Liver injury in COVID-19 : Detection, pathogenesis, and treatment
2021-06-24YueCaiLiPingYeYaQiSongXinLiMaoLiWangYanZhiJiangWeiTaoQueShaoWeiLi
Yue Cai, Li-Ping Ye, Ya-Qi Song, Xin-Li Mao, Li Wang, Yan-Zhi Jiang, Wei-Tao Que, Shao-Wei Li
Abstract In the early December 2019 , a novel coronavirus named severe acute respiratory syndrome coronavirus 2 was first reported in Wuhan, China, followed by an outbreak that spread around the world. Numerous studies have shown that liver injury is common in patients with coronavirus disease 2019 (COVID-19 ), and may aggravate the severity of the disease. However, the exact cause and specific mechanism of COVID-associated liver injury needs to be elucidated further. In this review, we present an analysis of the clinical features, potential mechanisms,and treatment strategies for liver injury associated with COVID-19 . We hope that this review would benefit clinicians in devising better strategies for management of such patients.
Key Words: COVID-19 ; Liver injury; Clinical features; Potential mechanism; Treatment
INTRODUCTION
Coronavirus disease 2019 (COVID-19 ), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2 ), which first broke out in Wuhan, China in December 2019 , has become a great threat to public health worldwide. As of August 4 ,2020 , more than 1000000 deaths from COVID-19 have been confirmed[1 ]. Although the lung is the main organ that is damaged in COVID-19 , approximately 60 % of the patients were reported to develop various degrees of liver injury in previous studies[2 -5 ]. Accumulating clinical data show that liver damage is related to the severity of COVID-19 and is also a major cause of death from COVID-19 , especially in the presence of hepatic failure[6 ,7 ]. Thus, early detection, effective treatment, and elucidation of the mechanisms underlying the pathogenesis of liver damage are urgently needed for COVID-19 patients. In this review, we summarize the characteristics of COVID-19 -associated liver injury from multiple perspectives, including clinical features (manifestation, laboratory examinations, liver biopsy,etc.), underlying pathogenesis (direct viral cytotoxicity, uncontrolled cytokine storm, drug-induced toxicity,etc.), special population of patients (those with cirrhosis, hepatitis B, liver transplantation,etc.), and clinical management (drugs, oxygen therapy, artificial liver blood purification,etc.). Based on the latest data, we hope to provide a feasible reference for follow-up clinical management of COVID-19 .
CLINICAL FEATURES OF HEPATIC INJURY IN COVID-19
In patients with COVID-19 , the most commonly used indicators of liver function impairment are liver transaminase, bilirubin, and albumin levels. Abnormal levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and total bilirubin were reported in 11 %-56 .3 %, 15 .0 %-86 .8 %, and 2 .7 %-30 .6 % of patients with COVID-19 ,respectively, whereas 2 %-11 % of such patients had pre-existing liver disease[3 -8 ]. In a recent study involving 228 patients with COVID-19 , who did not have chronic liver disease (CLD), abnormal liver function was observed in 129 (56 .3 %) patients, which included elevations in the levels of ALT [84 (36 .8 %)], AST [58 (25 .4 %)], total bilirubin[59 (25 .9 %)], and gamma-glutamyl transferase [67 (29 .5 %)][9 ]. In a study on 99 patients, Chenet al[10 ] reported elevated levels of ALT and AST in 28 (28 %) and 35 (35 %) patients, respectively, and hypoalbuminemia and hyperbilirubinemia in 97 (98 %) and 18 (18 %) patients, respectively. In addition, several studies revealed that liver damage is more prevalent in severe cases of COVID-19 than in mild cases. In a large sample multicenter study[11 ], abnormally elevated levels of ALT were observed in 28 .1 % of critically ill patients and in 19 .8 % of non-critically ill patients, and of AST in 18 .2 % of non-critically ill patients and 39 .4 % of critically ill patients. Huang et al[4 ]also indicated that patients admitted to the intensive care unit (ICU) not only had higher plasma levels of the inflammatory indices [interleukin (IL)-2 , IL-7 , IL-10 , tumor necrosis factor (TNF)-α,etc.], but also had abnormally high levels of AST [8 (62 %) of 13 patients] compared with non-ICU patients [7 (25 %) of 28 patients]. A recent descriptive study confirmed that the levels of ALT (35 vs 23 , normal range 9 -50 U/L, P = 0 .007 )and AST (52 vs 29 , normal range 5 -21 U/L, P < 0 .001 ) were significantly higher in ICU patients[12 ]. In a Japanese cohort study concerning COVID-19 , patients were classified into mild, moderate, and severe groups, respectively, based on gastrointestinal symptoms and severity of pneumonia; and the peak levels of AST (28 vs 48 vs 109 ,P<0 .001 ) and ALT (33 vs 47 .5 vs 106 , P = 0 .0114 ) were significantly stratified according to these criteria[13 ].
