Multidifferentiation potential of dental-derived stem cells
2021-07-02JingYaoYinXingHongLuoWeiQingFengShengHongMiaoTingTingNingQianLeiTaoJiangDanDanMa
Jing-Yao Yin,Xing-Hong Luo,Wei-Qing Feng,Sheng-Hong Miao,Ting-Ting Ning,Qian Lei,Tao Jiang,Dan-Dan Ma
Jing-Yao Yin,Xing-Hong Luo,Wei-Qing Feng,Sheng-Hong Miao,Qian Lei,Tao Jiang,Department of Stomatology,Nanfang Hospital,Southern Medical University,Guangzhou 510515,Guangdong Province,China
Ting-Ting Ning,Dan-Dan Ma,Department of Endodontics,Stomatological Hospital,Southern Medical University,Guangzhou 510280,Guangdong Province,China
Abstract Tooth-related diseases and tooth loss are widespread and are a major public health issue.The loss of teeth can affect chewing,speech,appearance and even psychology.Therefore,the science of tooth regeneration has emerged,and attention has focused on tooth regeneration based on the principles of tooth development and stem cells combined with tissue engineering technology.As undifferentiated stem cells in normal tooth tissues,dental mesenchymal stem cells(DMSCs),which are a desirable source of autologous stem cells,play a significant role in tooth regeneration.Researchers hope to reconstruct the complete tooth tissues with normal functions and vascularization by utilizing the odontogenic differentiation potential of DMSCs.Moreover,DMSCs also have the ability to differentiate towards cells of other tissue types due to their multipotency.This review focuses on the multipotential capacity of DMSCs to differentiate into various tissues,such as bone,cartilage,tendon,vessels,neural tissues,muscle-like tissues,hepatic-like tissues,eye tissues and glands and the influence of various regulatory factors,such as non-coding RNAs,signaling pathways,inflammation,aging and exosomes,on the odontogenic/osteogenic differentiation of DMSCs in tooth regeneration.The application of DMSCs in regenerative medicine and tissue engineering will be improved if the differentiation characteristics of DMSCs can be fully utilized,and the factors that regulate their differentiation can be well controlled.
Key Words:Dental mesenchymal stem cells;Regenerative medicine;Tissue engineering;Multipotency;Odontogenic differentiation;Osteogenic differentiation
INTRODUCTION
Over the past three decades,in the search for treatments for a variety of degenerative diseases and irreversible forms of tissue and organ damage,the emerging field of tissue engineering and regenerative medicine (TERM) has attracted a lot of interest,and great efforts have been made to realize the regeneration of different types of tissues and organs to restore normal physiology and body function.As one of the important aspects of regenerative medicine,tissue engineering mainly takes advantages of the following three methods:(1) Cell/biomaterial complex systems with cell-seeded biomaterials implanted into the body to restore and regenerate tissues/organs;(2) Cell systems,such as stem cell transplantation;and (3) Biomaterial systems implanted into the body and integrated into tissues[1].As a vital part of TERM,a suitable source of stem cells is a significant initial requirement.Since the 1990s,the field of stem cell biology has gradually developed and rapidly become a main research trend in regenerative medicine.Induced pluripotent stem cells,progenitor cells from various tissues,human embryonic stem cells and adult stem cells are all potential seed cells for TERM[2].Cells derived from induced pluripotent stem cells or differentiated from human embryonic stem cells can be used to build related tissue cell models.Progenitor cells and adult stem cells from various tissues can differentiate into mature tissues.
As adult stem cells,dental mesenchymal stem cells (DMSCs),including dental pulp stem cells (DPSCs),periodontal ligament stem cells (PDLSCs),stem cells from apical papilla (SCAPs),gingival mesenchymal stem cells (GMSCs),stem cells from human exfoliated deciduous teeth (SHED) and dental follicle stem cells (DFSCs) have been widely studied because of their ready availability,easy accessibility and lack of complex ethical issues.DMSCs have multiple differentiation potential and can differentiate into a variety of tissue-like cells under specific induction conditions,providing potential seed cells for TERM.For example,SHED are capable of inhibiting bone loss,decreasing neuronal apoptosis and forming pancreatic islet-like clusters[3-5].DPSCs can differentiate into myogenic lineage and corneal stromal-like constructs[6,7] and can also reduce bone loss in an osteoporosis mouse model,prevent retinal ganglion cell loss and repair spinal cord injury[8-10].
DMSCs,in particular,have great potential for application in engineering regeneration of dental tissues.In 2006,Sonoyamaet al[11] transplanted a hydroxyapatite/SCAP-Gelfoam/PDLSC structure into a swine alveolar socket,which regenerated mineralized root-like tissue and formed periodontal ligament space[11].In 2012,Guoet al[12] identified a method of combining DFSCs with treated dentin matrix scaffolds in the alveolar fossa that proved to be a promising strategy for tooth root regeneration[12].In 2013,Ioharaet al[13] transplanted autologous DPSCs with granulocyte-colony stimulating factor into a dog pulpectomized tooth and found that newly formed pulp tissue,including innervation and vasculature,fully filled in the root canal[13].
Efforts have been made to promote tooth regeneration by DMSCs,but many factors affect this complex regeneration process,such as correlative non-coding RNAs,signaling pathways,inflammation,aging and exosomes.In the process of induced differentiation of DMSCs,many non-coding RNAs,including microRNAs and long noncoding RNAs (lncRNAs) and related signaling pathways are involved to regulate the expression of odontogenic/osteogenic differentiation genes.In addition,donor age,cell senescence and the complex oral inflammatory microenvironment also pose great challenges to tooth regeneration by DMSCs.Moreover,the hot topic of research in recent years,exosomes,which carry a variety of contents,have also captured the attention of researchers in inducing the differentiation of DMSCs.If we can regulate these factors well,it will enable a big step forward in the application of DMSCs in the field of tooth regeneration.This review focuses on the multidirectional differentiation potential of DMSCs and the effect of the above-mentioned factors on the odontogenic/osteogenic differentiation of DMSCs in the field of tooth regeneration,hoping to provide a reference for the efficient use of DMSCs.
DIVERSE DIFFERENTIATION OF DMSCS
In addition to the odontogenic differentiation ability of DMSCs,in recent years the research on the differentiation of DMSCs into other tissue-like cells,such as osteogenesis,chondrogenesis,angiogenesis,neurogenesis and differentiation potential toward tendon-like cells,insulin-producing cells,hepatic-like cells,corneal stromallike cells,etc.has become popular (Figure 1).To explore the diverse differentiation ability of DMSCs is an issue worth exploring.

