Role of imaging in evaluating the response after neoadjuvant treatment for pancreatic ductal adenocarcinoma
2021-06-24YunZhangZiXingHuangBinSong
Yun Zhang, Zi-Xing Huang, Bin Song
Abstract Pancreatic ductal adenocarcinoma (PDAC) is a highly aggressive malignancy.Despite the development of multimodality treatments, including surgical resection, radiotherapy, and chemotherapy, the long-term prognosis of patients with PDAC remains poor. Recently, the introduction of neoadjuvant treatment(NAT) has made more patients amenable to surgery, increasing the possibility of R0 resection, treatment of occult micro-metastasis, and prolongation of overall survival. Imaging plays a vital role in tumor response evaluation after NAT.However, conventional imaging modalities such as multidetector computed tomography have limited roles in the assessment of tumor resectability after NAT for PDAC because of the similar appearance of tissue fibrosis and tumor infiltration. Perfusion computed tomography, using blood perfusion as a biomarker,provides added value in predicting the histopathologic response of PDAC to NAT by reflecting the changes in tumor matrix and fibrosis content. Other imaging technologies, including diffusion-weighted imaging of magnetic resonance imaging and positron emission tomography, can reveal the tumor response by monitoring the structural changes in tumor cells and functional metabolic changes in tumors after NAT. In addition, with the renewed interest in data acquisition and analysis, texture analysis and radiomics have shown potential for the early evaluation of the response to NAT, thus improving patient stratification to achieve accurate and intensive treatment. In this review, we briefly introduce the application and value of NAT in resectable and unresectable PDAC. We also summarize the role of imaging in evaluating the response to NAT for PDAC, as well as the advantages, limitations, and future development directions of current imaging techniques.
Key Words: Pancreatic ductal adenocarcinoma; Neoadjuvant treatment; Imaging;Resectability; Tumor response; Prognosis
INTRODUCTION
Pancreatic ductal adenocarcinoma (PDAC) has become a major public health issue globally[1 ,2 ]. Due to the late onset of symptoms, the high invasive potential of the disease, and the lack of accurate diagnostic markers to detect micro-metastasis, the prognosis of PDAC has not significantly improved over the past several decades[3 ].
Neoadjuvant treatment (NAT) is an emerging therapy for PDAC. It provides the theoretical advantages of downstaging borderline resectable or locally advanced pancreatic tumors, allowing more patients to benefit from surgery, increasing the possibility of R0 resection, treating occult micro-metastasis, and prolonging overall survival (OS)[4 ,5 ].
With the advancements in imaging technology and the renewed interest in data acquisition and analysis, there are increasingly more studies reporting the accuracy of imaging for evaluating the response to NAT for PDAC. This review focuses on the role of imaging in the response assessment to NAT for PDAC, as well as the advantages,limitations, and future directions of current imaging techniques.
CLASSIFICATION AND TREATMENT STRATEGY OF PDAC
Various guidelines recommend the use of multiphase contrast-enhanced computed tomography (CT) imaging for staging PDAC and determining tumor resectability[6 ].According to the National Comprehensive Cancer Network guidelines, PDAC can be divided into four categories based on the preliminary assessment of tumor location and metastasis as follows[7 ]: (1 ) Resectable. The possibility of R0 resection is very high.The tumor has no contact with the adjacent arteries [superior mesenteric artery,hepatic artery, or celiac artery (CA)] and veins [superior mesenteric vein (SMV), portal vein (PV), or their confluence (SMV/PV)], or the degree of contact is less than 180 ° the circumference of the vessel wall; (2 ) Borderline resectable. The possibility of incomplete resection of R1 or R2 is high. The degree of contact between the tumor and arteries (superior mesenteric artery or CA) is less than 180 °, the tumor invades a short segment of hepatic artery that can be resected or reconstructed, but does not involve the CA, or tumor contact with the adjoining vein (SMV-PV) exceeds the circumference of the vessel wall by 180 °; (3 ) Locally advanced. The tumor cannot be resected due to invasion of the adjacent structures; and (4 ) Metastasis. The tumor has distant metastasis. As seen from this classification, the tumor–vessel contact is critical to determining whether the tumor is resectable. At the same time, the realization of R0 resection (marginal negative resection) is key to prolonging the life of patients with PDAC. Surgical resection is recommended for resectable PDAC, and the 5 -year survival rate of R0 resection is 18 %-24 %[8 ]. However, the 5 -year survival rate of borderline and locally advanced PDAC is poor, about 8 %-11 %[9 ,10 ]. In addition,almost 50 % of patients relapse within a short period of time after tumor resection[11 ].Other therapies such as chemotherapy and radiotherapy are usually used in unresectable cases to reduce the disease burden and prolong the survival time.However, even then, the prognosis remains poor with a median survival time of 6 .8 -11 .1 mo in patients with unresectable tumor[12 ,13 ]. Hence, to achieve better survival,downstaging of the disease by NAT followed by surgical resection if possible has been advocated for unresectable PDAC.
