1,a histone overexpressed in the inactivated Х chromosome of female cells[20],we were able to distinguish donor cells from the recipient.We determined that macro-H2A.1 expression levels reliably differentiated between the transplanted female cells and the host male cells (Figure 5A,5B,and 5D).Macro-H2A.1-positive cells were found both in the white and grey matter of the injured spinal cord in all animals of the NPC group.Double staining with macro-H2A.1 and SOX2 antibodies revealed that the majority of the cells were positive for both markers (Figure 5B),as confirmed using the colocalization feature of the NIS elements application (Pearson correlation=0.78).The donor cells were predominantly localized around endogenous NF200-positive growth cones (Figure 5C and 5D) as well as around reactive astrocytes (Figure 5B and 5F).In the LC group,neither expression of macro-H2A.1 nor SOX2 was detected (Supplementary Figures 4-6).Quantification of the number of drNPC-derived cells in confocal images revealed that the density of macro-H2A.1 and SOX2-positive cells was almost 2-fold higher around damaged axons,which formed growth cones and presented high intensity NF200 stainingvsareas of low NF200 staining intensity (Figure 6).The estimated total number of transplanted cells in a tissue slice was less than 1000.Taking into consideration a slice thickness of 100 μm and that the area of transplantation did not exceed 2 mm,it appears that the total number of surviving cells was less than 20 × 103cells per NHP,indicating an estimated survival rate of less than 1% at 12 wk.