Figure 1 Location of dental mesenchymal stem cells and their diverse differentiation potential.
DPSCs
In 2000,Gronthoset al[14] identified that DPSCs can form alizarin red-positive condensed nodules with high levels of calcium cultivated by L-ascorbate-2-phosphate,glucocorticoid,dexamethasone and inorganic phosphate[14].As a seed cell for bone regeneration,DPSCs usually attached to some materials for bone defect models.For example,Wongsupaet al[15] fabricated a scaffold combination of poly-εcaprolactone-biphasic calcium phosphate with the modified melt stretching and multilayer deposition technique seeded with human DPSCs (hDPSCs),which increased the newly formed bone in calvarial defects rabbit models[15].However,Jinet al[16] showed that adipose tissue-derived stem cells exhibited greater osteogenic differentiation potential compared to DPSCs[16].
In vitro,DPSCs can differentiate into chondroblasts,which suggests that it can be useful for cartilage injuries[17].CD146 marked DPSCs can express the chondrogenic inducing factor transforming growth factor (TGF)-β3 and form three-dimensional cartilage constructs when seeded on poly-L-lactic acid/polyethylene glycol electrospun fiber scaffolds[18].Costal chondrocytes are able to supply a chondroinductive niche that promote the DPSCs to undergo chondrogenic differentiation and enhance the formation of cartilage[19].Xenotransplantation of DPSCs in platelet-rich plasma and 3% alginate hydrogels significantly regenerated cartilage in rabbit models of cartilage damage[20,21].
In 2016,Chenet al[22] first identified expression of tendon-related markers such as scleraxis,tenascin-C,tenomodulin,eye absent homologue 2,collagen I and collagen VI in dental pulp tissues.Also,DPSCs seeded in aligned polyglycolic acid fiber scaffolds can promote the expression of tendon-related markers under mechanical stimulation and form mature tendon-like tissue in a mouse model[22].As neural crest-derived cells,DPSCs can be induced to differentiate into neuron-like cells with the use of growth factors,including basic fibroblast growth factor and epidermal growth factor,which are preferable to the chemical-induction method[23-25].DPSCs transplanted into a rat model of middle cerebral artery occlusion,peripheral nerve injuries and retinal injury expressed related neuronal markers[26-28].
Three-dimensional culture promoted the differentiation of hDPSCs into insulinproducing cells[29],and pancreatic islets were also generated from DPSCs[30].The potential toward insulin-producing cells of hDPSCs was superior to human PDLSCs(hPDLSCs)[31].DPSCs also exhibited angiogenic potential when implanted into mouse brain and into a rat model of acute myocardial infarction by promoting neovasculogenesis[32,33].Furthermore,DPSCs differentiated into bladder smooth muscle cells in a particular culture medium[34],while the Wnt-GSK3β/β-catenin pathway played an important role in this process[35].DPSCs had the potential to form a highpurity hepatic lineage when cultured in serum-free medium[36],and DPSCs derived from cryopreserved dental pulp tissue of vital extracted diseased teeth also showed the potential to differentiate into hepatic-like cells[37].Additionally,DPSCs had the capacity to differentiate into melanocyte-like cells when cultured in a specific melanocyte differentiating medium[38].
PDLSCs and GMSCs
PDLSCs have great osteogenic differentiation potential.Katoet al[39] observed that PDLSCs have the highest levels of some bone differentiation markers without osteogenic differentiation among mesenchymal stromal cells derived from bone marrow and adipose-derived mesenchymal stem cells[39].Seeded on nanohydroxyapatite-coated genipin-chitosan conjunction scaffold,PDLSCs exhibited significantly greater viability and alkaline phosphatase activity and promoted calvarial bone repair[40].Moshaveriniaet al[41,42] reported that PDLSCs and GMSCs capsulated in an injectable arginine-glycine-aspartic acid tripeptide-coupled alginate microsphere delivery system promoted bone regeneration and chondrogenesis,respectively,for a calvarial defect animal and subcutaneous implantation of nude mice,and PDLSCs showed significantly higher osteogenic and chondrogenic differentiation capability compared with GMSCs.
In 2021,Shenet al[43] showed that 6-bromoindir-ubin-3’-oxime promoted mineralized nodule formation in PDLSCs[43].PDLSCs from beagle dogs and humans can both be induced to differentiate into neural-like cells by various protocols[44,45],and the Wnt/β-catenin signaling pathway has been implicated in this process[46].Buenoet al[47] found that the nuclear shape of hPDLSC-derived neural-like cells was similar to cells in neurogenic niches from adult mouse brain,and no cell proliferation occurred in the course of neurogenesis.The potential for neurogenesis is improved by the addition of specific short peptides or phytocompounds[48-50].As another stem cell type derived from periodontal tissue,GMSCs also have neurogenic differentiation potential and displayed action potential capacity when tested by a neurospheremediated induction method[51],while hypoxia preconditioning activated more genes associated with neuronal development[52].In addition,over prolonged passages,human GMSCs have been found to spontaneously differentiate into neural precursor cells[53].
Encapsulated PDLSCs and GMSCs in an alginate/hyaluronic acid threedimensional scaffold promoted the regeneration of neurogenic tissue[54].Besides,PDLSCs had the ability to differentiate into corneal stromal keratocyte-like cells[55]and constructed a multilamellar human corneal stromal-like tissuein vitrowhen seeded onto orthogonally aligned,multilayered silk membranes and supplemented with the neuropeptide substance P[56].PDLSCs also could be directed to develop into retinal progenitors and islet-like cell clusters with competence for photoreceptor differentiation and secretion of insulin[57,58].Moreover,both PDLSCs and GMSCs differentiated into tendon-like cells using an injectable and biodegradable arginine-glycineaspartic acid tripeptide-coupled alginate hydrogel scaffold[59].The GMSCs could also be induced to differentiate into functional keratinocytes when treated withAcalypha indicain a three-dimensional microenvironment[60].
