Role of induced pluripotent stem cells in diagnostic cardiology
2021-07-02StevenKarchVittorioFineschiPietroFranciaMatteoScopettiMartinaPadovanoFedericoManettiAlessandroSanturroPaolaFratiMassimoVolpe
Steven B Karch,Vittorio Fineschi,Pietro Francia,Matteo Scopetti,Martina Padovano,Federico Manetti,Alessandro Santurro,Paola Frati,Massimo Volpe
Steven B Karch,School of Medicine,University of Nevada,Las Vegas,NV 89102,United States
Vittorio Fineschi,Matteo Scopetti,Martina Padovano,Federico Manetti,Alessandro Santurro,Paola Frati,Department of Anatomical,Histological,Forensic and Orthopaedic Sciences,Sapienza University of Rome,Rome 00185,Italy
Pietro Francia,Massimo Volpe,Division of Cardiology,Department of Clinical and Molecular Medicine,Sapienza University of Rome,St.Andrea Hospital,Via di Grottarossa,1035,00189 Rome,Italy
Federico Manetti,Paola Frati,Department SAIMLAL,Sapienza University of Roma,Rome 00185,Italy
Massimo Volpe,Department of Clinical and Molecular Medicine,Sapienza University of Rome,Rome 00197,Italy
Abstract Ethical concerns about stem cell-based research have delayed important advances in many areas of medicine,including cardiology.The introduction of induced pluripotent stem cells (iPSCs) has supplanted the need to use human stem cells for most purposes,thus eliminating all ethical controversies.Since then,many new avenues have been opened in cardiology research,not only in approaches to tissue replacement but also in the design and testing of antiarrhythmic drugs.This methodology has advanced to the point where induced human cardiomyocyte cell lines can now also be obtained from commercial sources or tissue banks.Initial studies with readily available iPSCs have generally confirmed that their behavioral characteristics accurately predict the behavior of beating cardiomyocytes in vivo.As a result,iPSCs can provide new ways to study arrhythmias and heart disease in general,accelerating the development of new,more effective antiarrhythmic drugs,clinical diagnoses,and personalized medical care.The focus on producing cardiomyocytes that can be used to replace damaged heart tissue has somewhat diverted interest in a host of other applications.This manuscript is intended to provide non-specialists with a brief introduction and overview of the research carried out in the field of heart rhythm disorders.Key Words:Human induced pluripotent stem cells;Diagnostic cardiology;Heart rhythm disorders;Microelectrode array;Stem cell research;Ethical principles
INTRODUCTION
Human induced pluripotent stem cells (iPSCs) are produced by reprogramming adult mesenchymal cells,most often fibroblasts.The process is complicated,requiring the introduction and activation of four gene regulatory networks,each comprised of molecular regulators that interact with each other and with other substances in the cell to control gene expression of mRNA and protein-specific genes.Acting together these four transcription factors can produce mature cells that behave in a completely different manner to the original fibroblasts used to form them,leading to the formation of multiple cell types[1-3].
Myocytes created from fibroblasts are basically identical to native cardiomyocytes.There are three different types of native cardiomyocytes,and iPSC production yields all three types in variable and unpredictable proportions,presenting difficulties for researchers.The most obvious and most publicized cardiological application of iPSCs is the production of new cardiac tissue to replace tissues destroyed by infarction or other diseases[4-9],but this goal has yet to be successfully realized in humans.Initially,this was partly because the subtype of iPSCs could not be assured.Obviously,atrial cardiomyocytes would not be a suitable substitute for damaged ventricular cardiomyocytes and,regardless,there is always the danger that introducing a mixture of cells might lead to teratoma formation[10].Nonetheless,substantial advances have already been made,and success seems to be mainly a matter of time.Once these problems have been fully resolved,iPSCs,in various configurations,could be used to repair damaged hearts.They could also be used to predict interactions between drugs and the cardiac conduction system.
The occurrence of any specific conduction abnormality - including QT prolongation,altered action potential duration,triggered activity,the blockade of human-ether-a-gogo-related channel (hERG) and other ion conduction channels-and the occurrence of lethal arrhythmias-such as Torsades de Pointes (TdP)-cannot reliably be predicted with currently available screening methods (Langendorf preparations,patch-clamp,or even arterially perfused isolated rabbit left ventricular wedge)[11].Animal models are problematic predictors of arrhythmia occurrence because of anatomic varia-tions[12-16].Such an issue poses a huge difficulty for drug makers trying to produce effective antiarrhythmic drugs;animal-to-human extrapolation is an uncertain process,which can pose a danger to patients if unrecognized differences emerge between animal and human models[17].
