Programmed cell death in stem cell-based therapy:Mechanisms and clinical applications
2021-07-02XiMinHuQiZhangRuiXinZhouYanLinWuZhiXinLiDanYiZhangYiChaoYangRongHuaYangYongJunHuKunXiong
Xi-Min Hu,Qi Zhang,Rui-Xin Zhou,Yan-Lin Wu,Zhi-Xin Li,Dan-Yi Zhang,Yi-Chao Yang,Rong-Hua Yang,Yong-Jun Hu,Kun Xiong
Xi-Min Hu,Qi Zhang,Rui-Xin Zhou,Yan-Lin Wu,Zhi-Xin Li,Dan-Yi Zhang,Yi-Chao Yang,Kun Xiong,Department of Anatomy and Neurobiology,School of Basic Medical Sciences,Central South University,Changsha 410013,Hunan Province,China
Xi-Min Hu,Department of Dermatology,Xiangya Hospital,Central South University,Changsha 410013,Hunan Province,China
Rong-Hua Yang,Department of Burns,Fo Shan Hospital of Sun Yat-Sen University,Foshan 528000,Guangdong Province,China
Yong-Jun Hu,Department of Cardiovascular Medicine,Hunan People's Hospital (the First Affiliated Hospital of Hunan Normal University,Changsha 410005,Hunan Province,China
Abstract Stem cell-based therapy raises hopes for a better approach to promoting tissue repair and functional recovery.However,transplanted stem cells show a high death percentage,creating challenges to successful transplantation and prognosis.Thus,it is necessary to investigate the mechanisms underlying stem cell death,such as apoptotic cascade activation,excessive autophagy,inflammatory response,reactive oxygen species,excitotoxicity,and ischemia/hypoxia.Targeting the molecular pathways involved may be an efficient strategy to enhance stem cell viability and maximize transplantation success.Notably,a more complex network of cell death receives more attention than one crucial pathway in determining stem cell fate,highlighting the challenges in exploring mechanisms and therapeutic targets.In this review,we focus on programmed cell death in transplanted stem cells.We also discuss some promising strategies and challenges in promoting survival for further study.
Key Words:Programmed cell death;Apoptosis;Autophagy;Stem cell;Therapeutic strategies
INTRODUCTION
Cell-based therapies have raised tremendous expectations and presented favorable curative effects in repairing damaged tissue and enhancing functional repair[1-3].Stem cells (SCs) could serve as a cellular reservoir to maintain,produce,repair,and even regenerate multiple tissues with the characteristic properties of self-renewal and differentiation.Thus,SCs are developed as the preferred sources for cell-based therapies due to their ability to differentiate into a wide range of cell types and their capacity of secretion regulated by the microenvironment,also termed the “niche”[4].Based on the stage of development,SCs can be divided into three types:Embryonic SCs (ESCs),induced pluripotent SCs (IPSCs),and adult SCs (ASCs)[5].ESCs are derived from the inner cell mass of a blastocyst[6].There are ethical limitations to the use of ESCs in therapy[7].Compared with ESCs,IPSCs derived from mature body cells could be regulated to dedifferentiate into pluripotent SCs as a renewable source of alternative cells and tissues[8].ASCs or somatic SCs (SSCs) can be found in various adult tissues,including neural SCs (NSCs),hematopoietic SCs (HSCs),mesenchymal SCs (MSCs),and epidermal SCs.Many trials have shown that ASCs can be used to treat diseases[9,10].For example,bone marrow mononuclear cells[11],NSCs[12],and MSCs[13] are usually used to treat stroke.
SCs-based therapies are widely used in the treatment of various diseases[14-18].Limbal stem cell therapy is used for treating burn-related corneal destruction[19],NSCs in gastrointestinal tract disorders[20],bone marrow-derived mesenchymal SCs(BM-MSCs) in diabetic cardiomyopathy[21],and MSCs in multiple sclerosis[22] and several clinical conditions.However,SC-based therapies also have limitations.Impaired cell homing regulatedviavarious factors (such as chemokines) causesin situtissue regeneration failure[23].Also,a high death rate of transplanted SCs limits the therapies[24,25].After MSC injection,over 99% of injected cells die in the left ventricular myocardium within 4 d[26].
Accumulated evidence shows a close tie between multiple types of programmed cell death (PCD) and SCs,including apoptosis,autophagy,ferroptosis,pyroptosis,and necroptosis.Studies demonstrate that p53 induces apoptosis of human ESCs (hESCs)through a mitochondrial pathway shown to be extremely sensitive to FasL-induced cell death in MSCs[27,28].Ohgushiet al[29] observed that Rho-associated coiled-coilcontaining protein kinase (ROCK)-dependent hyperactivation of myosin directly caused dissociation-induced apoptosis in hESCs and immediate activation of the Rho/ROCK/MLC2 signaling cascade.In 2010,the María group found that inhibitors of apoptosis proteins (IAPs) could promote the numbers of hematopoietic stem and progenitor cells and improve resistance to cell death[30].Moreover,reports suggest that high levels of pro-apoptotic B-cell lymphoma 2 (Bcl-2) family members were overexpressed in hESCs[31].Autophagy in SCs traces its history to 1980 where marrow cells revealed several abnormalities within an intrinsic myeloid precursor cell defect[32].Lately,the role of autophagy in SC fate and aging is drawing attention due to the ability of the autophagy activator rapamycin to restore the biological properties of aged SCs by increasing their differentiation and proliferation capacity and decreasing adipogenic differentiation capacity,including the molecular mechanisms targeting 5′ AMP-activated protein kinase (AMPK) and rapamycin (mTOR)[33,34].Research on necroptosis in SCs started relatively late but progressed rapidly to show that tumor necrosis factor α (TNF-α) could act on HSCs and progenitors for facilitating hematopoietic clearance and promoting regeneration.Furthermore,pharmaceutical inhibition of receptor-interacting protein kinase-3 (RIP3) showed a curative effect in promoting SCs,such as targeting necroptosis of intestinal SCs[35].Some other cell death-related molecules have been increasingly recognized in SCs,such as the PI3K/AKT signaling pathway[36],MAP kinases ERK[37],JNK,and p38[38].
Some methods have been used to control programmed cell death in SCs.The concept of preconditioning was proposed by Charles E.Murry in 1986[39].Presently,several strategies,such as using heat shock,free radical scavengers,over-expressing anti-apoptotic proteins,anti-inflammatory therapy,and co-delivery of extracellular matrix molecules,have been introduced[40-45].Besides genetic strategies,threedimensional culture technology and co-transplantation are novel ideas to enhance SCbased therapies.
Exploring cell death mechanisms in SCs and targeting these potential therapeutic molecules are vital to successful SC-based therapies (shown in Table 1[19-21,46-92]).In this review,we highlight the conditions or reasons leading to cell death in SC-based therapeutic approaches.Also,we demonstrate the cell death mechanism in SCs,which may provide a novel,efficient,reliable,and potential strategy in promoting SC-based therapy.