DFSCs
Human DFSCs can differentiate to osteogenic lineage cells in osteogenic induction medium without dexamethasone,and BMP6 is a key gene in the osteogenic differentiation[61].Plasma rich in growth factors and soluble silica can promote osteogenic differentiation of DFSCs[62,63].Lucaciuet al[64] indicated that DFSCs could be used for promoting bone regeneration on titanium implant surfaces[64].DFSCs were loaded into poly-ε-caprolactone scaffold and implanted into skulls defects of Sprague Dawley rats,and bone regeneration was observed[65].Undifferentiated DFSCs expressed some neural markers,such as nestin,β-III-tubulin and S100β and exhibited a spindle-like morphology[66].Using a two-step strategy for neuronal differentiation,DFSCs could be differentiated into neurosphere-like cell clusters,and finally developed a cellular morphology with small bodies and long cellular extrusions while exhibiting increased expression of neural cell markers[67].
It has been suggested that human DFSCs may have the potential to differentiation toward the glial lineage rather than the neuronal lineage[66].Induced cardiomyocytes derived from DFSCs,which were cultured in medium with suberoylanilide hydroxamic acid,could be intraperitoneally injected into experimental mice and exhibited homing capacity into the heart muscle[68].Comparing the differentiation potential toward pancreatic β cell-like cells among the stem cells from dental pulp,papilla and follicle,the DFSCs demonstrated higher potency and secreted more insulin upon glucose challenge[69].Furthermore,epithelial stem-like cells from the human dental follicle were able to differentiate into salivary gland acinar and duct cells[70].
SHED
SHED represent a promising cell source for bone regeneration,which are usually combined with many biomaterials.Combined hydroxyapatite scaffold and SHED can promote alveolar bone regeneration,and interleukin-17A can enhance osteogenic differentiation of SHED,both due to increasing osteoprotegerin/receptor activator of nuclear factor κB ligand ratio[71,72].FGF-2 pretreated SHED represent a faster formation of intramembranous bone after implanted in craniofacial bone defects than hypoxia pretreated[73].A carbon nanomaterial named graphene oxide quantum dots promotes osteogenic differentiation of SHEDviathe Wnt/β-catenin signaling pathway[74].In addition,SHED have the chondrogenic differentiation ability.After transplantation into the subcutaneous space on the back of nude mice,SHED recombined with β-TCP scaffolds were able to produce new cartilage-like tissues[75].
In 2011,SHED were successfully induced to differentiate into neural-like cells by a simple short-term growth factor-mediated induction protocol[76],and then in 2013,a novel three-stage method was established[77].Yanget al[78] found that Noggin overexpression combined with the Rho kinase inhibitor Y-27632 exhibited a synergistic effect in promoting differentiation of SHED into neuron-like cells[78].The lncRNA C21orf121 promotes SHED differentiation into neuronal cells by upregulating the expression of BMP2,acting as a competing endogenous RNA to compete with BMP2 binding to miR-140-5p[79].SHED in polyglycolic acid tubes combined with autografting can regenerate the mandibular branch of the rat facial nerve[80].Also,SHED have been used to repair a Parkinsonian rat model,an acute contused spinal cord injury model and a model of diabetic peripheral neuropathy[81-83].
In addition,SHED can differentiate into angiogenic endothelial cells,and when cultured with decellularized extracellular matrix of human umbilical vein endothelial cells can improve endothelial differentiation[84,85].Using shear stressviathe downstream pathway of vascular endothelial-derived growth factor-Notch signaling or by inhibiting TGF-β signaling in SHED can enhance endothelial differen-tiation[86,87].SHED transplanted into immunodeficient mice using Matrigel with human umbilical vein endothelial cells form extensive vessel-like structures[88].
SHED also have the potential for hepatic differentiation,which can be improved by using liquorice or angelica extracts in the culture medium[89].CD117+SHED hepatically differentiatedin vitrowere used to repair either acute liver injury or induced secondary biliary cirrhosis in a rat model[90].Meanwhile SHED or SHEDconverted hepatocyte-like cell-based spheroids transplanted into a CCl4-induced chronic liver fibrosis mouse model improved hepatic dysfunction[91,92].
Furthermore,SHED can differentiate into epidermal cells and accelerate wound repair when seeded onto polyvinyl alcohol/silk fibroin nanofiber dressings[93].CD117+SHED also have the potential to differentiate toward all functional endocrine and exocrine subsets of pancreatic cells in serum-free conditions[94].When cocultured with immortal corneal epithelium cellsin vitro,SHED display the potential for transdifferentiation to corneal epithelium-like cells[95].Liet al[96] indicated that SHED can transdifferentiate into retinal photoreceptor-like cellsin vitroand retain good viabilityin vivoafter transplantation into mice with a normal immune system[96].Moreover,functional smooth muscle cells can be differentiated from SHED by TGF-β1 induction,while the ALK5 signaling pathway may regulate this process[97].
SCAPs
In 2020,Denget al[98] reported that platelet derived growth factor BB promoted SCAPs osteogenic differentiation and enhanced bone formation in calvarial defects combined with a thermosensitive hydrogel[98].Both conditioned culture medium containing traditional Chinese herbal remedy,Yunnan Baiyao,and high glucose α-Minimal Essential Medium can promote the odonto/osteogenic differentiation of SCAPs through the nuclear factor κB signaling pathway[99,100].Depletion of lysinespecific demethylase 2A enhanced the adipogenic and chondrogenic differentiation potentials of SCAPs[101].In 2020,Yanget al[102] reported that DLX5 and HOXC8 enhanced the expression of chondrogenic markers including type II collagen,type V collagen and sex-determining region Y box protein 9[102].
In 2017,Kimet al[103] first formed a three-dimensional cell-based nerve-like tissue with axons and myelin structures using SCAPs through a three-dimensional organotypic culture method[103].The secreted frizzled-related protein 2,a Wnt signaling modulator,and insulin-like growth factor (IGF)-2 improved the neurogenic differentiation potential of SCAPs[104,105].Adding graphene dispersion and watersoluble single-walled carbon nanotubes to the neuroinductive medium enhanced the neural differentiation of SCAPs[106].
SCAPs show angiogenic potential,and SCAPs and/or DPSCs transplanted in threedimensional-printed hydroxyapatite scaffolds can form vascularized dentin/pulp-like tissue[107].Coculture of human umbilical vein endothelial cells and SCAPs under hypoxic conditions promotes the construction of vessel-like structuresin vitro,and ephrinB2 may play an important role in stabilizing the vascular-like struc-tures[108,109].Furthermore,erythropoietin enhances the endothelial differentiation of SCAPs[110].In addition,SCAPs also have hepatogenic potential[111],and mesenchymal stem cells derived from dental papilla can also be differentiated into pancreatic β cell-like cells[69].