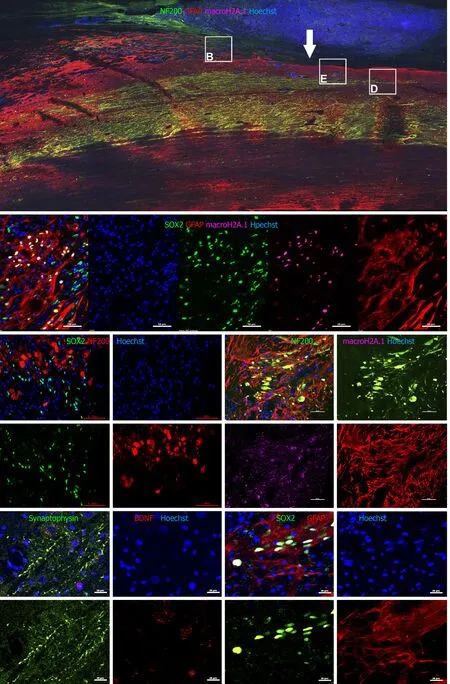
Figure 5 Immunohistochemical analysis on spinal cord sagittal sections 12 wk after transplantation.
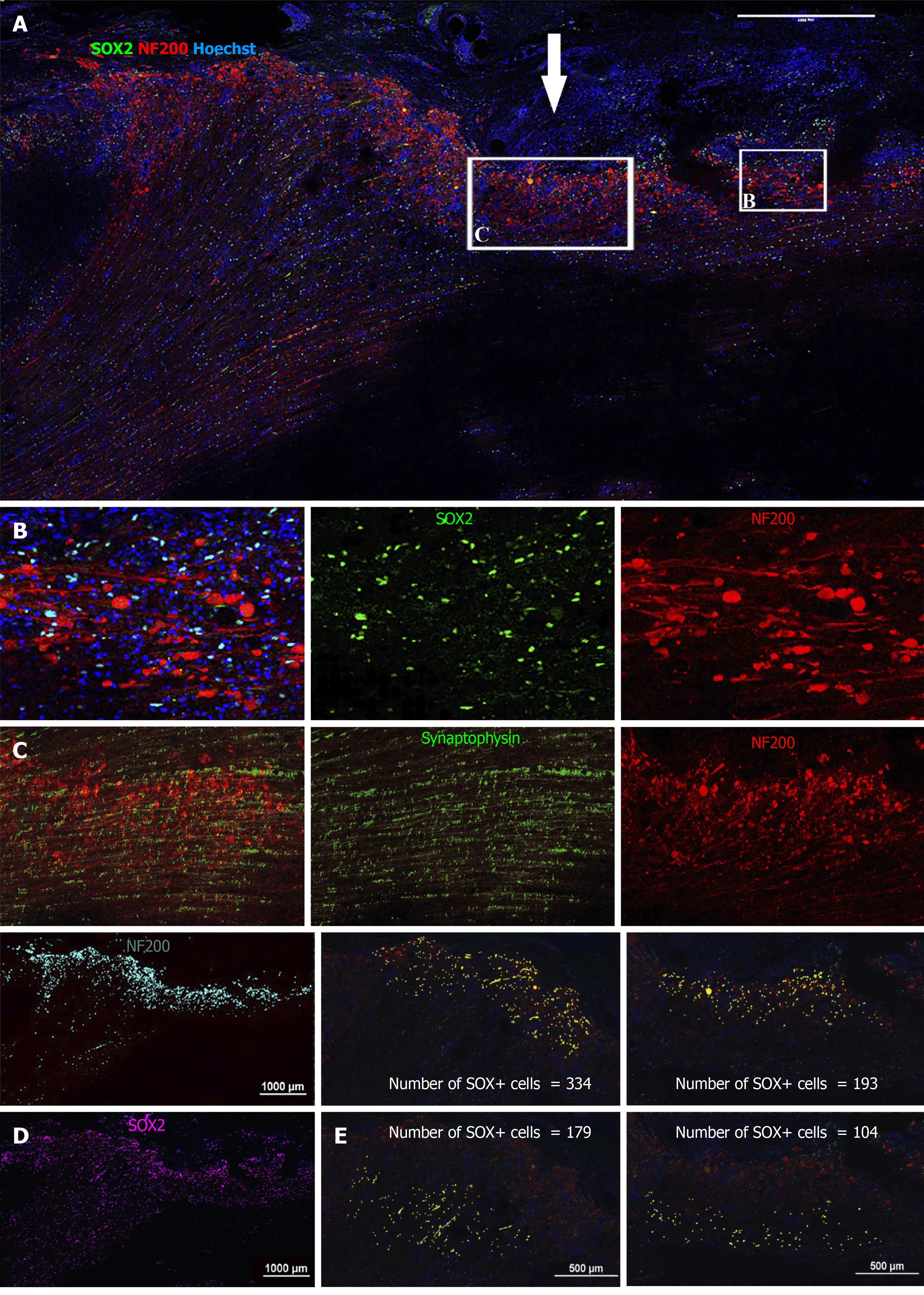
Figure 6 Immunohistochemical analysis of NF200 and SRY-box transcription factor 2 on spinal cord sagittal sections 12 wk after transplantation (animal NPC4).
Despite the abundance of macro-H2A.1+ cells in areas positive for NF200,there was no colocalization of the markers (Pearson correlation=0.20),indicating that none of the surviving drNPC derived cells were terminally differentiated neurons.Interestingly,we found high expression of synaptophysin and brain derived neurotrophic factor in the axonal growth cone-rich areas where macro H2A.1/SOX2-positive cells were located (Figure 5E).In astrogliosis foci,the SOX2-positive cells were located along GFAP-positive processes of reactive astrocytes and appeared GFAPpositive (Figure 7A).However,three dimensional reconstruction of confocal z-stacks showed that some SOX2/macro H2A.1-positive cells were GFAP negative (Figure 7BD) and oftentimes were surrounded by GFAP-positive processes (Figure 7D).Taken together,our careful single cell analysis indicated that surviving SOX2-positive cells had not differentiated into astrocytes.