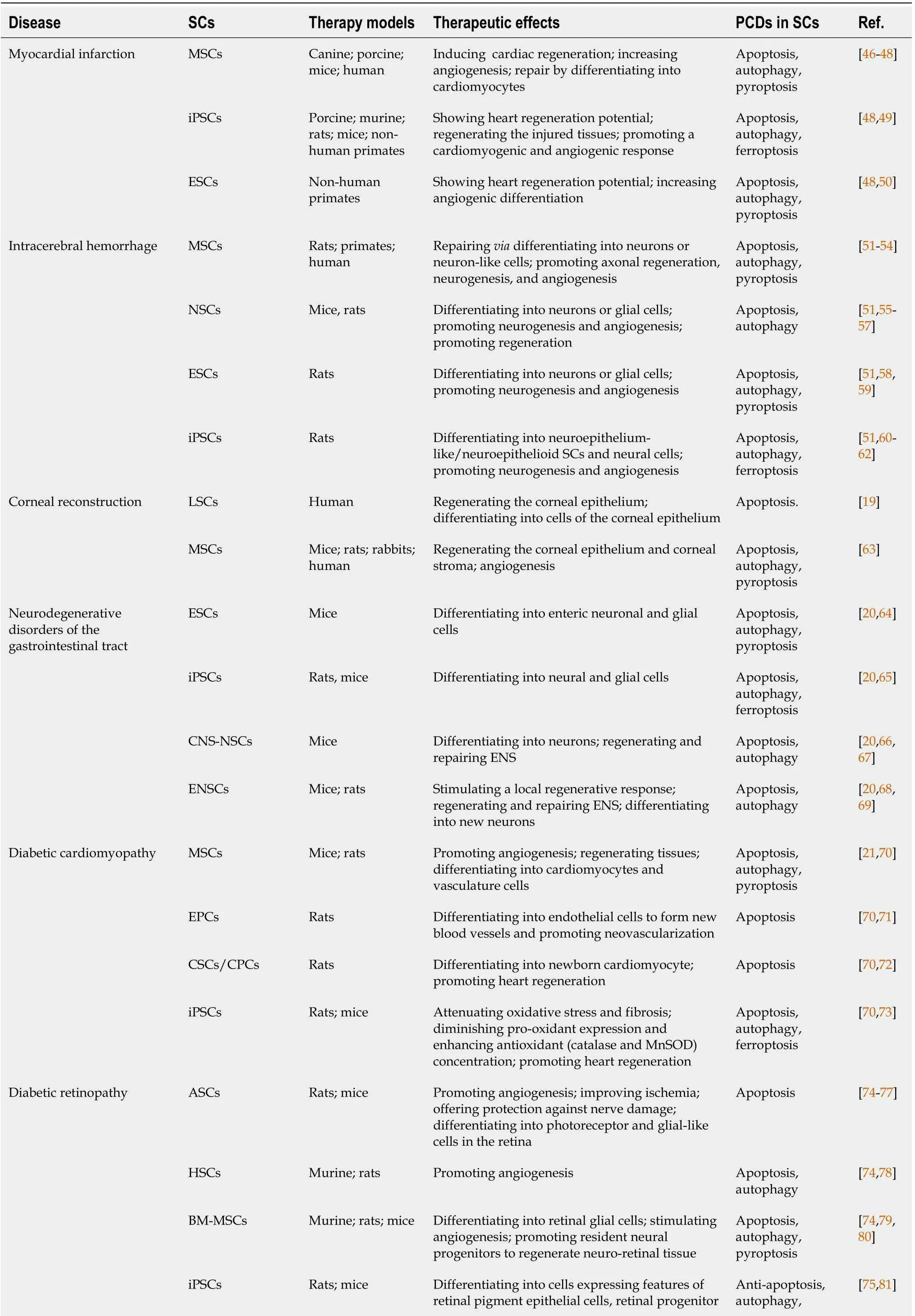
Table 1 Summary of programmed cell deaths in stem cell-based therapy
SC:Stem cell;MSCs:Mesenchymal stem cells;NSCs:Neural stem cells;ESCs:Embryonic stem cells;iPSCs:Induced pluripotent stem cells;LSCs:Limbal stem cells;CNS-NSCs:CNS-derived NSCs;ENSCs:Enteric neural stem cells;CSCs/CPC:Cardiac stem/progenitor cells;ASCs:Adipose stem cells;HSCs:Hematopoietic stem cells;BM-MSCs:Bone marrow derived mesenchymal stem cells;ENS:Enteric nervous system;EPCs:Endothelial progenitor cells;PCD:Programmed cell death.
A QUICK LOOK AT PCD
According to the death inducers,cell morphologic changes,and molecular mechanisms,cell death can be divided into two types:Non-programmed cell death caused by an external injury leading to instantaneous and irreversible cell damage[93,94],and PCD (e.g.,apoptosis,autophagy,necroptosis,and pyroptosis),a common occurrence in the development of organisms without strong immune responses[95].
PCD occurs extensively during the development of pathology in various tissues.It is closely related to the therapeutic efficacy and prognosis of SC-based treatment.Robeyet al[25] indicated that most cell death occurs in the first week posttransplantation.In NSC transplantation for neurological disorders in the brain,less than 4%-10% of primary NSCs survived within the first few days[96].Similarly,Yasuda and Hayashi’s groups showed that 15% of transplanted cells survived at 1 wk and 9% at 4 wk in a rat infarction model[97].A significantly high death rate occurred,and over 99% of MSCs died within 4 d after transplantation into the left ventricular myocardium of mice[26].Thus,cell death may be a significant concern that needs attention.
Apoptosis
Apoptosis is the classic form of PCD without spillage of contents into the surrounding environment[98].Apoptosis plays an important role in the orderly and efficient removal of damaged SCs to prevent cancer through two classical apoptotic pathways:The intrinsic pathway and the extrinsic pathway[99].The intrinsic pathway,also called the mitochondrial pathway,shows a close relation with SCs[100,101].It is closely regulated by a group of cytokines,especially the Bcl-2 family[102,103].The extrinsic pathway is triggered by ligand-receptor binding.TNF-family receptors and cysteineaspartic proteases,known as caspases,play a vital role in the extrinsic pathway[104].
Autophagy
Autophagy is a eukaryotic cell recycling process involving the degradation of cytoplasmic organelles,proteins,and macromolecules with the recycling of decomposition productsviathe mTOR/Ras-cAMP-PKA axis to maintain cellular homeostasis and enhance stem cell survival[105].Autophagy is divided into three major types:Microautophagy,macroautophagy,and chaperone-mediated autophagy(CMA)[106].During microautophagy,cargos are captured by lysosomal membrane invaginations or protrusions[107].In macroautophagy,autophagosomes are regarded as typical signatures[108].CMA focuses on molecular chaperones to identify cargo proteins containing specific pentapeptide sequences without using membrane structures to isolate cargo[109].
Necroptosis
Necroptosis is a pro-inflammatory lytic form of PCD.Necroptosis could be induced through several innate immune signaling pathways triggered by stimulating RIG-Ilike receptors,TLRs,and death receptors[110,111].Receptor-interacting serinethreonine kinases 1 and 3 (RIPK1 and 3) are phosphorylated and activated through these signaling pathways[112].Subsequently,mixed lineage kinase domain-like(MLKL) could be activated[113].
Others
Pyroptosis,dependent on multiple molecules,such as caspase-1 and caspase-11,is widely believed to play an important role in resisting the invasion of pathogens[114].Ferroptosis,an iron-dependent form of regulated cell death (RCD),is induced through an excessive accumulation (e.g.,ROS and lipid peroxidation products) characterized by mitochondria shrinkage or dysmorphic small mitochondria[115,116].Moreover,other types of cell death are also crucial during a series of events,such as failures in SCbased therapies.The biological correlations between the different PCD pathways are complex,where it is especially significant as a network among these pathways regarding PCD of transplanted SCs[117,118].
PCD AND ITS KEY MOLECULES IN STEM CELLS FOR TRANSPLANTATION THERAPY
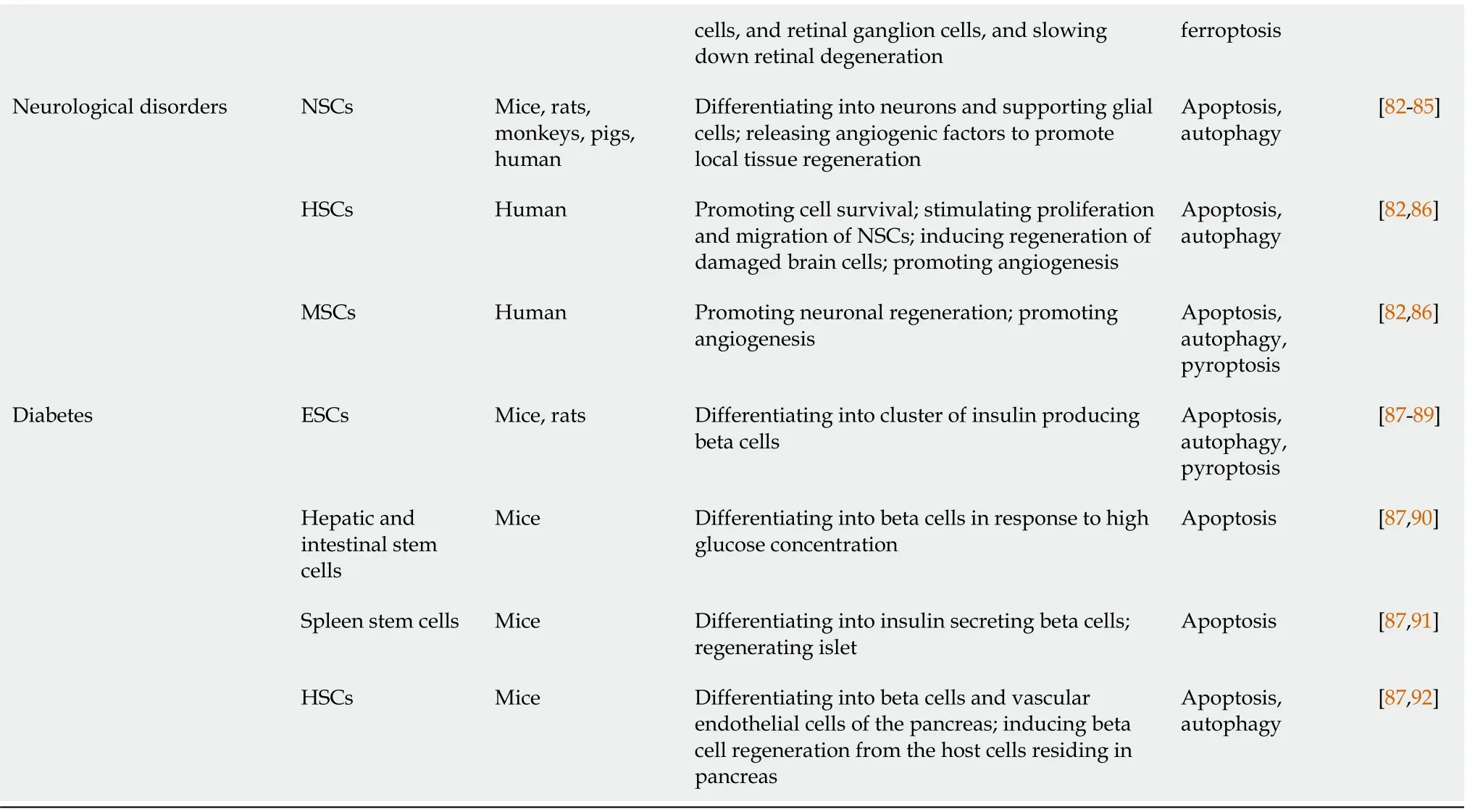
PCD of SCs is usually caused by a hostile pathological environment created due to multiple conditions,including apoptotic cascade activation,excessive autophagy,inflammatory response,ROS,excitotoxicity,and ischemia/hypoxia[39].This section systematically reviews the molecular mechanisms involved in cell death pathways and we also summarize these key molecules in Table 2[35,38,119-134].