MULTIPLE FACTORS INFLUENCING THE ODONTOGENIC/OSTEOGENIC DIFFERENTIATION OF DMSCS
MicroRNAs
MicroRNAs (miRNAs) play important roles in regulating the tooth regeneration process (Table 1).Downregulation of miR-143-5p and miR-143-3p promotes the odontoblastic differentiation of DPSCs through the osteoprotegerin/receptor activator of nuclear factor κB ligand signaling pathway[112,113].Actingviathe p38 mitogenactivated protein kinases (MAPK) signaling pathway,downregulated miR-143-5p and miR-488 are capable of inducing DPSCs to differentiate into odontoblast-like cells by targeting MAPK14 and MAPK1,respectively[114,115].Wanget al[116] found that miR-125a-3p regulates odontoblastic differentiation of DPSCs in an inflammation model by targeting Fyn,a member of the protein tyrosine kinase Src family[116].
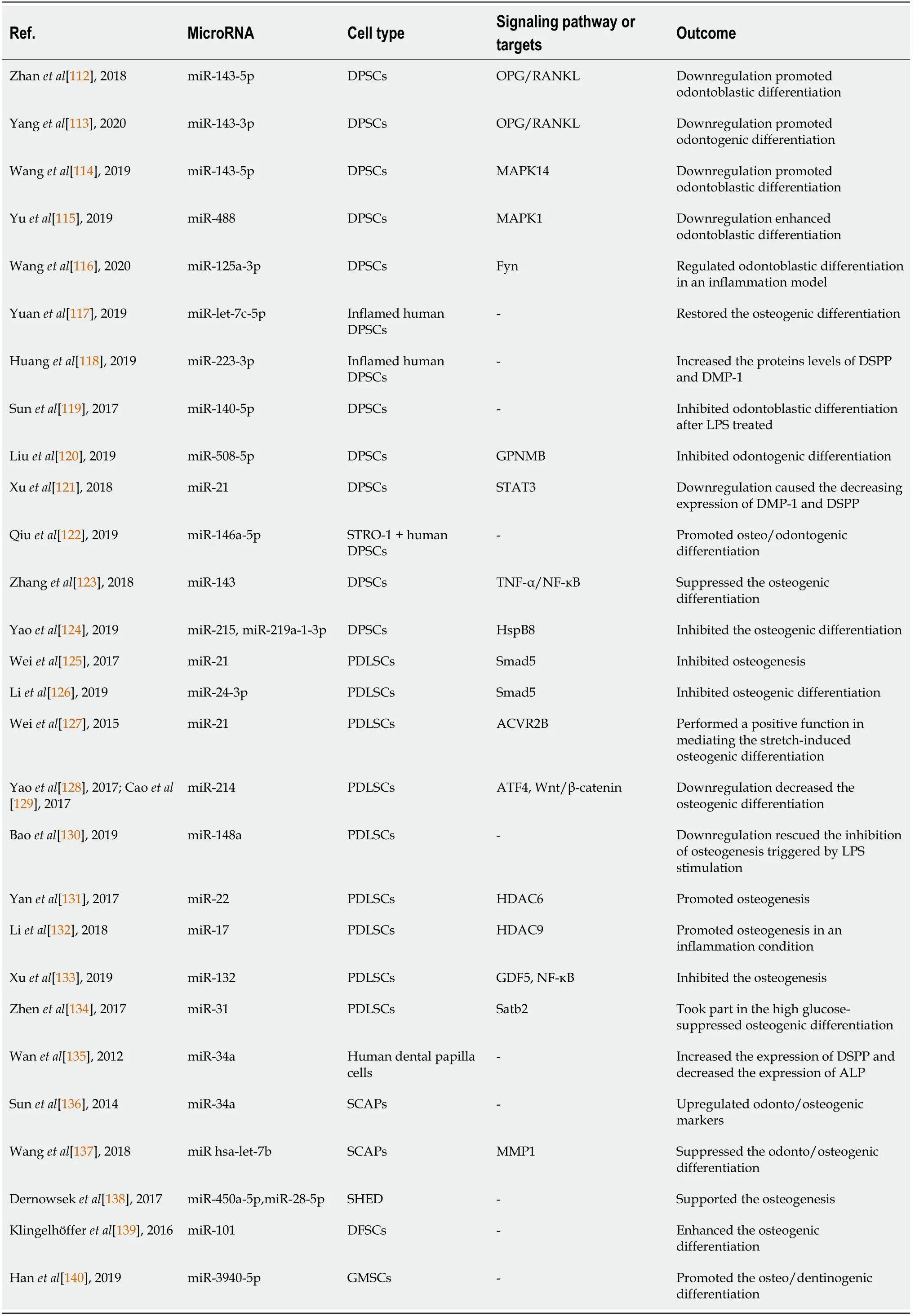
Table 1 Summary of the microRNAs influencing the odontogenic/osteogenic differentiation of dental mesenchymal stem cells
Meanwhile miR-let-7c-5p can restore the osteogenic differentiation of inflamed DPSCs by suppressing the lipopolysaccharide (LPS)-induced inflammatory phenomena[117].In inflamed pulp tissues,miR-223-3p is remarkably upregulated,and overexpression of miR-223-3p in DPSCs can increase the protein levels of dentine sialophosphoprotein (DSPP) and dentine matrix protein 1[118].Sunet al[119] found that during LPS-mediated odontoblastic differentiation of DPSCs,the expression of miR-140-5p is markedly decreased,while when miR-140-5p is expressed in DPSCs after LPS treatment,the odontoblastic differentiation ability is inhibited[119].
Additionally,during odontogenesis of hDPSCs,the expression of miR-508-5p decreases gradually,while significant inhibition of odontogenesis is observed after overexpression of miR-508-5p,which targets glycoprotein nonmetastatic melanomal protein B[120].Xuet al[121] reported that during odontoblast differentiation of DPSCs,the expression of miR-21 can be regulated by treating with TNF-α,while downregulation of miR-21 causes a decrease in the expression of dentine matrix protein 1 and DSPP by interacting with STAT3[121].Moreover,miR-146a-5p promotes odontogenic/osteogenic differentiation of STRO-1+DPSCs[122].miR-143 suppresses the osteogenic differentiation of DPSCs by regulating the TNF-α/nuclear factor κB pathway[123],while miR-215 and miR-219a-1-3p inhibit the osteogenic differentiation capability of DPSCs by downregulation of heat shock protein B8[124].