Apart from the liver enzyme tests mentioned above, there are characteristic clinical manifestations of liver damage. In China, it has been reported that some patients recovering from severe COVID-19 exhibited darkening and pigmentation during the recovery process. Multiple organ damage, especially liver damage, is the main cause of darkening and hyperpigmentation[14 ]. Abnormal liver function may lead to pigmentation through the following three pathways: (1 ) Impaired liver function leads to hypofunction of the adrenal cortex. When the liver is unable to metabolize the melanin-stimulating hormone secreted by the anterior pituitary gland, the secretion of melanin increases[15 ]; (2 ) abnormal liver function hinders the inactivation of estrogen,leading to an increase in its level. The increase in estrogen levels in the body reduces the inhibition of tyrosinase by thiamine, thereby increasing the conversion of tyrosine to melanin[16 ]; and (3 ) liver damage increases the iron content in the blood. Iron delivered to the facial skin causes the darkening of the face.
Liver biopsy is also important in the etiological diagnosis of hepatic injury in COVID-19 , particularly in cases where liver damage dominates the clinical manifestation, or where other alternative causes of damage need to be ruled out.Currently, most of the information on histological changes in the liver of patients with COVID-19 comes from autopsies. A case series by Bradley et al[17 ], based on ultrastructural findings and histopathology of samples from 14 fatal COVID-19 infections in the Washington State, showed centrilobular necrosis, consistent with hypoperfusion injury, in four patients; viral RNA was detected in the liver of these patients, as well. However, autopsies have several limitations. Death may occur long after the acute liver injury is noted; subsequently, histological changes may have been eliminated or obscured, and viral load would have diminished over time. Fielet al[18 ]presented their findings from two liver biopsies performed on patients infected with SARS-CoV-2 . Both patients had severe hepatic failure in the absence of obvious involvement of other organs. Detailed histological analysis,in situhybridization, and electron microscopy revealed that apoptosis, abundant mitosis, mixed inflammatory infiltration in the portal area, severe bile duct injury, apparent viral particles, and viral RNA within hepatocytes are typical. These findings suggested hepatic involvement in infections with SARS-CoV-2 . Another case report by Melquist et al[19 ] showed similar findings in a patient infected with SARS-CoV-2 , manifesting as acute hepatitis without any respiratory symptoms, rapidly progressing to fulminant liver failure. Acute hepatitis (panacinar hepatitis, zone 3 necrosis, and focal hemophagocytosis) with virallike changes was identified at the time of liver biopsy.
POTENTIAL MECHANISMS OF LIVER INJURY IN PATIENTS WITH COVID-19
The available evidence supports that hepatic injury in SARS-CoV-2 infection is a consequence of a multifactorial attack. The potential mechanisms of pathogenesis may be broad spectrum, ranging from direct cytotoxicity from viral infection to indirect involvement of the inflammatory cytokine storm, hypoxic changes caused by respiratory failure, endotheliitis, and drug-induced liver injury (DILI)[20 ] (Figure 1 ).
Direct effect of viral infection on the liver
Recently, it was determined by quantitative reverse transcription-polymerase chain reaction that SARS-CoV-2 RNA is widely present in other organs outside the respiratory tract, such as the liver[21 ], although the exact cell location of replication has not been determined because of the isolation of nucleic acids by whole tissue homogenization. Until recently, a typical hepatitis picture is yet to be observed, and hepatic tropism and direct cytopathic effects of SARS-CoV-2 should be considered as the underlying mechanism of COVID-19 associated liver injury[22 ]. A major determinant of viral tropism is the availability of viral receptors on the surface of host cells in specific tissues. Cellular entry of SARS-CoV-2 is mediated by the spike (S)protein of the virus, which is cleaved by transmembrane serine protease 2 /transmembrane serine protease 4 and specifically interacts with angiotensin converting enzyme 2 (ACE2 ) in the host[23 ] (Figure 2 ). According to Human Protein Atlas, ACE2 is highly expressed in the lung (type II alveolar cells), intestine, and gall bladder, but it seems to be almost absent in the liver. After in-depth research on ACE2 expression patterns, sinusoidal endothelial cells appear to be negative for ACE2 , but this protein is expressed in the central hepatic vein and portal vein endothelial cells[24 ]. The expression level of ACE2 in the bile duct epithelium is comparable to that in alveolar epithelial cells, being almost 20 -times higher than that in hepatocytes. Of note,Letkoet al[25 ] revealed that compensatory differentiation and proliferation of liver parenchymal cells derived from bile duct cells leads to the upregulation of ACE2 expression in liver tissues, which might be the underlying mechanisms in COVID-19 -associated liver injury.