Table 2 Molecular mechanisms and therapeutic targets of programmed cell deaths in stem cells
Apoptosis
Recently,an emerging body of evidence has highlighted a vital role of the apoptosis effect on several cell types,including SCs[135].Hence,it is crucial to investigate and understand the mechanisms underlying apoptosis for analysis of SC transplantation and the development of drugs targeting specific apoptotic molecules.According to the inducing signaling,apoptosis could be divided into two types:Intrinsic pathway initiated by intracellular stresses (shown in Figure 1),and extrinsic pathway responding to extracellular cues (shown in Figure 2).
The intrinsic pathway of apoptosis:In the intrinsic pathway,the initiators (e.g.,ROS and radiation induced DNA damage) cause various cascade reactions resulting in the release of cytochrome C (cyt C),p53,and mitochondrial outer membrane permeabilization (MOMP).For example,hematopoietic stem and progenitor cells (HSPCs) are used for treating acquired and primary immunodeficiencies,thalassemia,and sickle cell disease.However,the presence of intrinsic apoptosis is shown in HSPC-based therapy in which excess DNA damage can trigger cumulative p53 pathway,constraining proliferation,yield,and engraftment of HSPCs,while moderate damage can lead to reversible function impairment by transient p53 inhibition[136].According to the downstream activators of p53,two main pathways could be described:BH3-only activator (Way I shown in the left part of Figure 1) and active BAX from the Golgi(Way II shown in the right part of Figure 1) to the mitochondria.
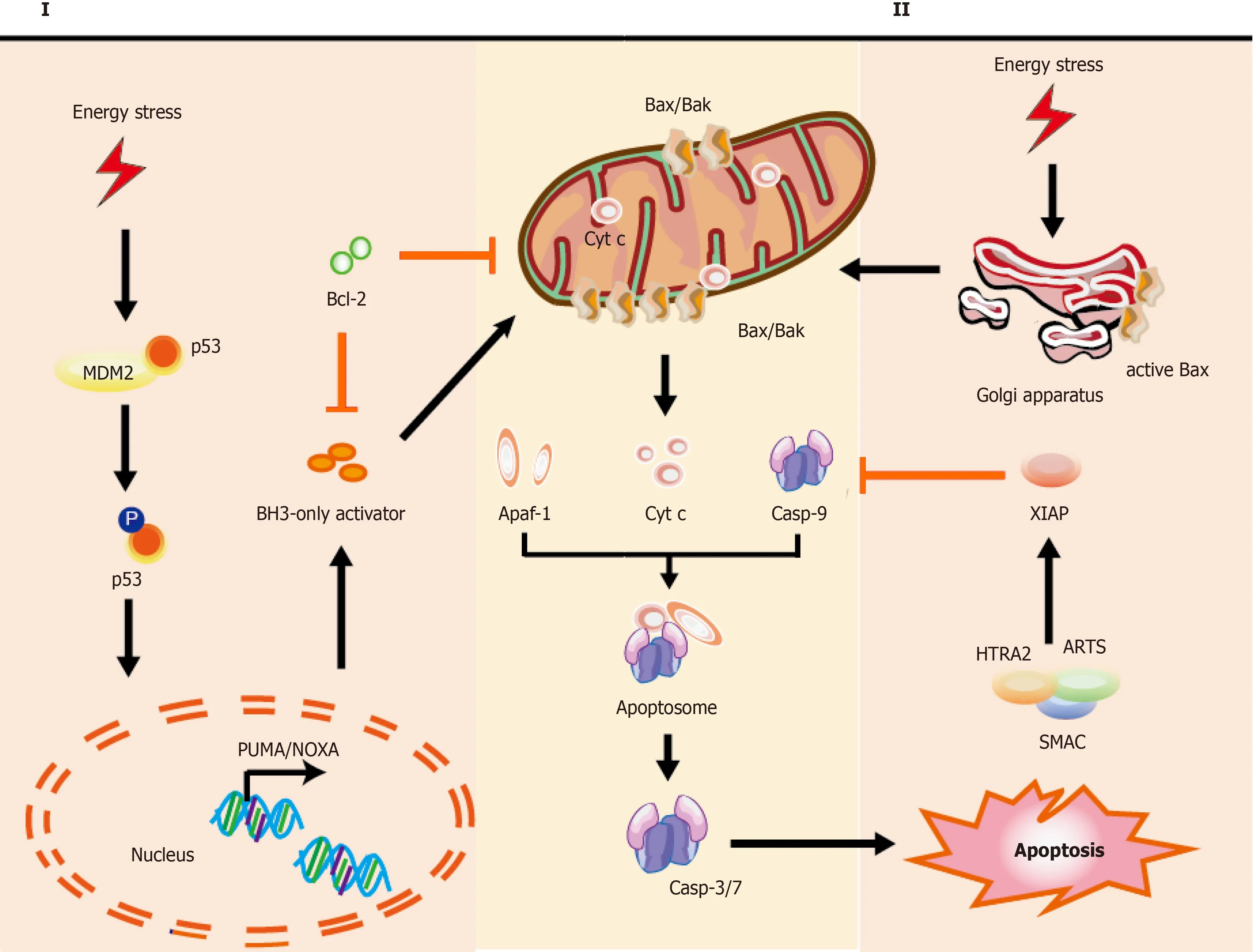
Figure 1 Mechanisms of intrinsic apoptotic pathways in stem cells.
Part I during the intrinsic pathway:During the intrinsic pathways,DNA damage,as a significant inducer,can stabilize and activate p53 by phosphorylation (for example,the phosphorylation of p53 at Ser46 can induce the p53-dependent apoptotic pathway caused by DNA damage[137]),leading to p53 nuclear translocation[119].Subsequently,p53 exerts an impact on transcription of apoptotic proteins (namely,the related proteins)viaDNA-binding activity and its transcriptional activity,such as the pro-apoptotic proteins p53 upregulated modulator of apoptosis (PUMA),NOXA (the pro-apoptotic BH3-only proteins,also known as PMAIP1 [phorbol-12-myristate-13-acetate-induced protein 1]),and apoptosis regulator Bcl-2 associated X protein (Bax)[138,139].
PUMA and NOXA can bind and activate Bax and Bcl-2 antagonist/killer-1 protein(Bak) in the cytoplasm,resulting in MOMP and release of cyt C[140].Further,p53 can directly interact with Bax and Bak to modulate MOMP[141,142].Of note,in the absence of cellular stress,p53 could rapidly produce and degrade in human pluripotent SCs (hPSCs),and the stabilization of p53 occurred upon DNA damage orviainhibition of MDM2 (the E3 ubiquitin ligase mouse double minute 2 homolog,which maintains low p53 levels through triggering p53 degradation)[143,144].Interestingly,the activation of p53 is also involved in other types of cell death,such as ferroptosis[134].