During osteogenic differentiation of PDLSCs,the expression of miR-21 and miR-24-3p decrease,and their downregulation markedly inhibits osteogenesis of hPDLSCs by targeting SMAD family member 5 (Smad5)[125,126].miR-21 also performs a positive function in mediating the stretch-induced osteogenic differentiation of hPDLSCs by regulating the expression of activin receptor type IIB[127].Inhibition of miR-214 in PDLSCs can decrease osteogenic differentiation by targeting activating transcription factor 4 and regulating the Wnt/β-catenin signaling pathway[128,129].Downregulation of miR-148a in PDLSCs rescues the inhibition of osteogenesis triggered by LPS stimulation[130].miR-22 and miR-17 promote osteogenesis of PDLSCs by inhibiting HDAC6 and HDAC9 expression,respectively,the latter under inflammatory conditions[131,132].In addition,in osteogenic differentiation of PDLSCs,miR-132 decreases,and overexpression of miR-132 inhibits osteogenesis by targeting growth differentiation factor 5 and activating the nuclear factor κB signaling pathway[133].Meanwhile miR-31 plays a role in the high glucose-suppressed osteogenic differentiation of PDLSCs by targeting Satb2[134].
Upregulation of miR-34a in human fetal dental papilla cells increases the expression of DSPP and decreases the expression of alkaline phosphatase (ALP)[135].In addition,miR-34a mimic transfection in SCAPs significantly upregulates odontogenic/osteogenic markers[136].miR-hsa-let-7b suppresses the odontogenic/ osteogenic differentiation of SCAPs partly by targeting matrix metalloproteinase 1[137].Moreover,overexpression of miR-450a-5p or miR-28-5p in SHED supports osteogenesis[138].miR-101 enhances osteogenic differentiation in human DFSCs[139],and miR-3940-5p promotes the osteo/dentinogenic differentiation of GMSCs[140].
LncRNAs
LncRNAs significantly regulate the multiple differentiations of mesenchymal stem cells,and there are several reports of the regulatory effect of lncRNAs in regenerative engineering of dental-tissue-derived stem cells (Table 2).In 2020,Liuet al[141]identified a total of 89 lncRNAs differentially expressed after osteo/odontogenic induction of hDPSCs,and downregulation of lncRNA SNHG7 was found to inhibit the differentiation of DPSCs,upregulating the expression of miR-1226-3p and miR-210-5p at the same time[141].In 2020,Chenet al[142] reported that 132 lncRNAs were differentially expressed between the odontoblastic-differentiated and undifferentiated hDPSCs and that lncRNA-G043225 exerted a positive regulatory effect through miR-588 and fibrillin 1[142].Additionally,47 lncRNAs were differentially expressed in hDPSCs between normoxic and hypoxic induction conditions,and 561 lncRNAs were differentially expressed between young and old donors in hDPSCs after osteoinduction[143,144].Overexpression of lncRNAs CCAT1 and lncRNA H19 promotes odontogenic differentiation of hDPSCs by inhibiting expression of miR-218 and regulating expression of theDLX3gene,respectively[145,146].Knockdown of lncRNA STL and lncRNA X-inactive specific transcript inhibits the osteogenic potential of DPSCs,and the latter is essential for efficient osteogenic differentiation induced by TNF-α[143,147].
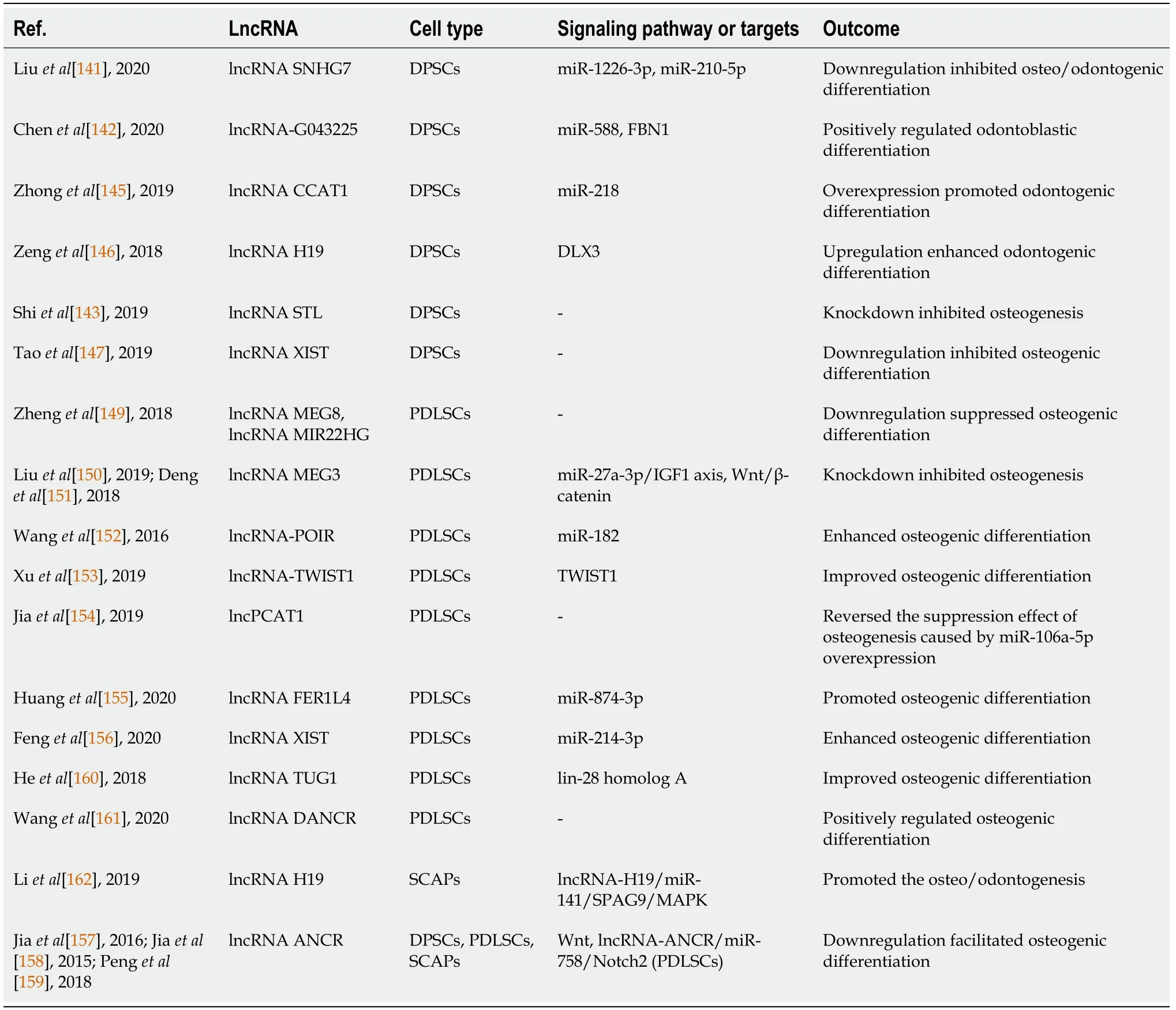
Table 2 Summary of the long noncoding RNAs influencing the odontogenic/osteogenic differentiation of dental mesenchymal stem cells
In 2016,Quet al[148] demonstrated that 2171 lncRNAs were differentially expressed between osteogenic-differentiated and undifferentiated PDLSCs,and 393 lncRNAs were strongly associated with osteogenesis-related mRNAs[148].Zhenget al[149]indicated that downregulation of lncRNA maternally-expressed 8 and lncRNA MIR22HG markedly suppressed the osteogenic differentiation of PDLSCs[149].Knockdown of lncRNA maternally-expressed 3 inhibits the osteogenesis of PDLSCs in periodontitisviathe miR-27a-3p/IGF1 axis,while it plays a positive role in human DFSCs by activating the Wnt/β-catenin signaling pathway[150,151].In 2016,Wanget al[152] identified a novel lncRNA named lncRNA-POIR,while Xuet al[153] first named lncRNA-TWIST1 in 2019;both are osteogenesis impairment-related lncRNAs of PDLSCs from periodontitis patients and can enhance the osteogenic differentiation of PDLSCs from healthy individuals and periodontitis patients by interacting with miR-182 and inhibiting TWIST1 expression,respectively[152,153].Prostate cancerassociated ncRNA transcript-1 upregulation reverses the suppression effect of osteogenic differentiation in PDLSCs caused by miR-106a-5p overexpression[154].