NAT FOR RESECTABLE PDAC
In the past, the standard treatment for resectable PDAC was surgical resection followed by adjuvant chemotherapy. In 2008 , a large retrospective study included surgically resectable PDAC and studied the impact of NAT on the prognosis of resectable PDAC[14 ]. The results showed that NAT followed by surgery had a survival advantage over upfront surgery with or without adjuvant treatment, which suggested the possible role of NAT in treating resectable PDAC. A recent metaanalysis[15 ] showed that the median OS of patients receiving NAT was longer than that with surgery (18 .8 mo vs 14 .8 mo). Although the overall resection rate of NAT was lower than that of previous surgery (66 % vs 81 .3 %), the R0 rate was higher (86 .8 %vs66 .9 %). In addition, other studies have reported that the tumor resection rate after NAT is between 50 % and 90 %, with a median survival time of 23 .5 mo[16 -19 ].
NAT FOR BORDERLINE AND UNRESECTABLE PDAC
Accumulating evidence indicates that patients with borderline PDAC can benefit from NAT, as it increases the chance of R0 resection, improves survival, and identifies cases of PDAC with rapid progression and poor response to treatment[20 -22 ]. An international consensus has proposed that patients with borderline PDAC undergo surgical resection after NAT when there are no anatomical contraindications or metastatic disease[23 ]. A multicenter trial showed that neoadjuvant S-1 therapy in combination with radiotherapy followed by surgery achieved an R0 resection rate of 63 %[24 ]. Inoueet al[25 ] reported that NAT with gemcitabine and nab-paclitaxel improved downstaging of the tumor and allowed patient selection. Angeret al[26 ] investigated the role of NAT in different types of borderline PDAC according to international consensus criteria. Their results showed that PDAC patients with biological(borderline resectable-B) have a relatively poor prognosis and should be considered for multimodal NAT.
In unresectable PDAC, palliative systemic chemotherapy is commonly used. In these patients, the main purpose is to extend the survival time under acceptable general conditions. FOLFIRINOX and gemcitabine in combination with other drugs are the preferred regimens for patients with good performance status, whereas capecitabine, gemcitabine, and 5 -fluorouracil monotherapy are usually given to patients in poor general condition[12 ,27 -29 ].
In summary, NAT for resectable PDAC performed before primary surgery increases the R0 resection rate and prolongs postoperative survival. In borderline and unresectable PDAC, NAT increases the chance of R0 resection, providing strong evidence for individualized patient treatment, eventually resulting in extending survival (Figure 1 ). However, there are some limitations in the current research. First,there is a lack of consensus on the best regimen for NAT. Second, some studies have not strictly separated resectable PDAC from unresectable PDAC during NAT. Finally,there is no clear consensus on the duration of NAT.
RESPONSE ASSESSMENT OF PDAC AFTER NAT
Conventional ultrasound
Conventional ultrasound (US) is an economical and radiation-free investigation. It helps to visualize pancreatic masses that are iso-attenuating on non-contrast CT images. However, its role in assessment of tumor response is very limited. US can be,however, a useful tool for detecting abdominal complications and drug toxicity during NAT in metastatic pancreatic cancer. The most common adverse effects of NAT regimens for metastatic pancreatic cancer are neutropenic colitis and venous thrombosis, which can be readily detected by abdominal US[30 ].
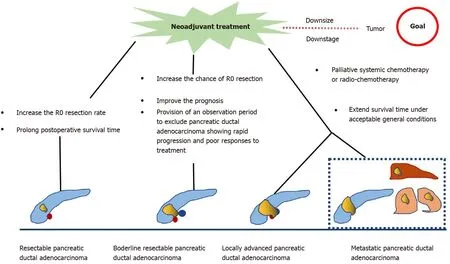
Figure 1 Role of neoadjuvant treatment for different types of pancreatic ductal adenocarcinoma.