Part II during the intrinsic pathway:Typically,Bax is monomeric in the cytoplasm.Studies show that active Bax localized to the Golgi held away from the mitochondrion in some hPSC lines,whereas active BAX could transform the mitochondria after cell stress as DNA damageviaa rapid p53-dependent pathway during apoptosis[145].Once instigated with the apoptotic signals,Bax could undergo dimerization and transfer to the outer membrane of mitochondria,leading to the alteration of MOMP[146],so that relevant proteins (such as cyt C) were released into the cytosol usually confined in the intermembrane space[147].The released cyt C is involved in apoptosome formationviabinding to the cytosolic apoptosis protease activating factor-1 (Apaf-1)[148].This complex recruits and activates initiator pro-caspase-9,and then act-caspase-9 activates downstream executor caspases-3/-6/-7,leading to apoptotic cell death[148,149].In the cytoplasm,the inhibitor of apoptosis (IAP) antagonists could bind and suppress XIAP (X-linked inhibitor of apoptosis,E3 ubiquitin-protein ligase),causing the activation of caspase-9 for the apoptotic pathway[121].These IAP antagonists include second mitochondria-derived activator of caspase (SMAC),apoptosis-related protein in the transforming growth factor-β signaling pathway(ARTS),and mitochondrial serine protease high-temperature-required protein A2(HTRA2)[121,148].Korenet al[121] found highly expressed ARTS in cells comprising the intestinal SC niche,which protects Paneth cells from undergoing apoptosis.
The extrinsic pathway of apoptosis:The extrinsic apoptotic pathway is also known as the death receptor-dependent pathway inducedviathe connection between death receptors exposed on the cell surface (one of the numbers in the tumor necrosis factor receptor (TNFR) family) and the specific TNF family ligands mentioned above[150].Previous research reported the effect of TNFα on the development of human hematopoietic progenitorsin vitrowithin the role of inhibition[151] or promotion[152].These TNFα-driven mechanisms play a vital role in HSC response to inflammatory stress for removing damaged cells and activating SCs[153].Recently,HSC transplantation for malignancy has shown anti-tumor activityviaTNFα-driven pathways[153,154].Death receptors and their ligands cause a conformational change,which leads to the recruitment of Fas-associated death domain (FADD)[155] and allows interactions between FADD and caspase-8 and/or the caspase-10,resulting in the cleavage and activation of caspase-3 and caspase-7 through interactions between their death domain (DD)[156].Finally,the active and cleaved caspase-3 induces changes in phosphatidylserine exposure,DNA fragmentation,and the formation of apoptotic bodies.However,reports suggest that caspase-3 activity could be elevated in nonapoptotic pathways in neural SCs[157].
Remarkably,caspase-8 can target the BH3-only protein Bid (BH3-interacting domain death agonist) and cleave Bid to a truncated fragment t-Bid[158].Capperet al[159] and Jiaet al[160] showed that decreased Bid could inhibit apoptosis,promote proliferation,and delay senescence in human periodontal ligament SCs (h-PDLSCs)viaactivated Yes-associated protein,and low levels of caspase-8 were detected in stem cell features through hypermethylation.Subsequently,t-Bid could directly translocate to the outer mitochondrial membrane after activating apoptotic regulator Bax and inhibiting Bcl-2,leading to co-engages between the intrinsic apoptotic pathway and the extrinsic apoptotic pathway[158].Some evidence shows that activation of the extrinsic pathway and inhibition of caspase-8 can induce necroptosis[161,162].
Emerging findings indicate that Bcl-2 family proteins play a vital role in SCs (e.g.,overexpression of Bcl-2 in MSCs[163],ESCs[164],and neuroepithelial SCs (NESCs)[165] improved their survival).The three functional groups Bak and Bax,BH3-only proteins,and Bcl-2 maintain a balance between SC survival and death.For example,high levels of Bcl-2 were measured in HFSCs for antiapoptosis in contrast to differentiated cells[166,167].In the SCs,Bax performs as an activated conformation sequestered in the Golgi apparatus held away from the mitochondrion.Following stresses such as DNA damage,active Bax translocates to the mitochondrial outer membrane to initiate MOMP and the apoptotic cascade,which bypasses the conventional intrinsic and extrinsic apoptotic pathways[168,169].However,the mechanism underlying the localization of active Bax at the Golgi and active Bax-induced pore formation in the Golgi stacks is unclear.
Autophagy
As a self-protective catabolic mechanism within the cells,autophagy exerts a key influence in sustaining SC homeostasis by maintaining stemness,upregulating quiescence,managing differentiationviaremodeling,and self-renewalviametabolic reprogramming[170-173].Autophagy contributes to metabolic regulation through increased glycolysis to generate ATP in the hypoxic milieu for balancing SC fate[174,175].For example,autophagy plays a vital role in maintaining the quiescence of SCs (e.g.,HSCs and muscle SCs (MuSCs))viarejuvenating aged quiescent SCs controlled by various autophagy pathways such as the p38/mitogen-activated protein kinase(MAPK) signaling pathway[176,177].Uncovering the autophagy mechanisms underlying SC quiescence presents novel therapeutic strategies to release the cells out of the quiescent state,promoting their proliferation and differentiation (such as induced activation of quiescent NSCs for neuron injury),or re-establishing quiescence to prevent aberrant proliferation and differentiation or premature senescence (such as anti-cancer therapeutics),which carry the risk of cancer SCs (CSCs)[178,179].These stressors (e.g.,starvation,oxidative stress,infection,and hypoxia) stimulate the cascade of autophagy as follows (shown in Figure 3)[180].
During autophagy,the formation of multi-protein complexes is associated with morphologic changes (shown in Figure 3).Initiation of autophagy is controlled by nutrient sensors,namely,mTOR and AMPK[173,181].Typically,the mTORC1 complex functions as an inhibitor for autophagy.Under environmental stresses and physiological stressors,AMPK is activated to inhibit the activity of mTORC1,leading to a release of the ULK1 (Unc-51-like kinase complex,also known as ATG1) complex to induce autophagy,which is usually inhibited by mTORC1[182].This initiation process is known as the phagophore assembly site (PAS) formation,which is regarded as indispensable for nucleation in the next stage.Compared with somatic mouse embryonic fibroblasts,whole-cell extracts of iPSCs and ESCs express high levels of AMPK and phosphorylated AMPK[183].Interestingly,AMPK inhibition in mouse bone marrow-derived MSCs can upregulate both autophagy and apoptosis in hypoxia and serum deprivation conditions,suggesting crosstalk between autophagy and apoptosis through AMPK-ULK1 pathways[184,185].Mutations in mTOR lead to smaller brains in mouse cortical development,and fewer proliferating neural progenitors result from disruption of NSC self-renewal[181].
Next,PI3 is phosphorylated to PI3Pviathe class III PI3-kinase-Beclin1 complex formed by core subunits of Beclin1 (Atg6),Atg14 L,and Vps34-Vps15,resulting in autophagosome formation[186,187].The Atg12-Atg5-Atg16L1 complex acts as a regulator for enveloping and translocating the cytoplasmic cargo to the lysosome within misfolded-protein degradation[188].Atg4 can cleave LC3 (Atg8) to generate cytosolic LC3-I.Atg3 (E2 enzymes) and Atg7 (E1-like enzymes) can lead the conjugation of PE to LC3-I to form lipidated LC3-II,which is combined with the autophagosome membrane to complete and elongate autophagosome formation[189].Finally,the autophagosome contents undergo degradation due to low lysosomal pH.Some evidence demonstrates that autophagy plays an important role in reprogramming to form iPSCs,while iPSCs colony formation shows reprogramming failure due to the lack of Atg3,Atg5,or Atg7[190,191].Autophagy is necessary for SC survival and sustenance.It is critical for SC differentiation in which co-localized dots of Tuj1-positive and GFP-LC3-positive cells are monitored and progress increasingly during NSC differentiation[192].
In microautophagy,misfolded or/and toxic proteins can be directly engulfed by the lysosomal membrane and degraded in the lysosome[193].During chaperone-mediated autophagy,the heat shock cognate 70 kDa protein (HSC70) chaperones attach to the pentapeptide motif KFERQ (namely Lys-Phe-Glu-Arg-Gln) for delivery to lysosomesviaa specific receptor LAMP2A.Reports suggest that targeting peptide HSC70 during autophagy can dramatically decrease amyloid-β (Aβ) oligomers in iPSCs with superior neuroprotective activity[194].However,the molecular mechanism between autophagy and SCs is still unclear and remains to be further explored.
Apart from these vital targets,key transcription factors are closely linked to the stem cell state and the occurrence of autophagy (shown in Figure 3).For example,FOXO3A can enhance autophagosome formationviaautophagy gene expression in hematopoietic SCs and breast cancer stem-like cells,which is needed to mitigate an energy crisis and allow cell survival[182,195].Moreover,an elevated level of SOX2 is detected in NSCs,which is important for self-renewal;downregulation of SOX2 is observed in differentiated neurons and glia[196].Besides SOX2,other transcription factors such as STAT3,OCT4,KLF4,and c-Myc are also vital for reprogramming in the initial creation of iPSCs at the genetic level[197].
