High fecal calprotectin levels are associated with SARS-CoV-2 intestinal shedding in COVID-19 patients: A proof-of-concept study
2021-06-24VerenaZerbatoStefanoDiBellaMauroGiuffreAnnaWladyslawaJaraczYleniaGobboDiegoLuppinoPaoloMacorLudovicaSegatRaffaellaKoncanPierlanfrancoAgaroMichaelValentiniLorySaveriaCroceMaurizioRuscioRobertoLuzzati
Verena Zerbato, Stefano Di Bella, Mauro Giuffre, Anna Wladyslawa Jaracz, Ylenia Gobbo, Diego Luppino,Paolo Macor, Ludovica Segat, Raffaella Koncan, Pierlanfranco D'Agaro, Michael Valentini, Lory Saveria Croce,Maurizio Ruscio, Roberto Luzzati
Abstract
Key Words: COVID-19 ; SARS-CoV-2 ; Obesity; Fecal calprotectin; Gut; Viral shedding
INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2 ) expresses a high affinity to human angiotensin-converting enzyme 2 (ACE2 ) receptors. High ACE2 expression was identified within the oral cavity, type II pulmonary alveolar cells, ileal and colonic enterocytes, myocardial cells, vascular endothelium, proximal tubule, and bladder urothelial cells[1 ].
One-third of coronavirus disease 2019 (COVID-19 ) patients have gastrointestinal symptoms, with diarrhea being the most common symptom[2 ]. Moreover, critically ill COVID-19 patients often develop intestinal complications. Ileus, gastrointestinal bleeding, and bowel ischemia are the most common[3 ,4 ]. Spontaneous intestinal perforations among COVID-19 patients are also increasingly seen in clinical practice[4 ].
SARS-CoV-2 RNA has been detected in stool samples of approximately 50 %COVID-19 individuals[5 ]. Often, viral detection in stools persists after viral clearance from respiratory samples[6 ], with a mean duration of fecal viral shedding of 17 d[7 ].
It is likely that SARS-CoV-2 can also be transmittedviathe fecal–oral route and that the potential of this mode of transmission has been widely underestimated[8 ].
The pathogenesis of gastrointestinal symptoms caused by SARS-CoV-2 is likely multifactorial, including disruption of the intestinal mechanical barrier integrity,alteration of the gut microbiome, increased translocation of bacteria and their metabolites, and systemic inflammatory response to the virus, which in critically ill patients could be disproportionate, with uncontrolled production and release of cytokines[1 ].
Calprotectin is a protein derived principally from neutrophils. Upon neutrophil activation or death, calprotectin is released extracellularly, where it has a role within the innate immune response with direct antimicrobial effects. It is present in many body fluids, in proportion to the degree of inflammation. The concentration of calprotectin in feces is about six times that in plasma, and its measurement is used as a surrogate marker of gastrointestinal inflammation[1 ,9 ].
Fecal calprotectin in COVID-19 patients was found to be a marker of intestinal inflammation, both in patients with and without gastrointestinal symptoms[10 ,11 ].Ojettiet al[12 ] also found a significant correlation between the development of pneumonia among COVID-19 patients and a high level of fecal calprotectin[11 ,12 ].
Serum calprotectin has been proposed as a severe COVID-19 progression marker,supporting innate immunity as a potential perpetrator of inflammation in COVID-19 .It should be kept in mind that neutropenia seen in the peripheral blood of COVID-19 patients should in part reflect neutrophil migration to the tissues[12 -14 ] and complement activation in different organs of COVID-19 patients[15 ] contributes to tissue damage and to the recruitment of neutrophils.
Given these premises, we aimed to investigate if fecal calprotectin correlates with SARS-CoV-2 intestinal shedding in hospitalized patients with COVID-19 pneumonia.
MATERIALS AND METHODS
We performed a prospective monocentric study enrolling consecutive adults (aged >18 years) with SARS-CoV-2 pneumonia admitted to the Infectious Diseases Unit of Trieste University Hospital, Italy, from September to November 2020 . Patients with inflammatory bowel diseases, gastrointestinal malignancy, and other known gastrointestinal disorders were categorically excluded.
SARS-CoV-2 detection in stool samples was determined by real-time polymerase chain reaction (RT-PCR) (LightMix®Modular SARS and Wuhan CoV E-gene and RdRp kit-TIB Molbiol, Berlin, Germany, with LightCycler Multiplex RNA Virus Master-Roche, Basel, Switzerland). According to the manufacturer’s specifications, fecal calprotectin levels were tested with the LIAISON®-Calprotectin (Diasorin, Vercelli,Italy) (normal value < 50 mg/kg). These tests were performed on hospital admission,regardless of gastrointestinal signs or symptoms.