Notably, typical coronavirus particles (characterized by S structures) were identified in the cytoplasm of hepatocytes in an ultrastructural examination by Wanget al[26 ]. In this study, typical lesions of viral infection, including conspicuous mitochondrial swelling, decreased glycogen granules, and endoplasmic reticulum dilatation, were also observed in the SARS-CoV-2 -infected hepatocytes, which indicated that hepatic impairment might be directly caused by SARS-CoV-2 . Interestingly, massive hepatic apoptosis and some binuclear hepatocytes were also identified in this study. In addition, autopsy results in patients with SARS, in the study by Guoet al[27 ], showed a large number of hepatocyte balloons, central lobular necrosis, and obvious apoptosis.Similar histological findings were also observed in a study of liver biopsy from patients with SARS by Chauet al[28 ], which suggested that SARS-CoV may induce apoptosis of hepatocytes, thereby leading to liver damage. Furthermore, Tanet al[29 ]demonstrated that overexpression of p7 a, a protein specifically expressed in SARSCoV-infected cells, could induce apoptosis in cell lines derived from different organs(including lung, kidney, and liver)viaa caspase-dependent pathway. This further confirmed the possibility that SARS-CoV can directly attack liver tissues and cause liver damage.
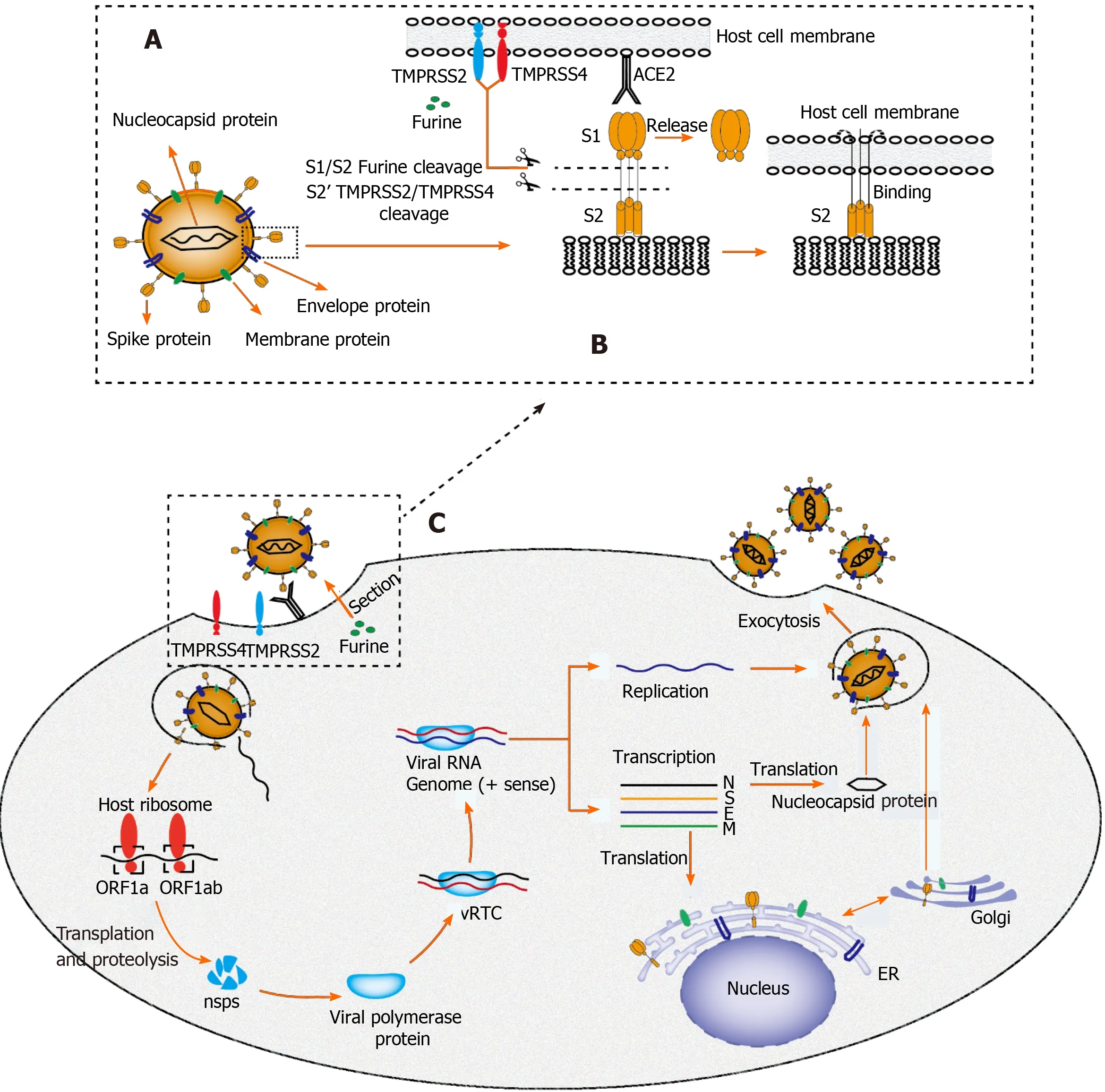
Figure 2 Proposed structure diagram of severe acute respiratory syndrome coronavirus 2 and its life cycle in host cells. A: Structural sketch of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2 ); B: Recognition and entry of SARS-CoV-2 into host cell. Transmembrane spike (S) glycoprotein on the surface of SARS-CoV-2 forms a homotrimer to recognize the human host angiotensin converting enzyme 2 (ACE2 ) receptor. The S protein is specifically cleaved by two mucose-specific serine proteases [recombinant transmembrane protease serine 2 (TMPRSS2 ) and TMPRSS4 ] and furine. The subunit of S protein(S1 ) is released, and another subunit (S2 ) is exposed and mediates the viral entry into host cells; C: Life cycle of SARS-CoV-2 in host cells. First, the S protein of SARS-CoV-2 binds to ACE2 to form an S protein-ACE2 complex, which directly mediates the cellular entry of virus and the process is facilitated by TMPRSS2 ,TMPRSS4 , and furine. Second, viral RNA is released into host cytoplasm. Open reading frame (ORF) 1 a and ORF1 