The problems associated with the use of amiodarone and sotalol illustrate the difficulties of drug development[18,19].Both drugs are used to treat atrial and ventricular tachyarrhythmias and can be life-saving,but can also produce lethal side effects.Unfortunately,predicting side effects is,at this moment,impossible.Amiodarone’s most feared side effect is fatal pulmonary interstitial fibrosis,but hepatitis,hypothyroidism (probably irreversible) and mixed sensorimotor polyneuropathy have all been reported with some regularity.Amiodarone’s most important complications are QT prolongation and TdP[20].If new and safer replacement drugs are ever to be developed and approved by the United States Food and Drug Administration and the European Medicines Agency,developers will first have to establish that new drugs do not produce predictable untoward side effects or exacerbate the conditions they were designed to treat.
The availability of iPSCs allows researchers to make reasonably accurate predictions about what effect any new drug will have on the heart and its electrical system.Cultured iPSCs,can be used to constructin vitromodels of the human cardiac conduction system.The effects observedin vitrocan then be used to predict how,and/or whether,a drug will alter electrical conduction,or produce structural alterations in humans.The process is not as simple as it sounds and some knowledge of the subject is crucial to clinicians for the safe use of new drugs.
UNDERLYING PHYSIOLOGY AND CLINICAL MANIFESTATIONS
Cures for cardiac conduction diseases will only be found when their root causes are fully elucidated.Even physicians who have nothing to do with arrhythmia research should retain some knowledge of the molecular biology that underlies cardiac conduction.
The cardiomyocyte repolarization/depolarization cycle begins with a current generated by the outward flow of potassium ions through specific pores or channels.Potassium pores exist in all life forms and many different types have been identified(more than 20).Two types of potassium channels are absolutely critical to the process of cardiac repolarization:The rapid delayed rectifier current (identified as IKr) and the slow delayed rectifier current (identified as IKs).If a drug or a mutation disrupts either of these two currents,the action potential of the cell is prolonged with an increase in the time required for electrical depolarization and repolarization of the ventricles[21].Prolonged repolarization leads to the occurrence of early after depolarization (EAD)currents.EADs are dangerous because they favor the occurrence of triggered activity(defined as the occurrence of spontaneous action potentials occurring during phase 2 or phase 3 of repolarization,leading to the production of inappropriate action potentials and arrhythmia)[12].Blockade of the IKr also causes the QT interval to be prolonged,leading to the triggered activityviaa slightly different mechanism[22].Such a situation is likely to occur when a drug molecule interferes with potassium channels as in the case of type III antiarrhythmic drugs.Slowing of the potassium current is associated with a repolarization dispersion,where one area of the myocardium recovers from depolarization faster than an adjoining region,which also makes TdP more likely to occur[23].Repolarization dispersion is thought to be the reason that myocardial hypertrophy is associated with arrhythmias[24].The farther the depolarization front has to travel,the greater the interval between depolarization and repolarization.Dispersion is especially likely to occur if the area of abnormal delay and dispersion is located within the Purkinje system or,alternatively,if the area is located in the mid-wall of the left ventricle where the “M cells” are located.These cells have prolonged action potentials that act to further increase the dispersion of repolarization,making the occurrence of TdP ever more likely[25].For a new antiarrhythmic drug to be introduced,it must first be proven that it exerts none of the effects enumerated above.