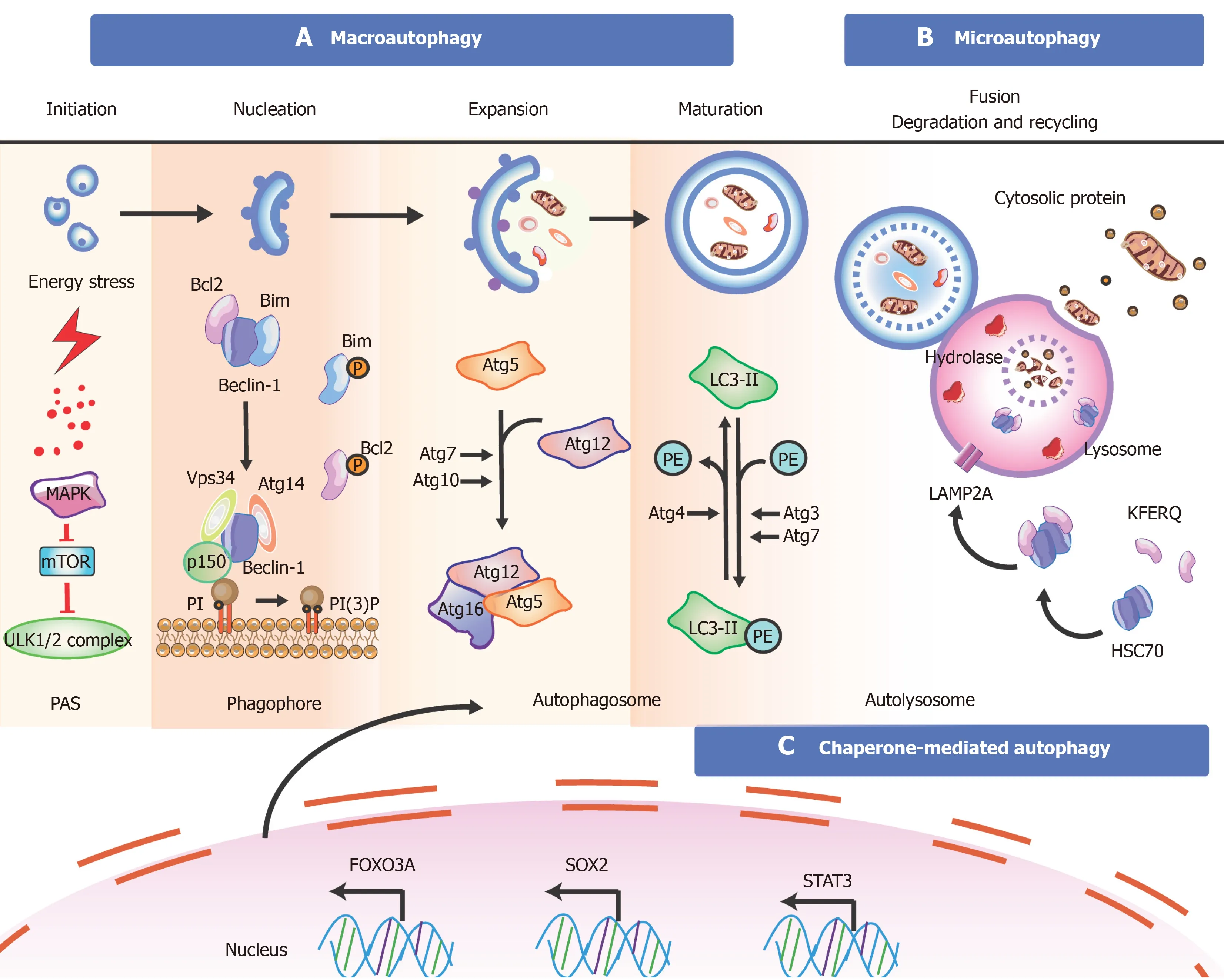
Figure 3 Overview of the mechanisms during autophagy in stem cells.
Necroptosis
The occurrence of necroptosis in SCs has recently been reported.Wanget al[35] found that gut stem cell necroptosis resulting from genome instability triggered bowel inflammation.Moreover,TNF-α could promote the survival and myeloid differentiation of HSCviaactivating a strong and specific p65-nuclear factor κB (NF-κB)-dependent gene program that prevents necroptosis rather than apoptosis to poise HSCs for myeloid cell production[153].
Others
In addition to apoptosis and autophagy (mentioned above),reports on other cell death types have led to studies exploring cell death mechanisms,such as ferroptosis and pyroptosis[35,132,198-203].Notably,different cell death mechanisms can simultaneously occur in disease (termed as ‘PANoptosis’),suggesting a complex but practical integrated network between various cell death mechanisms in SCs[204,205].
Ferroptosis had been observed in SCs with an imbalance of iron homeostasis,a significant upregulation of cytosolic free iron content,and DNA/protein/lipid oxidative damage,leading to an obvious senescence phenotype and spontaneous death in iPSC-derived neuronal precursor cells (NPCs)[134,206].iPSCs and genecorrection are used for treating Pelizaeus-Merzbacher disease (PMD) but subsequently undergo cell death after the pre-myelinating stage with evidence for caspase-3-dependent apoptosis in approximately 40% of cells and ferroptosis[205].Thus,iron chelators and lipophilic antioxidants can lead to downregulation of apoptosis and ferroptosis[205].Further,transfusional iron overload (IOL) may have clinical importance as a character close to transplant-related mortality in hematopoietic stem cell transplantation (SCT) for hematologic malignancies (HM)[198].
For pyroptosis (TLR4-NLRP3-mediated cell death pathway),a large body of evidence shows that stem cell transplantation can function as an inhibitor for pyroptosis,suggesting a novel approach called stem cell-derived exosome treatment[207,208],and numerous molecular pathways,such as exosome/LncRNA KLF3-AS1/miR-138-5p/Sirt1 axis and exosome/circHIPK3/FOXO3a axis,are presented[132,133,209].
All kinds of RCDs contribute to making a constant effort to maintain a homoeostatic balance,in which it is especially significant for the therapeutic effects of SC-based therapy.As for apoptosis in SCs,the intrinsic and extrinsic pathways play a synergistic role in ensuring the multi-cellular organisms to keep normal cells,and remove abnormally proliferating cells or other defective cells.Failure to regulate apoptosis would lead to the uncontrolled growth and division of cells during pathological process.In this regard,whether the SCs that we utilized in transplantation would be uncontrolled someday is also a potential challenge.Compared with apoptosis,autophagy could be regarded as a source of energy through digestion of cellular structures and/or organelles against multiple stresses such as nutrient deprivation(caloric restriction).These two main RCD pathways are widely studied and also some novel ways such as active-Bax in Golgi to inducing apoptosis will be further dug out.Remarkably,Bcl-2 as a co-regulator during these two pathways might be a potential target not only for apoptosis but also for autophagy.Others RCDs such as neroptosis,pyroptosis,and ferroptosis are also found in transplanted SCs,but their detail signaling and application need to keep digging.All in all,various cell death mechanisms are under investigation (apart from the cell death types described).Notably,it is necessary to focus on the overall network between different molecular cell death pathways.
STRATEGIES TO PROMOTE STEM CELL SURVIVAL FOR TRANSPLANTATION THERAPY
As mentioned above,the microenvironment exerts a vital role in the survival of SCs.Many studies have contributed to providing a wide range of strategies to enhance stem cell transplantation therapyviaimproving the microenvironment,including preconditioning strategy (e.g.,exposure to oxidative stress,heat shock,and ischemic/hypoxic injury),pretreatment (e.g.,drug treatment,cytokines,antioxidants,nitric oxide,glucose deprivation,growth factors,miRNAs,and exosomes),genetic modification,and co-transplantation of different cell types (shown in Figure 4 and Table 3[210-228).