ab are translated into large polyproteins by host ribosome, which are further proteolytically cleaved into 16 non-structural proteins (nsps). Viral polymerase protein is assembled by nsps and viral replication/transcription complex (vRTC) is subsequnently formed by polymerase protein and genomic RNA. Third, a negative sense viral RNA is synthesized and used as a template to replicate progeny (+) sense viral genome and transcribes to form various mRNAs. The nucleocapsid protein is translated in the cytoplasm,whereas the S protein, membrane (M) protein, and envelope (E) protein are translated in the endoplasmic reticulum and transported to the Golgi apparatus for further packaging. Finally, a completely new viral particle is assembled by viral RNA-nucleocapsid complex and S, M, and E proteins in endoplasmic reticulum–Golgi intermediate compartment and is released from host cell via exocytosis. SARS-CoV-2 : Severe acute respiratory syndrome coronavirus 2 ; M: Membrane; E: Envelope;S: Spike; ER: Endoplasmic reticulum; vRTC: Viral replication/transcription complex; ORF: Open reading frame; TMPRSS: Transmembrane protease serine; ACE2 :Angiotensin converting enzyme 2 .
It is noteworthy that the expression level of ACE2 on hepatocytes is regulated by many factors. Several experimental studies, in both mice and humans, have confirmed increased expression of hepatic ACE2 under conditions of liver fibrosis/cirrhosis[30 ].This may partially explain why pre-existing CLD increases the probability of liver damage in patients with COVID-19 . Hypoxia, a typical feature in severe COVID-19 cases, has been proven to be a main regulator of ACE2 expression in hepatic cells[31 ].This may explain why the dissemination of SARS-CoV-2 outside the lungs is mainly observed in patients with acute respiratory distress syndrome and other hypoxic conditions. Notably, the affinity of S protein in SARS-CoV-2 for its receptor can be increased when it is proteolytically activated by trypsin, a protein commonly expressed in liver epithelial cells[32 ]. A clinical drug trial by Fantini et al[33 ] indicated that ganglioside (GM1 ) might be another target that influences the S protein–ACE2 interaction, using a combination of structural and molecular modeling approaches. In the near future, new molecular and therapeutic insights concerning the S protein–ACE2 interactor are expected to be uncovered with the advancement of research.