Sudden cardiac death (SCD) due to ventricular tachycardia (VT) or ventricular fibrillation (VF) accounts for approximately half of all deaths in patients with heart failure (HF) and may be considered a heritable trait[26-32].Current guidelines[33]recommend an implantable cardioverter-defibrillator (ICD) in patients with symptomatic and severe left ventricular dysfunction of any origin.However,SCD may occur in asymptomatic patients with only mild HF.On the contrary,as many as twothirds of patients with severe HF implanted with an ICD do not experience device interventions over 3 to 5 years follow-up[34].A similar clinical scenario leaves unanswered the question of whether selected gene variants may affect the risk of SCD in HF patients.Genomic science provides us with new approaches to identify gene variants or mutations that predispose patients with inherited electrical diseases to SCD.However,a growing body of evidence suggests that DNA changes in the same genes that convey risk in primary electrical diseases may enhance susceptibility to VT/VF even in a polygenic condition such as HF.Sustained VT and VF often occur as a consequence of delayed after-depolarizations triggered by diastolic sarcoplasmic reticulum (SR) calcium leak[35].Genes encoding calcium handling proteins involved in electrical homeostasis of the failing heart may represent suitable candidates for defining individual susceptibility to life-threatening arrhythmia[26,27].However,only very few genes belonging to the major candidate systems have been characterized and screened for possible association with SCD in HF.The cardiac ryanodine receptor 2(RyR2),a calcium-releasing channel located in the SR membrane,plays a key role in the electrical homeostasis of cardiomyocytes.RyR2 dysfunction has been described in both HF patients and animal models and is critical to many of the aspects of the disease,including life-threatening arrhythmia[36].In a large cohort of HF patients,Ranet al[37] found that the A allele of RYR2 c.5656G>A was associated with an increased risk of SCD.Arvanitiset al[38] reported that the Ser96Ala variant in histidine-rich calcium-binding protein was associated with ventricular arrhythmia in idiopathic dilated cardiomyopathy.It is known that a serine residue replacing glycine at position 1886 (G1886S or rs3766871) in theRyR2gene prompts a significant increase in intracellular calcium oscillation and creates a site of phosphorylation for protein kinase C (PKC) entailing PKC-mediated calcium diastolic leak from the SR[39,40].While the RYR2 rs3766871 variant has been previously described only in the setting of arrhythmogenic right ventricular cardiomyopathy,a role of RyR2 rs3766871 minor allele for increased susceptibility to VT/VF has been recently reported also in patients with HF[41].The SERCA calcium ATPase (ATP2A2) belongs to a large family of Ptype cation pumps that couple adenosine triphosphate (ATP) hydrolysis with cation transport across membranes[42].Alternative splicing of theATP2A2gene produces two isoforms,SERCA2a (primarily located in the heart and slow-twitch skeletal muscle) and SERCA2b (present in smooth muscle and non-muscle tissues).Mutations in theATP2A2gene affect the expression level,ATP affinity,calcium affinity,and phosphorylation of ATP.In an attempt to investigate whether variants of the genes encoding major calcium handling proteins affect the occurrence of VT/VF in HF patients,it was found that the ATP2A2 c.2741+54G>A gene variant was associated with decreased susceptibility to life-threatening arrhythmia.Indeed,patients carrying the ATP2A2 c.2741+54A allele variant had an approximately 70% reduction in the relative risk of VT/VF during follow-up[43].Defective calcium handling in failing cardiomyocytes has long been recognized as a cause of ventricular arrhythmia,and recent evidence suggests that selected calcium gene variants may modify the risk of SCD even in a complex and polygenic disease such as HF.While statistically associated with a modified risk of SCD,the biological role of many of these gene variants is presently unknown.The recent breakthrough discovery of iPSCs could enable the investigation of mutated cardiomyocytes generated from patient’s somatic cells,allowing functional characterization of iPSC-derived mutated cardiomyocytes.A similar approach represents an interesting and promising solution for the biological relevance of genetic substrates in secondary arrhythmogenic conditions.
Microelectrode array
Microelectrode arrays are used in many fields of study,although the basics of the system are the same no matter what kind of test is being performed;improvements and refinements in this methodology are being reported almost continuously.These tests are performed in wells that look just like those in any clinical laboratory test plate used to observe chemical reactions,however,they differ in one important respect;electrodes are located at the bottom of each well.
When the electrodes and iPSCs are joined together they form the backbone of the system.The idea was derived from earlier networking studies,designed to test neural interactions.Networking electrodes were originally made of titanium salts and gold conductors[44],but other materials have been used.The system is now so advanced that these wells,indeed,the entire networking system,including software,are all available off the shelf.
IPSCs,can either be studied singly or as part of an integrated network.For most intents and purposes these cells have all the same capabilities as embryonic stem cells that have been allowed to mature.In 2012,Shinya Yamanaka outlined a method to induce pluripotency by inserting genes that acted as reprogramming factors,also called transduction factors,by attaching them to carrier viruses and inserting the virus into the cells,which eventually causes the cells to express the exogenous genes.The cells are then cultured and finally harvested[45].Since the technique was first introduced,many other iPSCs,and related transcription factors,have been identified and used,including,miRNAs (a type of non-coding RNA that inhibits translation in many species).Whatever the precise role of these diverse factors,other epigenetic processes are critical for the process of converting maturing stem cells back to inducible pluripotent cells[46].
Once the multiple electrode arrays have been constructed,beating cardiomyocytes,derived from pluripotent stem cells are plated over each well,without the electrodes ever actually penetrating the cells.Such a methodology essentially recreates many aspects of a working myocardium,including the generation of waveforms not very different from those seen on clinical electrocardiograms.Introducing an experimental drug into the system,the probable effect on a beating human heart can be confirmed with a high degree of accuracy.