The following data were collected on admission: Age, gender, comorbidities,gastrointestinal signs/symptoms, blood cell count, biochemical parameters, and clinical outcomes. In addition, administered drugs prior to and during hospitalization were collected. Diarrhea and obesity were defined as loose stools ≥ three times/day and a body mass index ≥ 30 , respectively.
According to the size of our sample, the Shapiro-Wilk test was performed to verify the normal distribution of variables. Inter-group differences (patients with diarrheavspatients without diarrhea and patients with fecal SARS-CoV-2vspatients without fecal SARS-CoV-2 ) were determined with the Mann-Whitney U test for continuous variables and the Pearson’s Chi Square Test for discrete variables. For all analyses,two-sided statistical significance was defined as aP< 0 .05 . Data were analyzed using SPSS (Statistical Package for Social Science) version 25 .0 (IBM SPSS Statistics for MAC OS. Armonk NY: IBM Corp.).
This study was conducted according to the declaration of Helsinki and approved by the Ethics Committee (Unique Regional Ethical Committee, Friuli Venezia-Giulia 16 April 2020 ), No. CEUR 2020 -OS-072 .
RESULTS
We enrolled 51 consecutive adults with SARS-CoV-2 pneumonia. Patient age ranged from 28 to 87 years [median 64 years, interquartile range (IQR) 57 ; 71 ] and 40 (78 %)were males. The most common comorbidities were: Hypertension (34 patients, 67 %),diabetes mellitus (5 , 25 %), obesity (5 , 25 %), heart disease (5 , 25 %), chronic kidney disease (5 , 10 %) and chronic obstructive pulmonary disease (5 , 10 %). Two (4 %)patients were active smokers. Ten (20 %) patients had diarrhea.
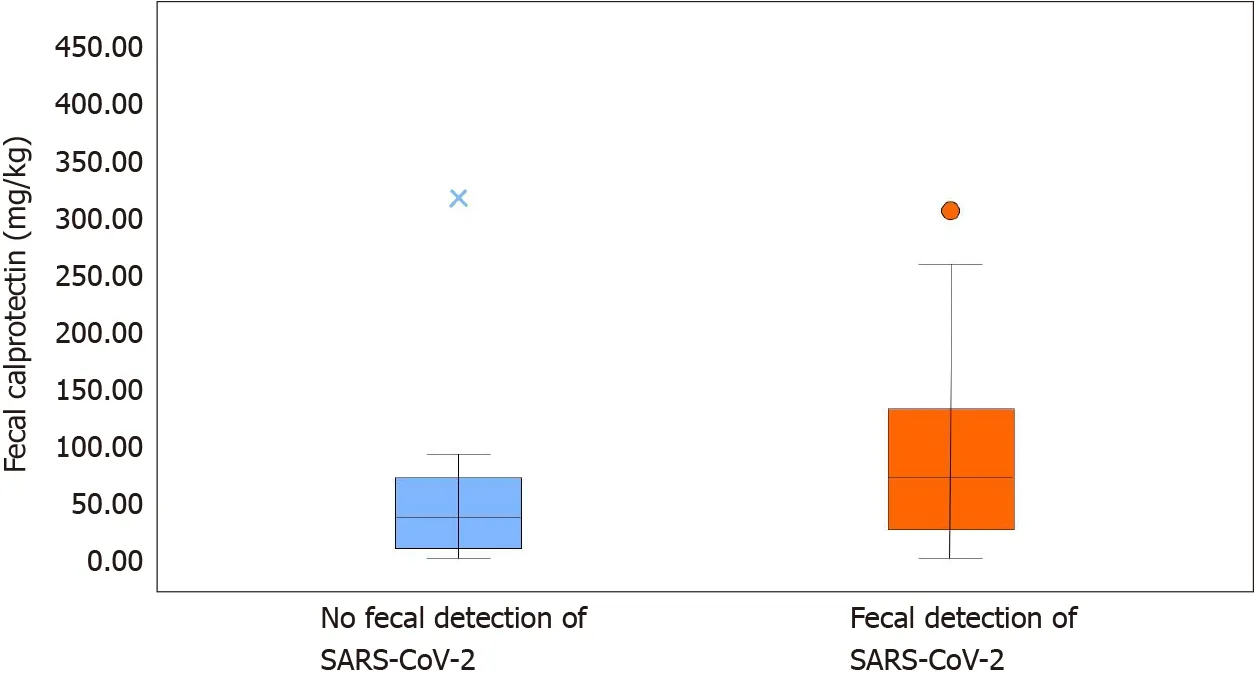
Figure 1 Fecal calprotectin content detected in stool with or without severe acute respiratory syndrome coronavirus 2 virus. SARS-CoV-2 :Severe acute respiratory syndrome coronavirus 2 .