Inflammatory storm in COVID-1 9 -associated hepatic injury
Inflammatory cytokine storm generated by the excessive immune response induced by coronavirus infection might also be one of the key factors in hepatic injury[34 ,35 ].Higher plasma levels of inflammatory cytokines (IL-2 , IL-7 , IL-10 , GSCF, IP10 , MCP1 ,MIP1 A, and TNF-α) and lower lymphocyte counts (both helper T cells and suppressor T cells) were commonly observed in patients with COVID-19 , especially in the critically ill ones[4 ,36 ]. Qin et al[37 ] showed that COVID-19 is a systemic inflammatory viral response, first during the viral infection period and subsequently during the inflammatory period. This may explain why the conditions of illness in some patients with COVID-19 are not serious in the early stage, but if they do not receive timely medical treatment, the disease deteriorates rapidly in a short time and enters a state of multiple organ failure[38 ]. A cohort study of 192 patients revealed that an increase in IL-6 and IL-10 and a decrease in CD4 + T cells were independent risk factors related to severe liver damage[39 ]. In another study recently published inWorld J Gastroenterol[40 ], lymphopenia and C-reactive protein levels were found to be independently associated with hepatic injury (Figure 3 ).
Burra[14 ] confirmed that the incidence of liver damage in patients with COVID-19 having elevated ferritin levels was significantly higher (52 .3 % vs 20 .0 %) than that of patients with normal ferritin levels. This suggests that ferritin could be employed as an easy-to-use tool to ascertain liver injury. A possible reason for this is that ferritin,acting as an inflammatory cytokine like IL-6 , participates in acute liver damage[41 ].Inflammasome activation and apoptosis/pyrolysis in SARS-CoV-2 induced inflammatory cells may cause multiorgan dysfunction[42 ]. Interestingly, pathological changes, such as spleen atrophy and lymph node necrosis, were observed in severe cases of SARS infection, which indicated the presence of immune-mediated injury[43 ].
Endotheliitis in COVID-1 9 -associated hepatic injury
COVID-19 is considered to be a thrombo-inflammatory disease that affects the lungs and, beyond that, endothelium, which is one of the largest organs in the human body.A variety of viruses, such as the HIV, dengue fever virus, and Ebola virus, have been previously reported to affect the coagulation system[44 ]. SARS-CoV-2 enters the endothelial cells by endocytosisviabinding to the ACE2 receptor as well[45 ]. A recent study from Switzerland[42 ] showed the presence of viral inclusion structures within endothelial cells and diffuse endothelial inflammation. The vascular endothelium is indispensable in regulating the vascular tone and in maintaining vascular homeostasis,and intact endothelial cells provide potent anti-coagulant properties[46 ]. When the vascular endothelium is destroyed, either directly by viral infection or through immune-mediated inflammation, vasoconstriction and procoagulant behavior can occur rapidly. Spieziaet al[47 ] found that plasma levels of fibrinogen and D-dimer in severe cases of COVID-19 were significantly higher than those in healthy controls. In this study, markedly hypercoagulable thromboelastometry profiles, as reflected by shorter clot formation time and higher maximum clot firmness, were also observed in patients with COVID-19 . In a recent study, multiple areas of microthrombi were revealed in a patient with COVID-19 by contrast-enhanced ultrasound of the lung[48 ],which confirmed the involvement of microvessels during the disease process. In addition, the frequency of acute pulmonary embolus in patients with COVID-19 was 30 %[49 ] higher than that usually occurring in critically ill (1 .3 %)[50 ] or emergency (3 %to 10 %)[51 ] patients without COVID-19 (Figure 3 ).