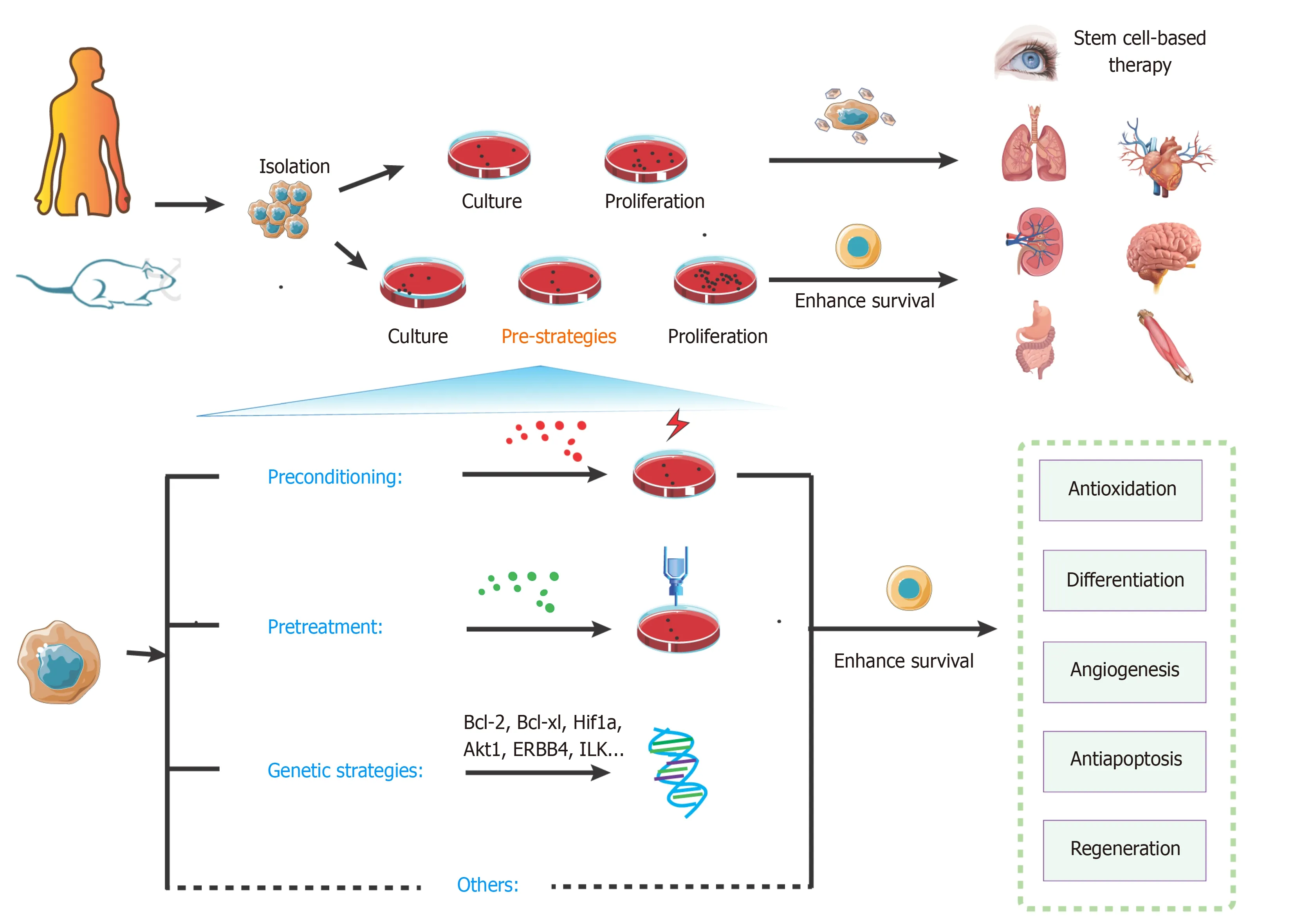
Figure 4 Overview of key strategies to enhance stem cell transplantation therapy.
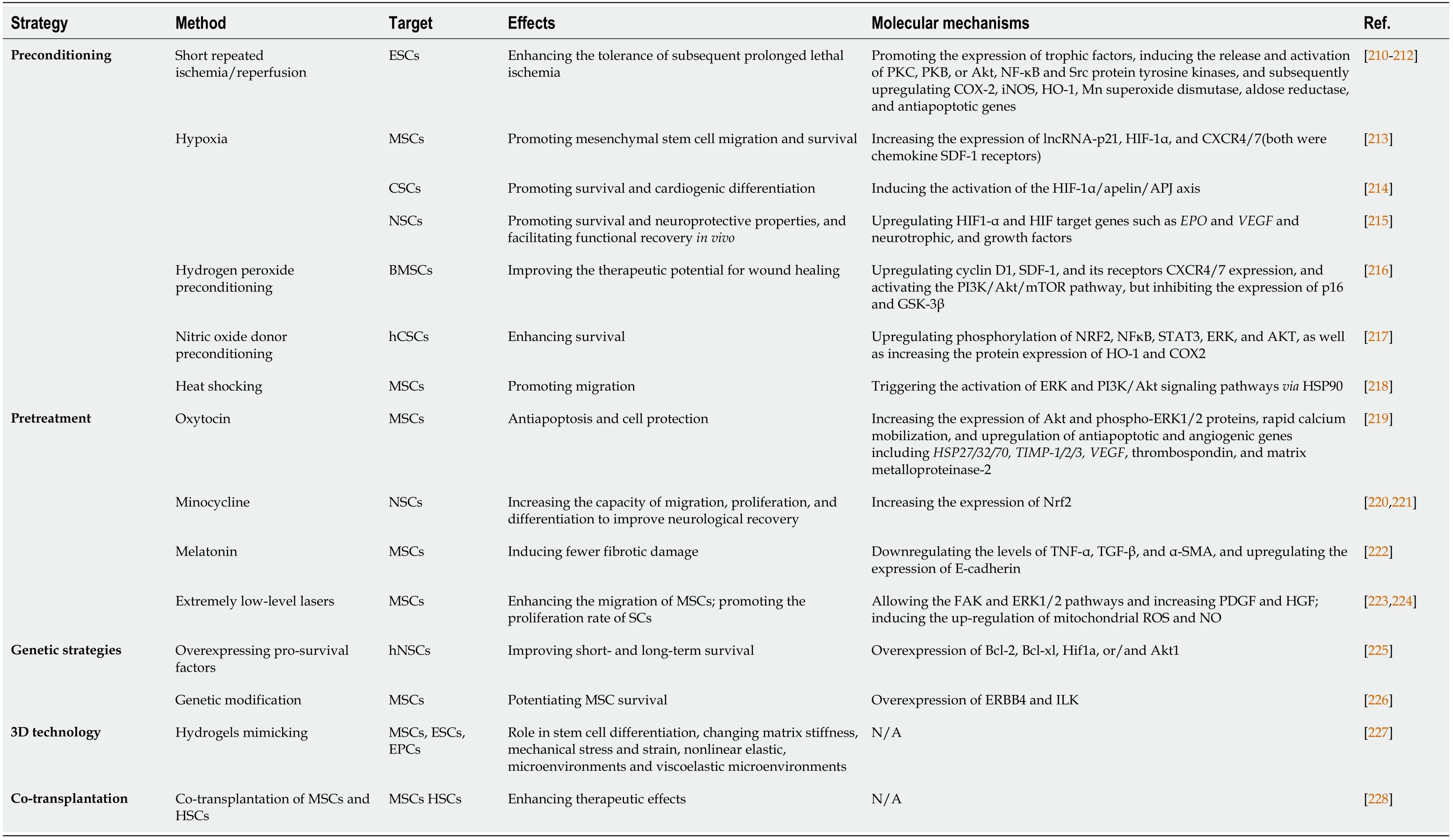
Table 3 Strategies to enhance stem cell transplantation therapy
Preconditioning strategy
Preconditioning strategies mainly help to promote tolerance of SCs and progenitor cells derived from SCs.These triggers aim to alter cell signaling and metabolism for adaptation to appropriate and mild stress conditions and sublethal insults [e.g.,ischemic preconditioning (IPC),hypoxia,anoxia,hydrogen sulfide (H2S),hydrogen dioxide (H2O2),and carbon monoxide (CO)].
In detail,IPC of SCs is considered an efficient method to promote cell survival.After a repeated short cycle of ischemic/reperfusion (I/R),some of the chemical signals (e.g.,ROS,NO,and adenosine) can release and trigger cell protectionviaa cascade of survival factors such as the activation of protein kinase C (PKC),protective protein kinase B (PKB or Akt),nuclear factor κB (NF-κB),and Src protein tyrosine kinases,and subsequent upregulation of cyclooxygenase-2 (COX-2),inducible NO synthase (iNOS),heme oxygenase-1 [HO-1],Mn superoxide dismutase,aldose reductase,and antiapoptotic genes (Bcl-xL,Mcl-1,c-FLIPS,andc-FLIPL)[210].During ischemia/hypoxia or heat shock preconditioning,the level of Hsp70 and Hsp90 is upregulated.Reports suggest that Hsp70/90 can inhibit SMAC in the myocardium to prevent activation of caspase-3/9 (pathway described above)[211,212].
Similarly,hypoxia-inducible factor (HIF-1) is upregulated during hypoxia preconditioning to inhibit tumor suppressor p53,reduce oxidative phosphorylation,upregulate VEGF receptor levels,and promote the activation of Akt to target caspases and Bcl-2 for anti-apoptosis[229,230].Recent findings reveal that OM-MSC (olfactory mucosa mesenchymal SC) with hypoxic preconditioning functions as an inhibitor for apoptosis and pyroptosis in microglial cells through activation of HIF-1αin vitro[231].Hypoxiapreconditioned SCs can also upregulate paracrine activity,and their exosomes are also considered a novel transplantation therapy.For example,MSC-derived exosomes with hypoxia preconditioning show promising potential as an effective means for optimized bone fracture healingviaexosomal miR-126 and the SPRED1/Ras/Erk signaling pathway[232].