LncRNA FER1L4 and lncRNA X-inactive specific transcript can promote the osteogenesis of PDLSCs by sponging miR-874-3p and miR-214-3p,respec-tively[155,156].In addition,downregulation of antidifferentiation noncoding RNA can facilitate the osteogenic differentiation of DPSCs,PDLSCs and SCAPs[157],while this regulatory effect on PDLSCs is related to the canonical Wnt signaling pathway[158].The antidifferentiation noncoding RNA/miR-758/Notch2 axis may also participate[159].Furthermore,lncRNA TUG1 improves osteogenic differentiation of PDLSCs by regulating the expression of lin-28 homolog A[160].Knockdown of lncRNA differentiation antagonizing nonprotein coding RNA positively regulates the osteogenic differentiation of PDLSCs[161].Moreover,lncRNA H19 overexpression promotes the osteo/odontogenesis of SCAPsviathe lncRNA-H19/miR-141/SPAG9/MAPK positive feedback loop[162].
Signaling pathways
Wnt signaling pathway:The Wnt/β-catenin signaling pathway plays an important role in regulating DMSC differentiation,which is a key signaling pathway.For odontoblastic differentiation,activating the Wnt/β-catenin signaling pathway partially reverses the vacuolar protein sorting 4B knockdown-driven suppression of odontoblastic differentiation of hDPSCs[163] and rescues the osteoblastic/odontoblastic differentiation of stathmin-deletion hDPSCs[164].These studies revealed that activation of the Wnt signaling pathway promotes osteogenic/odontoblastic differentiation of DPSCs.However,Schelleret al[165] first reported that Wnt/β-catenin inhibits odontoblastic differentiation of DPSCs in 2008[165].The reason for the conflicting effects of Wnt signaling on odontoblastic differentiation in these studies is undefined and needs to be further explored.For osteoblastic differentiation,Rolphet al[166] confirmed that ferutinin promoted osteoblastic differentiation of DPSCs by modulating the Wnt/β-catenin signaling pathway[166] when Wnt5a was reported to inhibit osteoblastic differentiation of human periodontal ligament stem cell-like cells[167].
MAPK signaling pathway:The MAPK signaling pathway includes the ERK signaling pathway and the p38/MAPK signaling pathway[168].In odontoblastic differentiation,one study showed that a combination of mineral trioxide aggregate and propolis significantly promoted the expression of DSPP and Dentine matrix protein 1 as well as mineralized nodule formation through activating the ERK signaling pathway in hDPSCs[169].Konget al[170] confirmed that a magnesium-enriched microenvironment enhanced the odontoblastic differentiation of hDPSCs by activating the ERK/BMP2/Smad signaling pathway[170].In osteoblastic differentiation,berberine was reported to bind to epidermal growth factor receptor in hPDLSCs to activate the ERK signaling pathway and upregulate the nuclear-related gene FOS,thus promoting osteoblastic differentiation of PDLSCs[171].In addition,mineral trioxide aggregate was confirmed to promote osteo/odontoblastic differentiation of SCAP through activation of the p38 and ERK signaling pathway.Another study showed that parathyroid hormone promoted the osteo/odontoblastic differentiation of DPSCs by activating the ERK and p38 signaling pathway[172].
Mechanistic target of rapamycin signaling pathway:Mechanistic target of rapamycin(mTOR),a highly conserved serine/threonine protein kinase,is involved in regulating interactions between proteins[173].The mTOR signaling pathway has been confirmed to play a significant role in the osteo/odontoblastic differentiation of DMSCs.Tanakaet al[174] confirmed that inhibiting mTOR signaling promoted osteo/odontoblastic differentiation of SCAPs[174].However,activation of the mTOR signaling pathway promoted osteogenic differentiation of hDPSCs in the process regulated by IGF-1 in which rapamycin blocked osteogenic differentiation induced by IGF-1[175] while inhibiting mTORC1 limited mineralized nodule formation by SHED[176].Taken together,these data suggest that the mTOR signaling pathway plays different roles in different cell types of DMSCs.