Endoscopic ultrasound
Endoscopic US (EUS) has developed from a strictly imaging modality to one that allows for tissue diagnosis through fine needle aspiration. It has been proved to be a valuable means for early detection and staging of PDAC, especially for lesions ≤ 3 cm,which is superior to multi-detector CT[31 ,32 ]. Recently, the role of EUS in the delivery of NAT in PDAC and its response assessment is rapidly emerging. Daset al[33 ]conducted a large sample study to investigate the value of EUS in preoperative tumor response prediction of PDAC after NAT. The results showed that the change in the tumor size after NAT on EUS was a sensitive marker for tumor response evaluation,and tumor size reduction ≥ 47 % was an independent prognostic factor for OS in these patients. A systematic review from Barretoet al[34 ] compared the accuracy of imaging modalities to predict resectability and R0 resection for borderline or locally advanced PDAC after NAT. They showed that effective imaging evaluation allowed prediction of tumor resectability. Moreover, decrease in tumor stiffness of PDAC on EUS elastography may be used as a potential marker for NAT response and tumor resectability assessment. In addition, Figueiredoet al[35 ] reported the role of EUS-guided technology in the implementation of NAT for PDAC. The authors indicated that EUSguided placement of fiducial markers for stereotactic body radiation therapy in PDAC helped to ensure the feasibility and security of subsequent NAT. The above studies illustrate the importance of EUS in the process of NAT for PDAC.
Multi-detector CT
Multidetector CT (MDCT) is the most frequently used imaging method to evaluate the response of PDAC after NAT. Compared with other imaging techniques, its advantages include higher spatial resolution and multiplanar reconstruction ability[36 ]. However, recent studies have shown that the diagnostic performance of MDCT in evaluating tumor resectability and re-staging of borderline tumors is not very satisfactory. In a study of 129 patients with borderline PDAC, the authors found that the commonly used response evaluation criteria in solid tumors (RECIST) criteria were not suitable for evaluating tumor response after NAT, because there were few morphological changes in the imaging after treatment[37 ]. A systematic review reported that only a small number of patients showed tumor shrinkage after NAT(Figure 2 ), and most patients (53 %-80 %) had stable disease[34 ]. Similar results were reported by a recent study[38 ] that showed that the assessment of resectability by MDCT after NAT is relatively insensitive and non-specific to predict R0 resection,because MDCT cannot accurately distinguish between residual tumor and tissue scarring after tumor regression[39 ]. Moreover, local inflammatory pancreatitis also cannot be distinguished from tumor infiltration and the area of tumor infiltration is replaced by fibrotic tissue, which does not lead to apparent changes in tumor size(Figure 3 ). All of these factors lead to under-evaluation of tumor resectability[40 -42 ].

Figure 2 Response assessment with contrast-enhanced computed tomography after neoadjuvant treatment. A-C: A 55 -year-old man with a 2 .4 cm tumor in the pancreas with non-uniform low density (A), showing no obvious enhancement relative to the surrounding pancreatic parenchyma in the arterial phase(B), without hyperenhancement or distinct ‘wash-out’ appearance in the portal venous phase (C); D-F: After 20 d of neoadjuvant treatment (FOLFOX), tumor size was reduced to 2 .1 cm, with low enhancement relative to the surrounding pancreatic parenchyma on contrast-enhanced computed tomography images.
Recently, some studies have started to explore whether imaging features other than tumor size and enhancement on MDCT images can be used to assess tumor response in PDAC. A study by Cassinottoet al[43 ] showed that the partial regression of tumor–vessel contact after NAT indicates suitability for surgical exploration,regardless of the reduction in tumor size or residual vascular involvement. Another study by Ameret al[44 ] suggested that changes at the PDAC/parenchyma interface may be used as an early predictor of response to NAT. A recent study from Weiet al[45 ] showed that the largest tumor diameter and radiological tumor volume on posttherapy MDCT were associated with the pathologic tumor staging and tumor response to NAT.
Although MDCT has a high resolution in displaying morphological characteristics of the tumor and the surrounding vascular structures, it has low specificity due to the lack of obvious tumor reduction after NAT in PDAC, as well as the presence of fibrous tissue and local pancreatitis. Hence, MDCT has low specificity and sensitivity in restaging of PDAC after NAT. However, further quantification and evaluation of imaging indicators on MDCT images can significantly improve the assessment of tumor response and prognostic value of patients with PDAC after NAT.