Besides preconditioning with ischemia and hypoxia,oxidative stress and heat shocking are also the most common preconditions for SCs within a similar rationale.Chronic exposure to oxidative stress (e.g.,H2O2,H2S,and CO) produces protective effects by activating mitochondrial ROS production,resulting in ERK activation and anti-apoptotic protein expression for cell proliferation,migration,anoikis,autophagy,and survival[216,233,234].Moreover,heat shocking precondition of mesenchymal SCs can induce HSPs to activate ERK and PI3K/Akt signaling pathways,resulting in increased expression of trophic factors,proteins,and genes for cell protection[218].
Pretreatment strategy
Pretreatment is a strategy for successfully protecting transplantable SCs,using various factors before implantation,whereas preconditioning refers to providing a specific environment within sublethal insults.These factors include antioxidants,cytokines,growth factors,and drug therapy (phosphodiesterase inhibitors,glucose deprivation,pro-survival protein expression,and anti-apoptotic proteins).
To date,various drugs have been developed for the pretreatment of SCs.Pretreatment with pharmacological inhibitors can result in increased expression of survival signaling and a high Bcl-2/Bax ratio in the early phase (2 h),and activation of the JAK/STAT signaling pathway in the late phase (24 h) for cardioprotection[210].Also,Ji group has reported the protective effect of histochrome pretreatment against oxidative stress in cardiac progenitor cells (CPCs)viaupregulating Bcl-2 and Bcl-xL and downregulating Bax and H2O2-induced cleaved caspase-3[235].Moreover,shortterm incubation either with an antioxidant N-acetyl-L-cysteine (NAC) or a specific inhibitor of TNFR 1 signaling can prevent TNF-α-mediated ROS accumulation in HSCs[154].MSC pretreatment with oxytocin (OT) [10(-10) to 10(-6) M] in response to signaling events can induce Akt and phospho-Ras-dependent extracellular signalregulated kinase (ERK)1/2,rapid calcium mobilization,and upregulation of antiapoptotic and angiogenic genes,includingHSP27/32/70,tissue inhibitor of metalloproteinase (TIMP)-1/2/3,vascular endothelial growth factor,thrombospondin,and matrix metalloproteinase-2[219].Minocycline preconditioning increases Nrf2 expression and neuroprotective paracrine secretion.It promotes migration,proliferation,and differentiation of NSCs to improve neurological recovery after NSC transplantation[220,221].The molecular mechanism involves upregulation of antioxidant genes and reduced oxidative stress grafted cell death following transplantation,resulting in low-rate cell death[221].Some studies have shown the benefits of melatonin pretreatment on MSC-based therapy with a reduction in the levels of TNF-α,TGF-β,and α-SMA,and upregulation of E-cadherin expression that induces less fibrotic damage[222].
Trophic factors and cytokines are also considered effective pretreatment approaches for regulating MSC fate.For example,SC pretreatment with IL-1β can promote migration and survival of MSCs and improve function in type 2 diabetes,acute myocardial infarction,and neural disordersviaupregulating the expression of various cytokines,chemokines,and adhesion molecules [e.g.,IL-6/8/23A,TNF-α,CCL5/20,CXCL1/3/5/6/10/11,VCA-1 (vascular cell adhesion molecule 1),and ICAM-1/4(intercellular adhesion molecule 1 and 4)].IL-1β can induce phosphorylation of NF-κB,but not PI3K/AKT and ERK1/2 pathways[236].In the NSC pretreatment strategy,a series of experiments using IL-6 show that it can reprogram NSCs to tolerate hostile environmentsviaactivating STAT3 to increase the levels of superoxide dismutase 2(SOD2) for anti-apoptosis against inflammatory cytokines and oxidative stressviamitochondrial-dependent apoptotic pathways[237,238].Some other molecular targets,including Rho-associated kinase inhibition,TGF-β2 treatment,SDF-1 signaling of PI3K/Akt,and p38 MAPK inhibitionviaanti-apoptotic pathways,also enhanced SC survival during treatment[239].
Compared with chemical pretreatment methods discussed above,physical factors such as extremely low-level lasers,pulsed electromagnetic fields (PEMF),mechanical stretch,and nanochelating-based nanocomplexes (e.g.,GFc7) are also used as pretreatment methods to enhance SC-based therapy[240-243].For example,pretreatment with extremely low-level lasers improves the migration ability of MSCsviaactivation of FAK and ERK1/2 pathways and increased expression of plateletderived growth factor (PDGF) and HGF.Furthermore,it also promotes the proliferation rate of SCs by inducing the upregulation of mitochondrial ROS and NO and enhancing the expression of the S-phase proportion in MSCs[223,224].
Genetic strategy
Genetic strategies have raised hopes for better SCs-based therapy since they were introduced more than a decade ago[244,245].The core idea of this technology is to target key genes and the expression of factors related to the fate of SCs.Under different death stimuli,overexpression of various factors such as TNFR,Akt1,stromal cell-derived factor-1 (SDF-1),and hepatocyte growth factor (HGF) is beneficial for the repopulation of SCs[246].Studies on modified transplanted hNSCs show improved short- and long-term survival of transplanted hNSCsviaoverexpression of these prosurvival factors,including Bcl-2,Bcl-xl,Hif1a,or/and Akt1[225].Genetic modification for ERBB4 (erb-b2 receptor tyrosine kinase 4) and ILK overexpression could potentiate MSC survival[226].In recent years,the CRISPR/Cas9 system has been widely used for genome editing applied in genetic modification of SCs forin vivoapplications such as neural regeneration,bone regeneration,treatment of blood disorders,and cartilage tissue engineering[247].Although gene modification promises to enhance tolerance to damage "at the root," there are still formidable predictability challenges and potential long-term side effects.
Others
Recently,three-dimensional culture technologies (e.g.,MSC encapsulation technique)mimicking the physical environment to sustain the viability of SCs to induce multilineage differentiation are used to protect SCs from PCD as an innate immune system and provide favorable mediators such as cytokines and growth factors[227,248].However,the time,cost,and labor efficiency of three-dimensional technologies for SCs may be non-negligible challenges,and a combination of biocompatible materials based on simple and easy methods is needed for SC-based therapy.Moreover,cotransplantation of different cell types offers an alternative strategy to improve outcomes of SC-based treatment.Studies show promising results with cotransplantation of human fetal mesenchymal and hematopoietic SCs in type 1 diabetes,epidermal neural crest SCs (EPI-NCSC),and olfactory ensheathing cells(OEC)[228,249].However,the significance of co-transplantation for SC-based therapy is still unclear[250,251].
As described above,these pre-strategies could provide transplanted stem cell with a certain microenvironment to improve the survival.The core ideas of these methods are to upregulate the survival factors (e.g.,Bcl-2,Akt,SMAC,mTOR,SOD2,STAT3,HSC 70,ERK,and Nrf2) and downregulate the death catalyzers (e.g.,caspase,p53,TNFa,Bax,cyt C,XIAP,MAPK,and Atg) (shown in Figure 5).Bcl-2 might be regarded as a key molecule that raised tremendous expectations,which plays a vital role in both apoptotic and autophagy pathways.Given the fact that gene strategies seem to be hardly accepted in clinical trials to improve effectiveness of SC-based transplantation,preconditioning and pretreatment may provide a cost-effective and handy option.Remarkably,distinct types of transplanted cells or distinct aiming organs show noticeable differences not only in their signaling but also their response to the local area,so studies need to find a right composition as well as an effective target of any applied transplanted SC system.