AKT signaling pathway:The AKT signaling pathway is critical for cell proliferation,growth,metabolism and differentiation,especially in differentiation of DMSCs.Recent studies have shown that metformin and miR-let-7c-5p enhance the osteogenic differentiation of PDLSCs by activation of the AKT signaling pathway[117,177].Another study reported that activation of the AKT signaling pathway could enhance the osteogenic differentiation of DPSCs in LPS-induced inflammation.In short,the AKT signaling pathway may play a positive role in odontogenic/osteogenic differentiation of DMSCs.
Notch and shh signaling pathway:The Notch signaling pathway is critical for development and cell differentiation.Notch signaling has been confirmed to inhibit odontoblastic differentiation of hDPSCs[178].Interestingly,another study showed that overexpression of CCN3 activated the Notch signaling pathway to promote odontoblastic differentiation of DPSCs,which suggested that Notch signaling pathway activation promotes odontoblastic differentiation of DPSCs[179].The reasons for these contradictory effects in odontoblastic differentiation of DPSCs remain undefined and need to be explored.
It is worth noting that the Shh signaling pathway is also involved in odontogenic/osteogenic differentiation of DMSCs.A recent study has shown that stathmin regulates odontogenic/osteogenic differentiation of DPSCsviathe Shh signaling pathway[180].
Inflammation
In an inflammatory microenvironment,DMSCs from inflamed tissue contact and interact closely with extrinsic irritants,local cells or their components,immune cells and multiple soluble regulatory molecules[181].For example,dental caries are one such gram-negative microbial infection that is primarily responsible for pulpal inflammation.LPS was used to createin vitroinflammatory conditions that initiate infectionstem cell interaction,which has been used widely to induce an inflammatory microenvironment[182].
Immunophenotyping of cell surface antigens by flow cytometry showed that DMSCs and inflamed DMSCs have similar expression patterns of surface markers[181,183].The cells are positive for STRO-1,CD105,CD73,CD90,CD29 and CD44[184] and negative for CD45,CD34,CD14 and HLA-DR,indicating a mesenchymal stem cell phenotype[183,185-187].In addition,inflamed DMSCs have the potential to differentiate into multiple lineages.Mesenchymal stem cells isolated from inflamed pulp possess stemness and multidifferentiation potential similar to DPSCs from healthy pulp[185].Like DPSCs,inflamed DPSCs are capable of adipogenic and osteo/dentinogenic differentiation under the correspondingin vitroinduction conditions.However,chronic inflammation impairs differentiation of DPSCs[188].On the other hand,inflamed DPSCs show increased ALP and osteocalcin.In the inflammatory microenvironment,PDLSCs from inflamed periodontal tissue show higher proliferation rates but express lower levels of osteogenic differentiation markers[189-191].Both inflamed hPDLSCs and hPDLSCs have been successfully differentiated under osteogenic and adipogenic conditions[192].Because of evident similarities in their immunomodulatory properties,inflamed PDLSCs can provide a promising alternative to PDLSCs[193].Cells isolated from human periapical cysts demonstrate a strong osteogenic but weak adipogenic capacity[184,194].Osteogenic differentiation of inflamed DFSCs results in decreased ALP activity and alizarin red S staining compared to normal DFSCs[195].Similarly,the osteogenic differentiation of LPStreated DFSCs is suppressed,and the cells display low levels of TGF-β1 and high levels of TGF-β2.
Aging
Aging is an intricate degenerative process during which the regenerative capacity of MSCs progressively declines[196].Unavoidably,DMSCs also undergo physiological age-related changes with declines in proliferation and osteo/odontogenic differentiation potentials with increased age[197,198].Improving the performance of aging DMSCs is important for tissue regeneration engineering.Yiet al[144] demonstrated that the osteogenic potential of DPSCs from young donors was superior to that of those from old donors,and 304 mRNAs and 561 LncRNAs were differentially expressed between age-groups[144].Wanget al[199] found that miR-433 may be one of the important senescence-related miRNAs of human dental pulp cells,which inhibits mineralization of human dental pulp cells by negatively regulating GRB2 and the RAS-MAPK signaling pathway[199].SHED and DPSCs undergo senescence,including declines in the proliferation rate and osteogenic differentiation capability,following serial expansion from P4 to P20.SHED exhibit a better performance than DPSCs,which indicates that mineralization capacity is related to replicative senescencein vitroand to donor age[200].
As a significant factor regulating the function of differentiated odontoblasts[201],sclerostin advances the aging process of human dental pulp cells through the Wnt/βcatenin pathway and reduces the proliferation and odontoblastic differentiation capability of senescent human dental pulp cells[202].The Wnt/β-catenin signaling pathway is one of the important pathways that regulates cell differentiation,increasing the osteogenic/dentinogenic differentiation potential of DPSCs[203].It has been reported that the rate of dentin deposition and neurogenic differentiation potential declines with advanced age,which may be related to a decrease in endogenous Wnt/β-catenin signaling[204,205].
In 2014,Fenget al[206] compared the characteristics of DPSCs from five different age groups (5-12 years,12-20 years,20-35 years,35-50 years and>50 years) and found that the expression of p16INK4Amarkedly increased with age and inhibited osteogenic/odontogenic differentiation when upregulated[206].Then in 2017,Mas-Bargueset al[207] indicated that p16INK4Aalso played a part in oxidative stress-related premature senescence of DPSCs caused by long-term culture in 21% ambient oxygen tension compared with 3%-6% physiological oxygen tension[207].Replicative senescence of DPSCs resulted in decreases of B-lymphoma Mo-MLV insertion region 1,organic carbon,DSP and bone sialoprotein compared with rapidly proliferating cells and increases of p16INK4A,while B-lymphoma Mo-MLV insertion region 1 transduction promoted the expression of organic carbon and DSP,ALP activity and mineralized nodule formation.Therefore,this may indicate that the odontogenic differentiation potential of DPSCs weakens during senescence,partly due to decreased B-lymphoma Mo-MLV insertion region 1 expression[208].
In contrast,Maet al[209] reported that adult DPSCs cultured in juvenile dental pulp cell-conditioned medium demonstrated decreased osteogenic differentiation capability,whereas juvenile DPSCs induced by adult dental pulp cell-conditioned medium showed improved osteogenic differentiation capability,indicating that the activity of DPSCs can be modulated by the extrinsic microenvironment[209].A certain degree of inflammatory stimulation promoted the proliferation and mineralization of both adult and juvenile rat DPSCs,but this effect declined with age[210].Furthermore,Horibeet al[211] isolated a type of mobilized dental pulp stem cells induced by granulocyte colony-stimulating factor from young and old donors,which showed minimal characteristic changes with aging,suggesting that mobilized dental pulp stem cells act as an advantaged source in dental pulp regeneration[211].