Magnetic resonance imaging
Magnetic resonance imaging (MRI) provides better visualization of the soft tissues and pancreatic and biliary ductal abnormalities. In PDAC, the high cellularity and potential fibrosis of the tumor hinders the free movement of water molecules. This can be quantified by diffusion-weighted imaging (DWI) on MRI, which results in a low mean apparent diffusion coefficient (ADC) on the ADC map. Several studies have investigated the utility of DWI for assessment of the NAT response in patients with PDAC[46 -50 ]. A previous study conducted by Cuneo et al[46 ] reported that there was an obvious correlation between the mean ADC values before treatment and the amount of destruction of tumor cells after neoadjuvant chemoradiation. The mean pretreatment ADC (161 × 10 -5 mm2 /s) of patients with a good response was significantly higher than that of non-responders (125 × 10 -5 mm2 /s), which may provide evidence for candidate selection of intensified therapy. Dalahet al[47 ] investigated the relationship between ADC values after neoadjuvant chemoradiation and the pathological treatment response in PDAC and showed that the post-mean ADC values were moderately correlated with the pathological tumor responses. The study also noted that, compared to tumors with poor pathological response, tumors with a good pathological response had a higher ratio of fibrosis to tumor cells. However, different results were reported by a recent study from Zimmermannet al[48 ]. The authors found that mean ADC cannot be used as a reliable marker to identify PDAC patients with a good response to NAT.
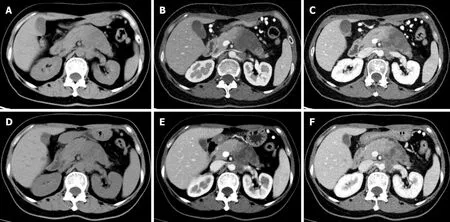
Figure 3 Response assessment with contrast-enhanced computed tomography after neoadjuvant therapy. A-C: A 58 -year-old woman with a 5 .6 cm × 4 .2 cm tumor in the pancreatic body and tail with non-uniform low-density (A), showing slightly enhancement relative to the surrounding parenchyma in the arterial phase (B), with invasion of the left renal vein as seen in the portal venous phase (C); D-F: After 2 .5 mo of neoadjuvant treatment (nab-paclitaxel combined with gemcitabine), the tumor size was reduced to 4 .7 cm × 4 .2 cm (D), small patchy enhancement was seen in the original lesion (E), and the degree of invasion of the left renal vein was reduced (F).
A prospective study by Okadaet al[49 ] of 28 patients with borderline PDAC found that the post-treatment whole-tumor ADC value predicted R0 resection with 75 %accuracy and histological response with 89 % accuracy. Based on these findings, the authors suggested that whole-tumor ADC value can be used as the new marker for treatment response evaluation in PDAC. Baliet al[50 ] compared the effectiveness of DWI and RECIST criteria in evaluating tumor response to systemic chemotherapy in unresectable PDAC. This study selected three imaging biomarkers, ROI-ADC, DW volume, and diffusion parameters derived from histograms, and showed that these markers more accurately classified between responders and non-responders compared to RECIST 1 .1 criteria. So, the ADC value of DWI shows better performance than morphological features in evaluating the tumor response to NAT in PDAC. However,at present, there are few related studies with inconsistent conclusions, and there are no unified standard criteria for the selection of DWI parameters. In addition, DWI has the disadvantages of large motion artifacts, long time-consuming procedures, and high cost, limiting its clinical application.
Positron emission tomography
18 F-fluorodeoxyglucose positron emission tomography (18-F-FDG-PET) is a diagnostic test that reflects the genetic, molecular, metabolic, and functional status of the lesions.The maximum standardized uptake value (SUVmax) obtained by PET imaging reflects the glucose metabolism of the tumors. Choiet al[51 ] explored the relationship between the early treatment response after neoadjuvant chemo-radiotherapy using FDG-PET and surgical outcome in locally advanced PDAC. The results suggested that FDG-PET is helpful to monitor the clinical efficacy of NAT in the treatment of locally advanced PDAC. In patients with a good tumor response (≥ 50 % decrease in SUV after cycle 1 ),surgical resection was accomplished. Leeet al[52 ] investigated the role of pre-operative18-F-FDG PET/CT in predicting survival of patients with PDAC. The results showed that metabolic tumor volume and total lesion glycolysis were independent prognostic factors for predicting recurrence-free survival and OS of patients, and PET/CT provides useful prognostic information for patients after resection of PDAC with curative intent irrespective of NAT.