Figure 3 Underlying molecular mechanisms of coronavirus disease-19 -associated liver injury caused by systematic inflammatory response syndrome and hypoxic ischemia. (a): Complement and interleukin-23 are released into the blood during the systemic inflammation, which subsequently activate Kupffer cells and induce their production of tumor necrosis factor α (TNF-α). As a pro-inflammatory cytokine, TNF-α aggravates the inflammation responses by up-regulating the expression of endothelial cell adhesion molecules and inducing hepatocytes to secrete chemokines. Under the induction of chemokines, CD4 T cells and neutrophils are rapidly recruited to the liver, in which CD4 T cells assist mucosal molecules to promote neutrophils into the liver parenchyma. Finally, neutrophils directly damage liver cells by releasing oxidants and proteases, leading to necrotic cell death; (b): Acute respiratory distress syndrome and endotheliitis are the two main causes leading to hypoxic-ischemic liver injury in the period of systematic inflammatory response syndrome. Increased anaerobic glycolysis leads to a decrease in ATP production, which ultimately leads to the death of hepatocytes by inhibiting hepatocyte signal transduction. ARDS:Acute respiratory distress syndrome; TNF-α: Tumor necrosis factor α; IL: Interleukin; IFN-γ: Interferon-γ.
SARS-CoV-2 infection causes inflammation of the vascular endothelium, which in turn leads to vascular dysfunction, especially in capillaries. Subsequently,microvascular dysfunction leads to a hypercoagulable state, tissue edema, and organ ischemia[44 ,52 ]. Liver ischemia reperfusion injury, a pathophysiological process commonly occurring after rapid recovery of blood circulation, may be the underlying mechanism of COVID-19 -associated hepatic injury. Liver ischemia-reperfusion can activate neutrophils, Kupffer cells, and platelets, inducing a series of destructive cellular reactions, such as reactive oxygen species and calcium overload, which ultimately lead to an inflammatory response and cell damage. It has also been reported that hepatic sinusoidal endothelial cell damage causes microcirculation disorders and further aggravates liver ischemia and hypoxia. Wanget al[9 ] observed different degrees of hypoxemia by blood gas analysis in more than 40 % of patients with COVID-19 . Patients receiving oxygen therapy have a faster recovery of liver function, and the average length of hospital stay is considerably shortened. In addition, lymphatic vessels are also reported to be involved in the pathological process of acute liver injury, prevent the occurrence of acute liver damage, and delay the progression of COVID-19 [53 ]. Lymphatic vessels participate in the clearance of virus through absorption and transportation of inflammatory exudates, inflammatory cytokines, dead cell debris, and immune cells[54 ].
Drug-induced hepatic injury during treatment
DILI is defined as liver damage caused by the drug and/or its metabolites, or by hypersensitivity or reduced tolerance of the drug owing to special physique during the use of drugs[55 ]. Based on the available data, a variety of drugs widely used to treat COVID-19 , such as antibiotics (macrolides and quinolones), antiviral drugs (ribavirin and lopinavir/ritonavir), and non-steroidal anti-inflammatory drugs, have been reported to cause liver damage[56 ]. A meta-analysis by Kulkarni et al[57 ] showed a pooled incidence of DILI of 25 .4 % in patients with confirmed SARS-CoV-2 infection.Interestingly, Fanet al[58 ] found that the proportion of patients with abnormal liver function who received lopinavir/ritonavir after admission (57 .8 %) was significantly higher than that of patients with normal liver function (31 .3 %). Moreover, the average hospital stay of patients with abnormal liver function was significantly longer than that of patients with normal liver function (15 .09 ± 4 .79 d vs 12 .76 ± 4 .14 d, P = 0 .021 ).Previous studies have indicated that patients with severe COVID-19 disease are prone to hepatic injury[4 ,5 ]. The reason for this might be that severe and critically ill patients require long-course and/or more metered administration of antiviral drugs,antibiotics, or other potentially hepatotoxic drugs during hospitalization. Similarly,Qinet al[37 ] reported that up to 70 % of critically ill patients received systemic corticosteroid therapy. Furthermore, a case report from Italy suggested that tocilizumab, a drug used to reduce inflammation by blocking the IL-6 signal transduction pathway,played a beneficial role in the management of severe COVID-19 disease[59 ]. In the case of poor efficacy of azithromycin, hydroxychloroquine, and lopinavir, the administration of tocilizumab rapidly improved the clinical condition. However, mild to moderate elevations in transaminases have been observed in patients with COVID-19 treated with tocilizumab. A possible reason is that the use of immunosuppressive drugs, such as tocilizumab, tofacitinib, and dexamethasone, can reactivate hepatitis B virus (HBV) in patients with occult infection and induce liver damage[57 ]. However,randomized controlled clinical trials evaluating the safety of remdesvir and tocilizumab in the treatment of COVID-19 have not yet revealed any significant difference in the incidence of liver injury between the treatment and placebo groups[60 ,61 ].