For example,experimental drugs have been tested in networked iPSCs that alter the duration and shape of the QT interval in almost exactly the same pattern as seen in humans.Not only do drugs produce the same electrocardiographic changes,but physiological stressors also produce changes similar to those that occurin vivowith the same rate and QT interval alterations seen in humans[47,48].If animal studies suggest that a drug can cause dangerous QT prolongation,it is simple enough to test the drug on networked beating human cardiomyocytes.Another obvious application of this technology is the measurement of calcium transients by using fluorescence microscopy.Calcium indicators are introduced into the cells and the resulting fluorescence can be quantitated noninvasively and used to measure calcium ion flux,which controls inotropy.In the past,such experiments required the use of isolated small animal muscle[49].
The same type of cellular network can be used to study the effect of genetic mutations known to cause cardiac arrhythmias,including channelopathies such as hERG;more than 90 long QT syndrome (LQTS) mutations have been mapped to date.It is possible to measure the effect of mutations on IKr and IKs,although debate still exists over the exact mechanism by which some mutations alter potassium flow,answers to at least some of these questions should soon be forthcoming[50].With the availability of high-throughput networked cardiomyocytes,it is now possible to evaluate a drug’s effects on potassium flow before it is ever given to an animal,let alone evaluated in human clinical trials.
IPSCs from a patient with a novelKCNQmutation were used by Egashiraet al[51] to identify the mutation.The patient had survived VF,thanks to the nearby presence of an automated external defibrillator.Using a slight variation multi-electrode array system (where the electrical activity of clumps of cells,rather than sheets of cells was measured),abnormal repolarization,as manifested by electrical field potential duration,was observed in the spontaneously beating iPSC cardiomyocytes.Egashiraet al[51] then added an assortment of potassium ingress and egress blockers to prove that the repolarization abnormality lay within the slow inward potassium channel[51].At present,the technology is too cumbersome for routine clinical use.In the future,however,it should be possible to use this approach when exome screening fails to identify one of the usual culprits.
The recent discovery of theTECRLgene,an arrhythmia-inducing gene that produces features of catecholaminergic VT (CPVT) and LQTS,was accomplished using much the same technology[52].Three patients were studied;two with a history of cardiac arrest and one with an episode of recorded CPVT.Once iPSCs had been produced and the mutation identified and sequenced,electrophysiological studies were then performed.These demonstrated exactly the same features (catecholamine sensitivity,triggered activity,delayed afterdepolarizations as had been seen in the patients.The abnormalities were all reversed by the addition of flecainide,a class 1c antiarrhythmic drug.Had iPSCs not been available,finding a remedy would have been purely by empiric trial and error.However,the real significance of the study is that there is now a reliable methodology with which to screen drugs for effectiveness.
Even without going to the effort of creating an entire iPSCs network,it is still possible to clinically diagnose some disorders from the electrical behavior of a single iPSC.A very recent report describes two patients with known Brugada Syndrome.When compared to the findings in two healthy controls,it was observed that each of the Brugada Syndrome patients carried one of two different sodium voltage-gated channel alpha and subunit 5 variants.The electrical characteristics of iPSCs produced from the patient's own skin fibroblasts were studied.The studies showed reductions in inward sodium current density and reduced maximal upstroke velocity of action potential when compared with healthy controls.Furthermore,iPSC cardiomyocytes from the Brugada Syndrome patients demonstrated increased triggered activity,abnormal calcium (Ca2fl) transients,and beating interval variation,the very same abnormalities previously reported in other studies,using different methodologies[53].Late in 2016,a study using individual iPSCs was used to confirm results observed in a previous knock out mouse study.The studies had suggested the existence of a new cardiac regulatory mechanism that appeared to play a key role in the association between arrhythmias and myocardial hypertrophy.When the mouse studies were repeated in human iPSCs,it was possible to confirm that the same stress-activated kinase was operative in human cells[54].
Heart disease screening
Another obvious application for iPSCs is screening for suspected heart disease,and for determining the significance of a mutation once it has been identified.Hypertrophic cardiomyopathy (HCM) is a very good example.The clinical diagnosis can be difficult to make (left ventricular hypertrophy with wall thickness>15 mm,in the absence of ventricular dilation or any apparent disease that could cause hypertrophy)[55].Unfortunately,it is not uncommon for there to be a complete disconnect between phenotype and genotype:Abnormal genes may be present but symptoms and signs absent.