Median fecal calprotectin levels were 60 mg/kg (IQR 21 ; 108 ). RT-PCR of SARSCoV-2 in stools was positive in 39 patients (76 %) (Figure 1 ). The clinical features and biochemical parameters of enrolled patients are reported in Table 1 .
No statistically significant differences in the compared variables between the two groups (patients with diarrheavspatients without diarrhea) were found (Table 1 ).Both groups showed increased fecal calprotectin levels: 58 mg/kg (IQR 31 ; 75 ) in the diarrhea group and 64 mg/kg (IQR 18 ; 108 ) in those without diarrhea. Fecal SARSCoV-2 was detected in all patients with diarrhea and in more than two thirds (29 /41 ,71 %) of patients without diarrhea.
Comparing patients with and without fecal SARS-CoV-2 shedding (Table 2 ), we found higher fecal calprotectin levels in the former (74 mg/kg, IQR 29 ; 132 .5 )compared to the latter group (39 mg/kg, IQR 14 ; 71 ) (P < 0 .001 ) (Figure 2 ). None of the patients without SARS-CoV-2 shedding had diarrhea. Neutrophil count was higher in patients with fecal SARS-CoV-2 shedding (P = 0 .035 ), as well as D-dimer levels (P=0 .011 ) and white blood cell count (P = 0 .038 ). C-reactive protein was higher in patients without fecal SARS-CoV-2 shedding (P = 0 .029 ). Fecal SARS-CoV-2 was found in all obese patients (P= 0 .021 ).
DISCUSSION
SARS-CoV-2 fecal shedding is a common finding among COVID-19 patients irrespective of gastrointestinal symptoms[16 ,17 ]. Our results confirmed that fecal SARS-CoV-2 was present in approximately three quarters of our patients with COVID-19 pneumonia.
Fecal calprotectin was found to be a marker of intestinal inflammation, both in COVID-19 patients and in the general population[10 ,11 ]. Our work demonstrates that hospitalized COVID-19 patients with pneumonia have high fecal calprotectin levels,regardless of gastrointestinal symptoms.
To our knowledge this is the first study to investigate whether fecal calprotectin correlates with SARS-CoV-2 intestinal shedding in COVID-19 patients. In our study,calprotectin levels were significantly higher in those with SARS-CoV-2 fecal shedding.This finding supports the hypothesis that bowel inflammation can lead to the “leaky gut” syndrome with potential distribution of the virus to other organs[18 ].
While the detection of SARS-CoV-2 in feces does not necessarily lead to more gastrointestinal symptoms, the presence of SARS-CoV-2 in gastrointestinal tissue generally correlates with more severe symptoms[19 ].
High fecal calprotectin in COVID-19 patients is likely secondary to increased neutrophil activation in the intestinal tract. In fact, SARS-CoV-2 can activate neutrophil extracellular traps and increase levels of intracellular reactive oxygen species[20 ].