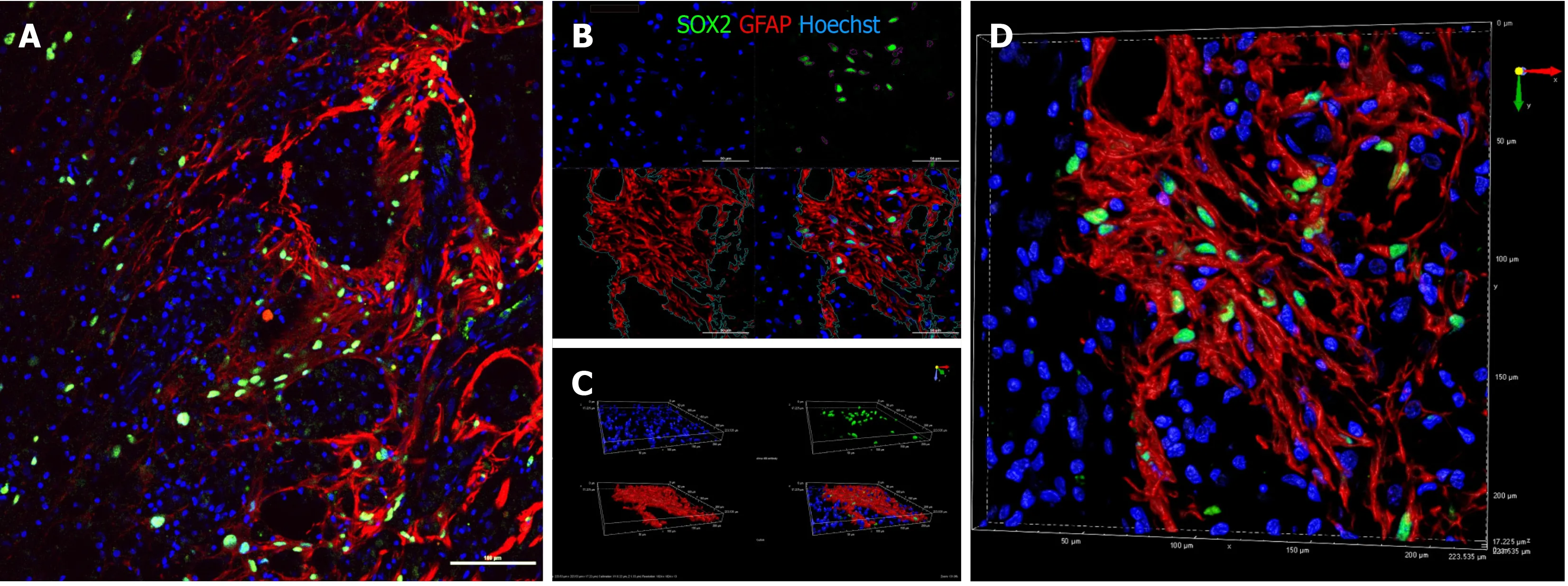
Figure 7 Immunohistochemical analysis of SRY-box transcription factor 2 (green) and glial fibrillary acidic protein (red) on sagittal sections of animal NPC4 spinal cord.
DISCUSSION
Research focused on regenerative approaches for central nervous system diseases in general and SCI in particular indicates upon close examination of the published literature on this topic that one of the most important aspects driving clinical translation of promising preclinical data is the choice of thein vivomodels.The data obtained in small rodents has not always been successfully reproduced in large animals,and therefore its extrapolation to humans is still challenging[21,22].Because of this,research conducted on anthropoid primates has experienced renewed interest from the scientific community based on the rationale that the phylogenetic proximity of NHPs to humans may provide a basis for more successful clinical trans-lation[19,21-23].Based on these considerations,we had previously developed a bioethically acceptable NHP model of regionally complete SCI with the use of intraoperative EP detection,resulting in a regionally complete and irreversible model of SCI[18].This was evidenced in the current study by the absence of EP from nervus tibialis,AH,and TA both intraoperatively,i.e.immediately after excision of a fragment of the spinal cord,as well as 2 wk after surgery,except for some residual potentials.