The Novel Coronavirus Pneumonia Diagnosis and Treatment Scheme (Trial Version 8 ) issued by the National Health Commission of the People's Republic of China on August 19 , 2020 [62 ], provides a brief summary of the antiviral drugs under trial. Some drugs have been shown to have certain therapeutic effects in clinical observation studies, but no antiviral drugs have been determined to be effective in strict“randomized, double-blind, placebo-controlled studies.” It is recommended that drugs with potential antiviral effects should be used early in the course of the disease and should be applied to patients with high-risk factors for severe illness and severe illness tendencies. It is not recommended to use lopinavir/ritonavir and ribavirin alone, as well as hydroxychloroquine, in combination with azithromycin. The trial of alphainterferon, ribavirin (recommended to be used in combination with interferon or lopinavir/ritonavir), chloroquine phosphate, and arbidol can be continued, and their efficacy, adverse reactions, contraindications, and interactions with other drugs should be evaluated in further clinical applications. It is not recommended to use more than three antiviral drugs at the same time.
COVID-19 -ASSOCIATED LIVER INJURY IN SPECIAL POPULATIONS
As of date, there are approximately 400 million patients with CLD in China, including those with chronic viral hepatitis, fatty liver, alcoholic liver disease, cirrhosis, or other liver diseases. Therefore, in patients with SARS-CoV-2 infection, the effect of preexisting liver disease on the liver injury status cannot be ignored.
Previous studies have shown that patients having cirrhosis with relatively lower immunity are vulnerable to liver decompensation or acute chronic liver failure after influenza virus infection[63 ]. Notably, similar results were also reported in a large,multicenter, international cohort study; cirrhosis in patients with COVID-19 was closely associated with a poor model for end-stage liver disease score and decompensated events[64 ]. The authors indicated that mortality was strongly correlated with hepatic decompensation following SARS-CoV-2 infection; 63 .2 % of patients with new decompensated events died, whereas the proportion of death in those without new decompensation events was 26 .2 %. According to a study by Sarinet al[65 ], decompensating one-fifth of cirrhosis was observed, in which 57 % of patients had progression of liver damage and the mortality rate was 43 %. Notably, among patients with liver cirrhosis, the mortality rate of COVID-19 was significantly higher than that in patients hospitalized for bacterial infection[66 ]. However, in a contemporaneously enrolled study conducted in the United States[67 ], Bajaj et al[67 ] determined that the mortality rate in patients with cirrhosis + COVID-19 was similar to that in patients with cirrhosis alone (30 % vs 20 %, P = 0 .16 ), but was higher than that in patients with COVID-19 alone (30 % vs 13 %, P = 0 .03 ) after matching for age/sex. In this study, the Charlson Comorbidity Index, a prognostic comorbidity score, was identified as the only independent variable predictive of mortality in the entire matched cohort [odds ratio 1 .23 , 95 % confidence interval (CI): 1 .11 -1 .37 ; P < 0 .001 ].Thus, whether the mortality rate in patients with cirrhosis infected with SARS-CoV-2 is higher than in those infected with other viruses or bacteria is yet to be determined.
Based on previous studies[68 ], patients with chronic hepatitis B co-infected with severe acute respiratory syndrome (SARS) virus are more likely to develop severe hepatitis. A possible reason is that the SARS virus triggers HBV reactivation and massive replication, and chronic hepatitis B patients co-infected with SARS virus may require a longer time to fully clear the SARS virus from their bodies. Very recently, an observation from China[69 ] revealed that two patients with HBV infection had a slower clearance of SARS-CoV-2 (mean difference 10 .6 d; 95 %CI: 6 .2 -15 .1 d). The mechanism may involve the dysfunction of T cells in HBV-infected patients, which causes the body's immune response to other viruses to weaken, but whether there is an exact connection between the two remains to be elucidated. However, Chenet al[70 ]reviewed the clinical characteristics of patients with SARS-CoV-2 /HBV coinfection and found no significant difference in the hepatic function index between 20 patients with HBV infection (6 .1 %) and 306 patients without HBV infection (93 .9 %). Moreover,there is no evidence that SARS-CoV-2 /HBV coinfection would reduce the discharge rate and lengthen the hospital stay. A similar phenomenon was observed in another study[71 ] showing that patients with COVID-19 co-infected with hepatitis B were not significantly associated with worse outcomes compared to those without hepatitis B.