Exosomes
Exosomes are vesicles secreted by different cells with a diameter of 30-100 nm.They can function as carriers for different components to impact intercellular communication,including various miRNAs,lncRNAs and proteins.Exosomes play an important role in mediating some signaling pathways to influence the physiological function of cells.In recent years,increasing research into the effect of exosomes on the odontoblastic/osteogenic differentiation of DMSCs has been proposed (Figure 2).

Figure 2 Reported extracellular vesicles that mainly contributed to the odontogenic/osteogenic differentiation process of dental mesenchymal stem cells.
In 2016,Huanget al[212] indicated that the exosomes derived from hDPSCs cultured with growth (DPSC-Exo) or odontogenic differentiation media (DPSC-ODExo) enhanced the odontogenic differentiation of DPSCsin vitro,and DPSC-OD-Exo showed stronger induction differentiation-inducing ability than exosomes derived from hDPSCs cultured with growth media in a three-dimensional environment consisting of type I collagen hydrogels and a tooth root-slice regeneration model[212].In 2019,Huet al[213] further identified the miRNA profile of human exosomes derived from hDPSCs cultured with growth media and DPSC-OD-Exo by miRNA sequencing,and the results indicated that miR-27a-5p was highly expressed in DPSC-OD-Exo,promoting odontogenic differentiation of DPSCs through the TGF-β1/Smad signaling pathway[213].
In 2019,Chewet al[214] reported that human MSC exosome-loaded collagen sponge used in an immunocompetent rat model with periodontal intrabony defects significantly repaired the defects by regenerating newly formed bone and periodontal ligament as a result of periodontal ligament cell migration and proliferation[214].Meanwhile in 2020,Wanget al[215] reported that conditioned SHED-Exos derived from a 3 d osteogenic supernatant improved the osteogenic ability of PDLSCs by activating the BMP/Smad and Wnt/β-catenin signaling pathways and that BMP2 and Wnt3a carried by SHED-Exos played a pivotal part in this process[215].
Moreover,extracellular vesicles (EVs) are a type of mixed vesicles,consisting of endosome-derived exosomes and cell membrane-derived ectosomes.In 2017,Liet al[216] demonstrated that the EVs derived from Schwann cells promoted the osteogenic differentiation of hDPSCs[216].In 2019,Čebatariūnienėet al[217] indicated that hPDLSC EVs did not influence osteogenic mineralization of PDLSCs but reversed the inhibitory effect on PDLSC osteogenic differentiation of an anti-TLR4 blocking Ab.They also revealed that the EVs may have a potential regulatory ability of genes related to osteogenesis and interfere with TLR4 signaling[217].Additionally,Pizzicannellaet al[218] reported that EVs derived from human GMSCs combined with a three-dimensional polylactide biomaterial enhanced the osteogenic differentiation of human GMSCsin vitro[218].
CONCLUSION
At present,most studies of the multidirectional differentiation of DMSCs focus on the following areas:the regeneration of teeth,bone,cartilage,tendon and blood vessels;the repair of nerve injury;the formation of retina and cornea;and the secretion of insulin.Different types of DMSCs have different abilities towards differentiation into diverse lineages.It is significant to explore the potential of DMSCs to differentiate into various tissues.In addition to the application of oral tissue regeneration,these studies are helpful to the future application of DMSCs in neurovascular injury-related diseases,retinal and corneal injury-related diseases and endocrine diseases such as diabetes.The induction of DMSCs to differentiate insulin-producing cells and neuronlike cellsin vitrorequires the conditioned-culture medium with a variety of auxiliary inducing factors,like some growth factors and peptides,and sometimes it needs to be induced in several steps,which takes a long time and is relatively complex.The cells induced by the conditioned culture medium express the specific molecules of related tissue-like cells.Researchers detect the specific expression molecules to determine whether the cells differentiate into specific tissue-like cells.Suchin vitrodifferentiation is often limited and may not represent the true differentiation of the cell itself.It is of great significance to improve the induction mode and shorten the induction time for the application of DMSCs in the future.In addition,combining DMSCs with materials possessing good biological compatibility may provide a better approach to tissue regeneration.
Making full use of the odontogenic/osteogenic differentiation ability of DMSCs is of great significance to the application of DMSCs in dental tissue regeneration engineering.In this review,some factors related to the regulation of DMSCs in odontogenic/osteogenic differentiation are reviewed.The regulation process of DMSC odontogenic/osteogenic differentiation is complex.A variety of non-coding RNAs and multiple signaling pathways participate in the differentiation process of DMSCs.The application of DMSCs should consider the donor age and cell aging.With increasing donor age and number of cell passages,differentiation ability may decrease accordingly.At the same time,the future clinical application of DMSCs should account for the impact of the inflammatory microenvironment.How to increase the antiinflammatory ability of DMSCs is a difficult problem for clinical application of DMSCs in the future.In addition,exosomes,as a crucial medium for communication and transmission of information between cells,have become a hotspot in recent years.In the process of normal tooth development,exosomes also seem to play an important role in regulating gene expression of target cells through their rich and varied contents.Utilizing the characteristics of exosomes endocytosed by cells,discovering other exosomes or transforming contents to promote DMSC odontogenic/osteogenic differentiation will be a future research direction.If we can positively regulate the related factors that advance the odontogenic/osteogenic differentiation of DMSCs and make full use of their differentiation potential,there will be great progress in the application of DMSCs in dental tissue regeneration engineering.Future research should emphasize effectively combining the various types of DMSCs with odontogenic/osteogenic,neurogenic,vascularization and other multipotencies to provide a potential scheme for dental tissue regeneration with normal functions.
杂志排行
World Journal of Stem Cells的其它文章
- Role of induced pluripotent stem cells in diagnostic cardiology
- Stem cell therapy in ocular pathologies in the past 20 years
- Programmed cell death in stem cell-based therapy:Mechanisms and clinical applications
- Low complexity domains,condensates,and stem cell pluripotency
- Different kinds of stem cells in the development of SARS-CoV-2 treatments
- Disease modifying treatment of spinal cord injury with directly reprogrammed neural precursor cells in non-human primates