In the current study we used allogeneic rather than autologous drNPC cells because that allowed us to carefully characterize donor cell behavior postmortem without relying on permanent genetic modification of the donor cells or pretransplant physical labeling that may lend itself to errors in the case of cell fusion or endocytosis of the label by host cells.This likely resulted in a lower number of surviving cells at 12 wk post transplantation and likely did not allow for the survival of any differentiated cells.The surviving SOX2+donor cells nevertheless allowed for significant functional recovery by supporting neurogenesis and synaptogenesis of the host neurons despite the absence of immunosuppression.
Previous studies in NHPs addressing the safety and efficacy of cell-based therapies in SCI have showed that xenogeneic (human) neural stem cells might improve spinal cord regeneration[9],but for clinical purposes,we argue that the most desirable cell type is autologous[7].The potential challenges of using nonautologous cells in the clinic was demonstrated in a recent study whereby pharmacological immunosuppression did not provide adequate long-term survival of transplanted cells and failed to improve functional recovery after SCI[24],indicating that both longer term and more extensive immunosuppression may be required than previously thought for nonautologous cell transplants for SCI.This may not be feasible for SCI patients who are already more prone to suffer from infections.To provide a solution to the challenge of generating autologous neural progenitor cells for large scale clinical use in SCI repair,a realistic source of such autologous cells are the patient’s own somatic cells that are obtained,e.g.,from the bone marrow and directly reprogrammed into neural precursor cells[11-13].Direct reprogramming skips the pluripotent state and therefore rapidly generates cells with considerable safety advantages over pluripotent derived cells[14].
Based on the hindlimb score[22],modified for our model,we observed restoration of function in the paralyzed limb for all animals in the drNPC transplanted group(NPC group),while the vehicle control group showed no recovery.The best recovery was observed in subject NPC1,which correlated with the restoration of MEP to over 50% of baseline (preinjury) levels.Importantly,none of the three control animals showed any recovery of SSEP and MEP up to 12 wk post vehicle injection.Therefore,the MEP restoration observed in all four animals and the SSEP restoration observed in three animals in the drNPC transplanted group suggests preliminary efficacy of drNPCs transplantation in SCI.
Because we transplanted female drNPCs into males,we used antibodies against macro H2A.1,a histone marker overexpressed in the inactivated X chromosome of female cells[20] to identify the donor cells.Transplanted cells could be identified by the high fluorescence intensity of macro H2A.1;it was further confirmed that the bright macro H2A.1-positive cells were indeed female drNPCs because they were also labelled with SOX2,a specific marker of neural multipotency that is not expressed in the naïve adult spinal cord[25].This was further confirmed by the absence of any SOX2+cells in the vehicle transplanted LC NHPs (Supplementary Figures 4-6).Using this approach,we were able to detect double positive macro H2A.1 and SOX2+cells(drNPCs) that mainly accumulated in the formation zone of axonal growth cones.This biased distribution of grafted drNPCs suggests active migration towards the spinal cord regeneration zone.Interestingly,our histology data estimated that less than 1% of the transplanted drNPC cells were detected at 12 wk post transplantation.Although the drNPC retained neural multipotency (SOX2 expression) and low human leukocyte antigen DR expression,the use of allogeneic rather than autologous drNPCs in the absence of immunosuppressive therapy (a major limitation of this study) may help explain the low survival rate of the graft.The development and testing of autologous NHP drNPC in SCI is the subject of ongoing studies.
Despite the regenerating axonal environment into which donor cells integrated,we were unable to detect macro H2A.1-positive cells that expressed neuronal markers.We cannot rule out the possibility that neuronal differentiation did occur before early immune detection and elimination by the host immune system.Nevertheless,our study suggests that drNPC-based efficacy does not depend on substantial survival of the grafted cells nor neuronal differentiation.While unravelling the mechanism of drNPC based efficacy was beyond the scope of our study,it is anticipated that it involves various factors,of which neurite outgrowth and synaptogenesis appear significant,as previously discovered in a recent study employing the cells in an unrelated rodent model of stroke[13].The mechanism may also have similarities to spinal cord regeneration mechanisms seen in more primitive vertebrates[26].The hypothesis according to which transplanted NPCs must undergo neuronal differentiation,integrate into functioning neural networks (i.e.be directly involved in conducting an electrical impulse[9,17]) to provide meaningful clinical benefit is the focus of intense debate and investigation.Our study with drNPCs suggests that significant functional recovery of anatomically disturbed pathways can potentially be accomplished through a regeneration mechanism that may be unique to drNPC-like cells.