In our cohort, all obese patients had SARS-CoV-2 RNA detected in stools. Obesity is one of the main risk factors for severe COVID-19 and poor clinical outcomes[20 ,21 ],and is associated with a strong inflammatory response both in the general population and in COVID-19 patients[22 ]. This could justify a more prolonged viral shedding inthis subgroup of patients.
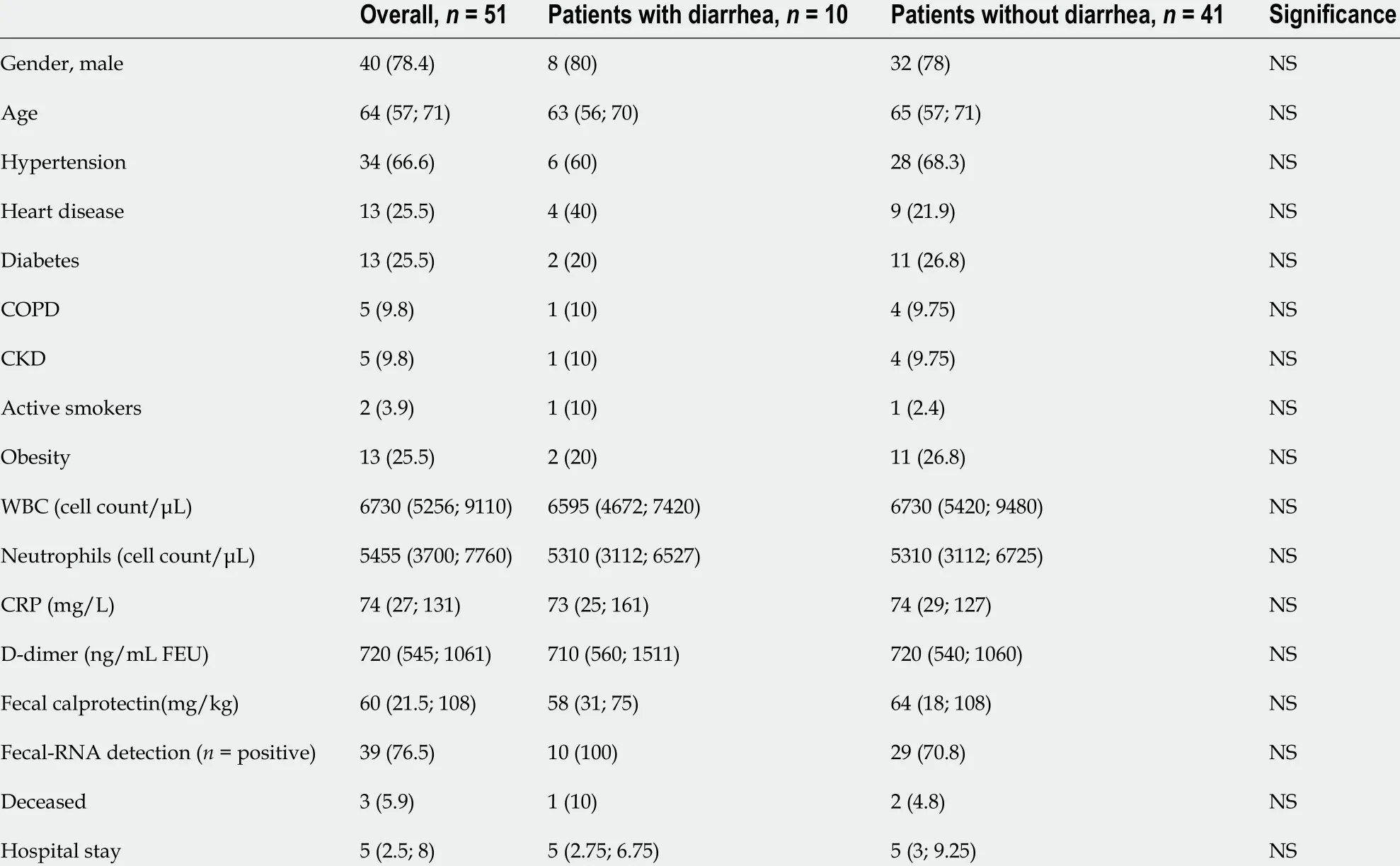
Table 1 Clinical and biochemical characteristics of coronavirus disease 2019 patients with and without diarrhea, n (%)
Our work has two main limitations. First, this was a monocentric study with a small sample of patients. Second, SARS-CoV-2 was detected in stools by PCR. This technique does not discriminate between live virus and non-infectious viral particles. Only a few studies have reported live SARS-CoV-2 in the stools of COVID-19 patients[23 -26 ].There is also limited evidence on the duration of fecal viral shedding[7 ].
Our study demonstrates that higher fecal calprotectin levels correlate with SARSCoV-2 fecal shedding in hospitalized COVID-19 patients with pneumonia. Our results support the role of neutrophils in SARS-CoV-2 pathogenesis.
CONCLUSION
In conclusion, our study provides two main results: (1 ) Fecal SARS-CoV-2 is present in approximately three quarters of hospitalized patients with COVID-19 pneumonia and in all patients with diarrhea; interestingly, all obese COVID-19 patients show fecal viral shedding; and (2 ) High fecal calprotectin levels are a common finding among hospitalized COVID-19 patients, especially those with SARS-CoV-2 fecal shedding. We believe that our results could strengthen the hypothesis that SARS-CoV-2 -induced intestinal damage mediated by innate immunity (complement activation and consequent activated neutrophil migration) could contribute to COVID-19 pathogenesis.

Table 2 Clinical and biochemical characteristics of coronavirus disease 2019 patients with and without severe acute respiratory syndrome coronavirus 2 fecal detection, n (%)

Figure 2 Comparison of patients with and without fecal severe acute respiratory syndrome coronavirus 2 shedding. SARS-CoV-2 : Severe acute respiratory syndrome coronavirus 2 .
ARTICLE HIGHLIGHTS

ACKNOWLEDGEMENTS
We thank Dr. Lisa Fusaro for her kind help.
杂志排行
World Journal of Gastroenterology的其它文章
- Role of imaging in evaluating the response after neoadjuvant treatment for pancreatic ductal adenocarcinoma
- Liver injury in COVID-19 : Detection, pathogenesis, and treatment
- Enhancer of zeste homolog 2 contributes to apoptosis by inactivating janus kinase 2 / signal transducer and activator of transcription signaling in inflammatory bowel disease
- Interplay between nuclear factor erythroid 2 -related factor 2 and inflammatory mediators in COVID-19 -related liver injury
- Helicobacter pylori promotes invasion and metastasis of gastric cancer by enhancing heparanase expression
- Role of bile acids in liver diseases mediated by the gut microbiome