Recently, some studies have further studied the value of SUVmax in PDAC to assess the tumor response after NAT[48 ,53 ,54 ]. The results showed that the post-NAT SUVmaxcan be an effective indicator to predict the prognosis and treatment response to NAT for PDAC. Moreover, the post-treatment metabolic parameters of PET/MRI also reportedly correlate with the pathological response. However, the sample size of the above studies was relatively small (< 50 cases in 2 /3 articles), and the results were not convincing. Barneset al[55 ] enrolled 201 consecutive patients with localized PDAC treated with NAT. The results showed that the pre-treatment SUVmax cut-off value of 7 .5 on PET/CT could accurately predict the OS. The results from Yokose et al[56 ]showed that PET response criteria in solid tumor was more accurate in determining the NAT effects for PDAC than RECIST criteria (72 .7 % vs 36 .4 %), which puts forward a new direction for future research in this field.
The application of PET imaging indicates that the response assessment to NAT in PDAC has changed from morphological to metabolic evaluation, especially with the proposal of PET/MRI, which plays a significant role in the prediction of treatment outcome and clinical decision-making in these patients. However, due to the lack of literature, the results need to be further investigated. In addition, the PET response criteria in solid tumor criteria need to be compared with traditional evaluation criteria in future studies.
Perfusion CT
PDAC is a matrix-rich tumor, characterized by the activation of pancreatic stellate cells, which deposit a large amount of extracellular matrix[57 ]. The accumulation of extracellular matrix, including collagen, fibronectin, proteoglycan, and hyaluronic acid, can induce the formation of rigid extracellular matrix to compress blood vessels,leading to perfusion damage and ultimately hindering the transmission of anti-cancer drugs to the tumor cells[58 ] (Figure 3 ). Based on the above theoretical assumptions,Hamdyet al[59 ] investigated the value of perfusion CT in predicting the response of PDAC to neoadjuvant chemotherapy and radiation therapy. The results showed that participants who responded to NAT had higher baseline blood flow than those who did not respond (median, 44 mL/100 g/min vs 28 mL/100 g/min, respectively). In responders, the perfusion parameters increased after treatment, whereas there were no significant changes in perfusion parameters of non-responders. The authors suggested that pre-treatment perfusion CT can be helpful to predict the histopathologic response of PDAC to NAT. However, the exact role of perfusion imaging in PDAC needs to be further evaluated in a larger number of patients.
Texture analysis and radiomics
In recent years, many studies have emphasized the role of radiomics in various aspects of pancreatic tumors, such as tumor characterization, assessment of resectability, risk of recurrence, and prediction of survival. A previous study from Chenet al[42 ] showed changes in the CT radiomic features, such as the histograms of mean CT number,skewness, and kurtosis during the chemoradiation in patients with PDAC. The authors suggested that these changes may potentially be used for the early evaluation of the treatment response and patient stratification to achieve accurate and intensive treatment. Chakrabortyet al[60 ] conducted a preliminary study to investigate the value of CT texture analysis in quantifying tumor heterogeneity and predicting 2 -year survival in patients with PDAC. The results revealed that CT texture features can predict the heterogeneity in pancreatic tumors. Using fuzzy minimum-redundancy maximum-relevance feature selection and a naïve Bayes classifier, the area under the curve scaled up to 90 %. At the same time, the accuracy of CT texture analysis in predicting the 2 -year survival rate can reach 82 .86 %. Thus, it can be used to formulate the optimal treatment plan for PDAC patients. Ciaravinoet al[61 ] investigated the added value of CT texture analysis in estimating tissue changes in PDAC downsized and resected after chemotherapy. The results suggested that the change of kurtosis before and after treatment showed a statistically significant difference, suggesting that CT texture analysis can assess tumor heterogeneity, tissue changes, and tumor downstaging in PDAC cases with no significant alteration in tumor size after NAT.