CONCLUSION
There was no evidence of safety concerns regarding drNPC transplantation into the spinal cord for at least 12 wk post transplantation,as evidenced by the absence of pathological changes in the spinal cord and cerebrospinal fluid as assessed by MRI and histological analysis.There were also no observed ectopic cell colonies.
drNPCs injection contributed to significant improvement of spinal cord function after subacute complete SCI,based on neurological status assessments and neurophysiological recovery during 12 wk post transplantation.Functional improvement was not associated with the neuronal or glial differentiation of drNPCs but rather by the presence of multipotent SOX2+drNPCs.Directed drNPC migration to the areas of active host growth cone formation,including in the areas of corticospinal axons,may provide some paracrine trophic support that activate the regeneration processes.
ARTICLE HIGHLIGHTS
Research background
The research is based on two points:the discovery of direct reprogrammed neural progenitor cells (drNPCs) and the development of an evoked potential-driven model of spinal cord injury in non-human primates.
Research motivation
The key problem to be solved is the restoration of brain-spinal cord connection and functions after the complete spinal cord injury.
Research objectives
The main objective of the study was to investigate the safety and efficacy of intraspinal transplantation of drNPCs in the treatment of complete spinal cord injury on nonhuman primates.
Research methods
Experiments were conducted on non-human primates with behavioral,neurophysiological,histological,and immunohistochemical assessment.
Research results
Injections of drNPCs were accompanied by restoration of anatomically resected afferent and efferent neuronal pathways.No evidence was found that drNPCs were directly involved in the restoration of neuronal pathways.
Research conclusions
Using a primate evoked potential guided spinal cord injury model we demonstrated safety and efficacy of intraspinal injections of allogeneic drNPCs.
Research perspectives
At the next stages of research,it is necessary to increase the survival rate of transplanted cells,such as by transplanting predifferentiated tissue-engineered constructs.
ACKNOWLEDGEMENTS
The authors would like to sincerely thank the staff members of the Institute of Biomedical Problems,Svetlana Eremenko,Maria Rempel,and Dr.Yuri Vasilyevich Gordeev and the staff members of the Scientific Research Institute of Medical Primatology (Sochi),Igor N Klots and Tatyana E Gvozdik for the care of experimental animals and invaluable help in working with them.
The authors are grateful to Prof.Feodor G Zabozlaev,Inna V Isayeva and Dr.Nikolay N.Letunovsky,for their assistance in conducting pathohistological studies.
The authors are grateful to the staff members of the FRCC of FMBA of Russia,Dr.Dmitry Skvortsov,Dr.Tatyana Klypa,Dr.Alexander Shin,Dr.Sergei Kaurkin,and Natalia Nerusheva,for their help at various stages of the experiment.
The authors express their gratitude to the senior researcher of REC “Medical Nanobiotechnology” Maxim A.Abakumov for assistance with the magnetic resonance imaging in experimental non-human primates.
The authors are grateful to Dr.Vladimir Ushakov,Dr.Alexandra Capela,and Dr.Natalie Kushnir for their kind help with the manuscript preparation.
杂志排行
World Journal of Stem Cells的其它文章
- Role of induced pluripotent stem cells in diagnostic cardiology
- Multidifferentiation potential of dental-derived stem cells
- Stem cell therapy in ocular pathologies in the past 20 years
- Programmed cell death in stem cell-based therapy:Mechanisms and clinical applications
- Low complexity domains,condensates,and stem cell pluripotency
- Different kinds of stem cells in the development of SARS-CoV-2 treatments