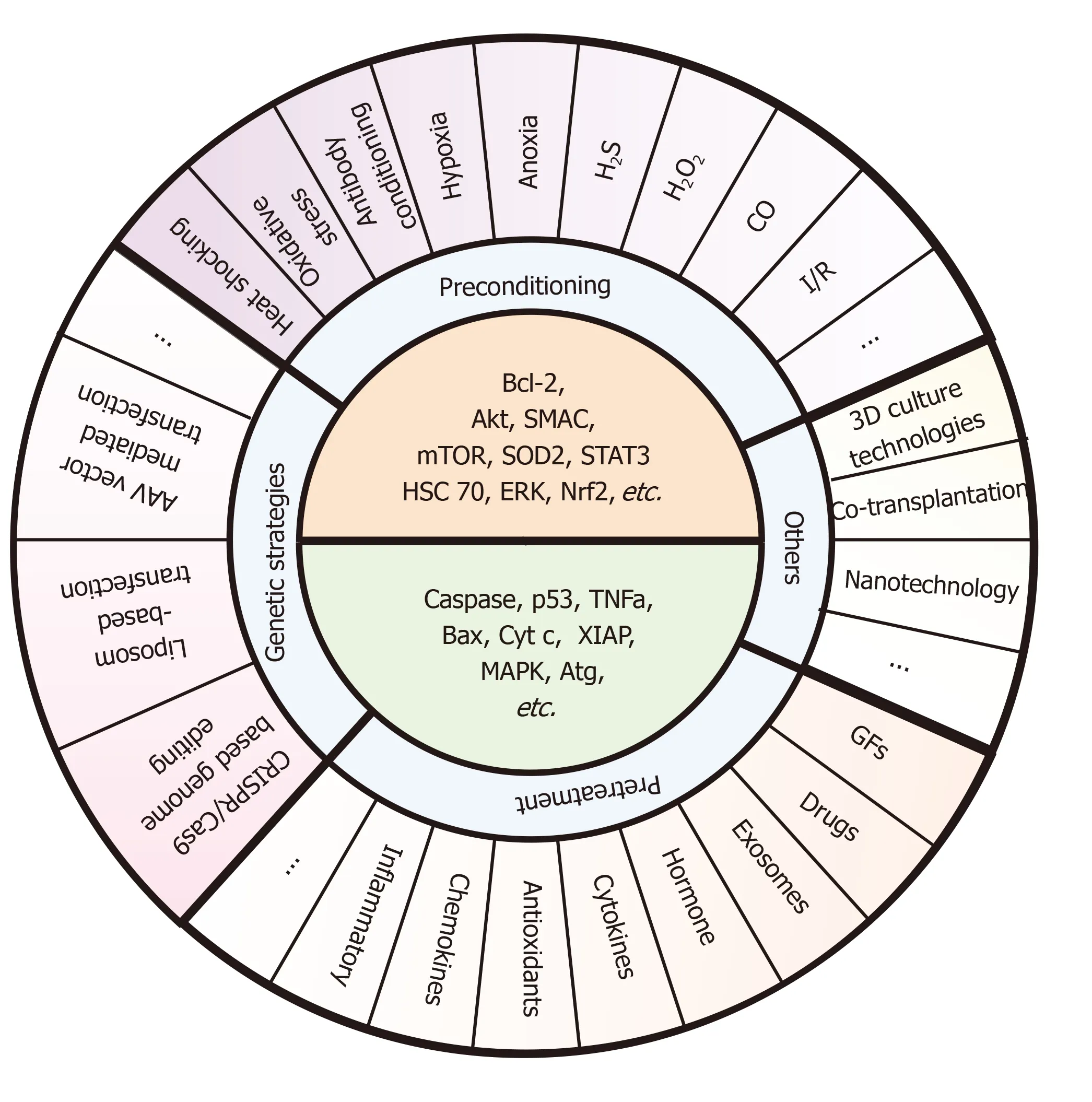
Figure 5 Specific pre-strategies and their key molecule targets for enhancing stem cell transplantation therapy.
CONCLUSION
The SC pool plays a driving role in tissue homeostasis and harm repair.Lately,SCbased therapies may be regarded as a potential strategy that raised tremendous expectations and presented favorable curative effects in enhancing functional repair and repairing damaged tissue.Given the fact that a considerable number of studies on SC-based therapy verify that RCDs occur extensively during the development of the transplanted SCs,RCDs show a crucial role in the therapeutic efficacy and progression of this treatment.Also,RCD interventions may offer opportunities for a better clinical application.
Recently,there have been tremendous strides in understanding the fate of SCs posttransplantation related to self-condition and microenvironment.Along this line,targeting multiple signal transduction pathways in PCDs and survival processes would provide novel approaches for enhancing SC-based therapies.However,the interactions are complex and involve multiple networks rather than one crucial pathway (as the recent term ‘PANoptosis’),thus necessitating further research.Moreover,various factors involved in specific pathways may change during stem cell differentiation or show microenvironmental divergence in different cell types,stages of development,and stimuli.
Several approaches can prevent the loss of a vast majority of transplanted SCs,such as preconditioning,pretreatment,and genetic strategies.Important insights into the molecular pathways that control PCD of SCs may unlock novel and potential avenues for regenerative drugs and more efficient therapy.These pre-strategies provide SCs with harsh or nutrient-rich environment to improve the SCsviaupregulating the survival factors and downregulating the death catalyzers.A summary diagram is shown in Figure 6.Recently,some of the novel technologies such as 3D culture technologies,co-transplantation,and nanotechnology also show promising prospects.Furthermore,safer use,better results,and highly feasible and beneficial methods are required for clinical applications.
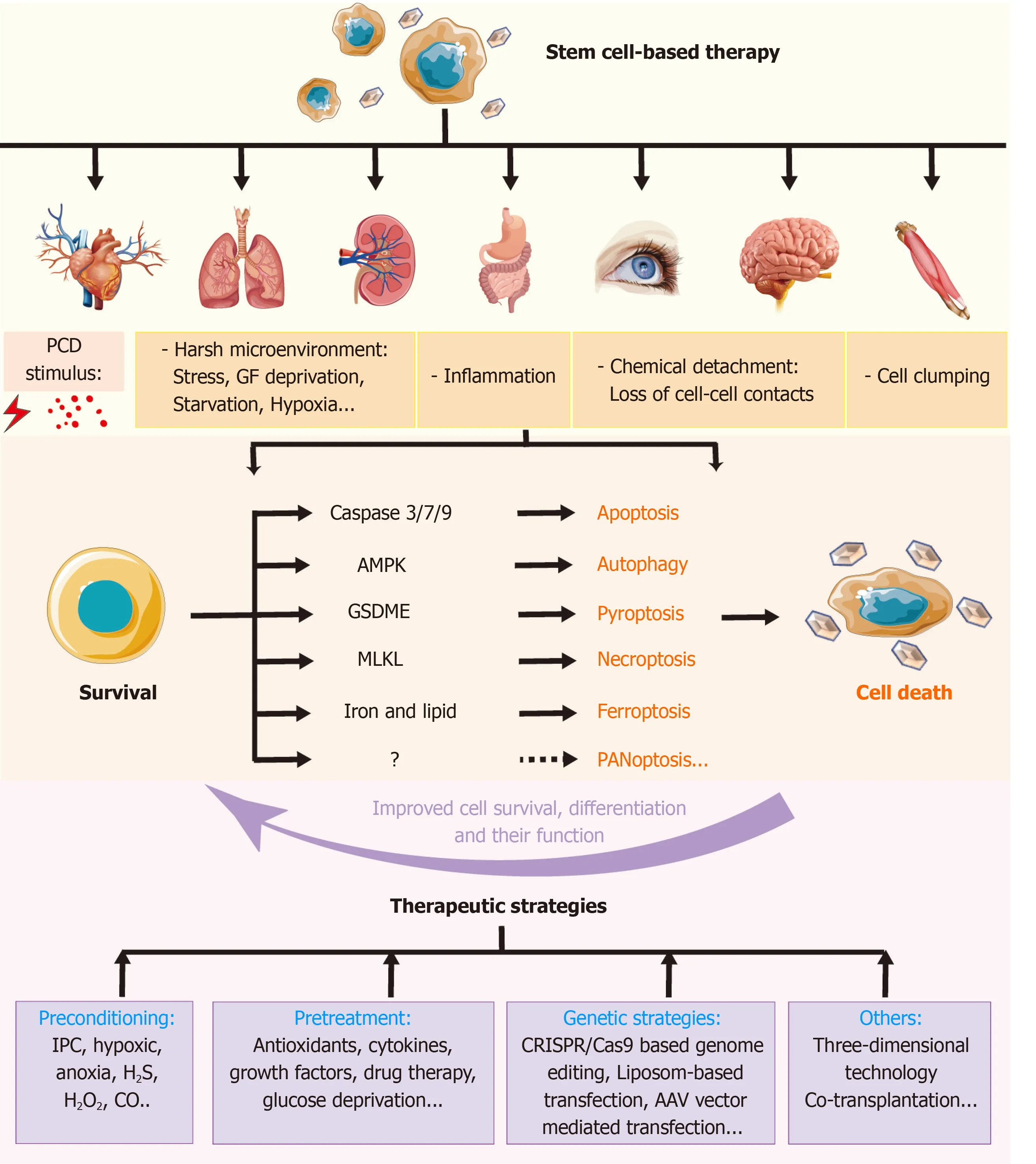
Figure 6 Role of regulated cell deaths in stem cell-based transplantation and therapeutic pre-strategies to improve the therapy.
杂志排行
World Journal of Stem Cells的其它文章
- Role of induced pluripotent stem cells in diagnostic cardiology
- Multidifferentiation potential of dental-derived stem cells
- Stem cell therapy in ocular pathologies in the past 20 years
- Low complexity domains,condensates,and stem cell pluripotency
- Different kinds of stem cells in the development of SARS-CoV-2 treatments
- Disease modifying treatment of spinal cord injury with directly reprogrammed neural precursor cells in non-human primates