Both sarcomeric mutations and non-sarcomeric mutations in HCM can be identified by whole-exome sequencing,and these studies demonstrate that the same genotype may be responsible for sudden death in one individual,but remain asymptomatic in another[56].Multiple mutations have been detected in patients with HCM:Nine sarcomeric genes are known to carry most HCM-related mutations and encode sarcomeric mutations,while an additional nine mutations code for sarcomeric Z-disc proteins such as muscle LIM protein,α-actinin,or telethonin[57,58].
Since iPSCs cardiomyocytes became available,the pathogenic effects of some mutations (MYH7 and MYBPC3) associated with HCM have already been identified[59],and calcium blockade has been found to be an effective treatment for another HCM mutation (MYH7-R663H)[60].Whole-exome sequencing almost never yields the identity of a single culprit gene,but rather detects multiple mutations,some of which may be relevant and some not.If one single mutation is responsible for the obvious phenotype of HCM,it has yet to be identified.It hardly needs saying,but exactly the same methodology used to identify culprit genes could be applied to genomic studies of countless other disorders,just by inducing the required cell type from transformed fibroblasts.
DISCUSSION
Overcoming the ethical problems related to the use of stem cells through the introduction of iPSCs opens up an interesting scenario on the study of the cellular basis of diseases[61,62].The use of pluripotent cells makes it possible to reproduce models for the study of cardiological pathologies which frequently cause SCD and are often diagnosed post-mortem such as structural cardiomyopathies and channelopathies[63-66].
Furthermore,iPSCs can be exploited in the personalization of therapies in relation to the possibility of carrying out pharmacological tests on cells derived from the patient[67-70].
Although the principles are easy to understand,at present there are some important caveats.One is that fibroblast generated iPSCs demonstrate an immature phenotype so that they more closely resemble mid-gestation human fetal hearts[71-73].These differences may well alter final experimental and clinical results,depending on the stage of development of the iPSCs being used.When used in other fields,the same caveat applies.Now that this difference has been recognized,finding ways to make sure the cells are organized and function as adult cells is the object of intense research,which has already begun to generate results.Recent reports indicate that iPSCs can be stimulated and made to mature by a combination of pacing and increasing mechanical stress[74-77].
Another issue that had been delaying progress is that protocols used to produce iPSCs do not produce just one kind of cell,but rather yield a mixed population of cardiomyocyte subtypes including ventricular-,atrial- and pacemaker-like cells[78-81].Birket and colleagues[82] made the early observation that even though the iPSCs can behave like normal human cardiomyocytes,the production process leads to unequal numbers of each of the subtypes.Obviously,different results will be generated depending on which type of cell predominates.Many laboratories are working on effective cell separation methods and standardized methods should soon be available.
CONCLUSION
In summary,the main applications of stem cells include disease modeling,cell diagnostics,and therapy personalization (Figure 1).Such tasks involve molecular profiling,the identification of biomarkers of the expression of the pathological phenotype,as well as the identification and testing of targeted therapies.The availability of pluripotent cardiac stem cells,especially networked beating cardiomyocytes,is likely to revolutionize our understanding of many cardiac rhythm disorders and diseases,provide a rational testing method for the development of drugs,permit clinicians to assess effectiveness before drug administration and,most importantly,save lives.
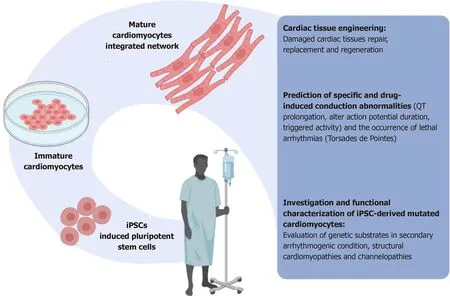
Figure 1 Induced pluripotent stem cells can provide new ways to study arrhythmias and heart disease in general,accelerating the development of new,more effective antiarrhythmic drugs,clinical diagnoses,and personalized medical care.
杂志排行
World Journal of Stem Cells的其它文章
- Multidifferentiation potential of dental-derived stem cells
- Stem cell therapy in ocular pathologies in the past 20 years
- Programmed cell death in stem cell-based therapy:Mechanisms and clinical applications
- Low complexity domains,condensates,and stem cell pluripotency
- Different kinds of stem cells in the development of SARS-CoV-2 treatments
- Disease modifying treatment of spinal cord injury with directly reprogrammed neural precursor cells in non-human primates