Recently, some studies have explored the utility of CT texture analysis in predicting resectability and prognosis in patients with PDAC[62 ] and the relationship between texture features and the tumor pathological response[63 ]. The results showed that the CT texture feature was more accurate in defining the tumor as resectable than unresectable. Moreover, higher subtracted entropy and lower subtracted gray-level cooccurrence matrix entropy indicated longer OS[62 ]. In the study by Borhani et al[63 ],texture parameters, such as pre-treatment mean positive pixel, pre-treatment kurtosis,changes in kurtosis, and pre-treatment tumor SD, were found to be statistically different between patients with poor histologic response and those with favorable histologic response. The authors concluded that pre-treatment textural features of baseline CT imaging and longitudinal changes in tumor heterogeneity can be used as biomarkers for predicting histologic response to neoadjuvant chemotherapy and disease-free survival. In addition, Nasiefet al[64 ] reported the value of radiomics combined with carbohydrate antigen 19 -9 (CA19 -9 ) in the evaluation of NAT for PDAC. The results showed that reduction of CA19 -9 levels and delta radiomics features were predictors of survival in these patients. The delta radiomics features-CA19 -9 combination has the potential to increase the possibility for response-based treatment adaptation. There is no denying that radiomics or texture analysis has wide prospects in the management of PDAC after NAT. The current limitations of radiomics include the time-consuming segmentation and non-robustness conclusion. Further large-scale studies are required to determine its real potential.
MERITS AND SHORTCOMINGS OF CURRENT IMAGING IN NAT RESPONSE FOR PDAC
In all stages of NAT for PDAC, imaging plays an essential role in the diagnosis,assessment of resectability, tumor re-staging, and response evaluation after NAT(Table 1 ). The change in tumor size as assessed by EUS after NAT can provide valuable information on tumor response and survival prediction. Moreover, change in tumor stiffness of PDAC on EUS elastography may be used as a potential marker for NAT response and tumor resectability assessment. MDCT, which relies on its highdensity resolution and the speed of data acquisition, is often used to evaluate the resectability and tumor response of PDAC after NAT. However, because of the fibrotic and infiltrative nature of PDAC, changes in the size of the tumor after NAT are not apparent, and it is difficult to distinguish accurately between residual tumor and scarring from tumor regression. Hence, the value of conventional CT imaging features in evaluating NAT for PDAC is limited. ADC value quantified by DWI on MRI can reflect the cellularity and potential fibrotic changes of PDAC after NAT. Moreover,pre-treatment ADC can be used as an imaging biomarker to distinguish responders from non-responders after NAT. However, due to the shortcomings of large motion artifacts, the time-consuming process and high cost, as well as the inconsistency of existing results, the application of DWI in NAT for PDAC needs further confirmation.PET has great prospects for the prediction and evaluation of NAT for PDAC, which represents the transformation of imaging markers from morphological to metabolic.However, due to the lack of substantial evidence and the costly nature of the PET technique, the role of PET in PDAC is limited and needs further investigation.Perfusion CT, as a new imaging method to assess the response to NAT in PDAC, has proved to be beneficial. It can reveal the changes of extracellular matrix by monitoring the changes in the tumor blood perfusion, so as to predict and evaluate the efficacy of NAT in PDAC. However, more research is needed to confirm the findings of the preliminary studies. Radiomics and texture analysis have gradually become research hotspots in the field of NAT for PDAC, providing additional values for evaluating tumor heterogeneity. However, the time-consuming segmentation and non-robustness of current data cannot be denied.
CONCLUSION
The role of conventional CT imaging features in the evaluation of NAT response for PDAC is limited. Other imaging techniques, including EUS, DWI, PET, and perfusion CT, have enormous potential to become powerful tools for the assessment of tumor resectability and survival prediction of PDAC after NAT. In addition, the derivate techniques based on artificial intelligence, such as texture analysis and radiomics, have gradually begun to show their prominence in the field of NAT for PDAC. Although current research is limited and the conclusions are inconsistent, additional research conducted in this field will address the shortcomings of the existing evaluation system for PDAC and promote the implementation of precision medicine.

Table 1 Role of current imaging in the response assessment after neoadjuvant treatment for pancreatic ductal adenocarcinoma
杂志排行
World Journal of Gastroenterology的其它文章
- High fecal calprotectin levels are associated with SARS-CoV-2 intestinal shedding in COVID-19 patients: A proof-of-concept study
- Liver injury in COVID-19 : Detection, pathogenesis, and treatment
- Enhancer of zeste homolog 2 contributes to apoptosis by inactivating janus kinase 2 / signal transducer and activator of transcription signaling in inflammatory bowel disease
- Interplay between nuclear factor erythroid 2 -related factor 2 and inflammatory mediators in COVID-19 -related liver injury
- Helicobacter pylori promotes invasion and metastasis of gastric cancer by enhancing heparanase expression
- Role of bile acids in liver diseases mediated by the gut microbiome