Patients with non-alcoholic fatty liver disease (NAFLD) were previously reported to be more prone to liver damage when infected with SARS-CoV-2 ; many of these were cases of mild to moderate liver damage, and severe disease was rare[72 ,73 ]. Ji et al[73 ]showed that patients with pre-existing NAFLD infected with SARS-CoV-2 had a higher possibility of abnormal hepatic function during hospitalization, higher risk of disease progression, and longer duration of virus shedding compared to those without NAFLD. In this study, the pattern of liver injury was mainly hepatocellular rather than cholestatic, which was contrary to the existing finding that SARS-CoV-2 has a high affinity for the ACE2 receptor highly expressed in biliary tract cells. Metabolic diseases, such as obesity, hypertension, diabetes, and cardiovascular disease, are common in patients with NAFLD. Studies have shown that coexisting metabolic risk factors in patients with NAFLD are independent risk factors for severe COVID-19 disease, and the risk of severity increases with the number of metabolic risk factors present[74 ]. To further verify whether NAFLD itself affects liver function in patients with COVID-19 , Hashemi et al[75 ] adjusted for potential confounding factors (age, sex,hypertension, diabetes, obesity, hyperlipidemia, heart diseases, and pulmonary disorders), and determined that NAFLD was still independently associated with ICU admission (49 .3 % vs 35 .0 %, P = 0 .028 ) and mechanical ventilation (47 .8 % vs 30 .3 %,P=0 .0055 ), but was not associated with mortality. Patients with NAFLD who were infected with SARS-CoV-2 had a higher prevalence of elevated transaminases on admission. Coincidently, a very recent study[76 ] revealed a similar result that NAFLD is an independent predictor of liver injury in COVID-19 , but not a predictor of death and disease severity (presentation or progression). However, the debate concerning whether NAFLD increases the risk of death in patients with COVID-19 continues.
Liver transplant recipients are also a special population affected by the global spread of COVID-19 . Long-term immunosuppressive treatment may increase the risk of contracting respiratory viruses, especially in patients with preoperative organ decompensation and chronic disease[77 ]. A prospective cohort study of 111 cases[75 ]showed that liver transplant patients had an increased risk of contracting SARS-CoV-2 owing to chronic immunosuppression, but the mortality rate was lower than that in the matched general population. Mycophenolate, a baseline immunosuppressive drug,was identified as an independent predictor of severe COVID-19 , but no such deterioration was observed with calcineurin inhibitors or everolimus. In a series of cases in Brazil[78 ], a negative effect of COVID-19 on liver transplantation was reported,especially in elderly patients with comorbidities. One case was a 69 -year-old patient with severe cardiovascular disease who showed a rapid deterioration after being diagnosed with COVID-19 , and another case was a patient with NAFLD complicated with kidney failure, who eventually died of a secondary bacterial infection. However,different opinions have been forwarded in other studies. A large international observational study conducted by Webbet al[79 ] indicated that liver transplantation did not significantly increase the proportion of ICU admission and the risk of death. Similarly,D'Antigaet al[80 ] also showed that patients with COVID-19 receiving liver transplantation were not at an increased risk of severe pulmonary disease, despite their immunosuppressed status. Moreover, three COVID-19 -related deaths observed at an Italian transplant center were of patients undergoing long-term treatment with a minimal immunosuppressive regimen, rather than of fully immunosuppressed patients who recently received transplants[81 ].
TREATMENT STRATEGIES FOR LIVER INJURY IN PATIENTS WITH COVID-19
Although liver damage is a common complication of COVID-19 , most cases of COVID-19 show mildly abnormal liver function, which is usually temporary and can return to normal without any special treatment[7 ]. According to the Chinese Pharmaceutical Association, the “Four-Anti and Two-Balance” strategy is recommended, which includes antivirus, anti-shock, anti-hyoxemia, anti-secondary infection therapy, and maintenance of water, electrolyte, acid–base, and microecological balance. Patients with COVID-19 exhibiting obvious liver damage could be treated with hepatoprotective, anti-jaundice, or anti-inflammatory drugs, such as polyene phosphatidylcholine, glycyrrhizic acid, ursodeoxycholic acid, and adenosylmethionine. For liver injury in critically ill patients infected with SARS-CoV-2 , one or two kinds of drugs can be chosen to avoid drug abuse and aggravation of liver burden, and reduce drug interactions. A recent study by Hoeveret al[82 ] revealed that glycyrrhizic acid derivatives, which are preferred anti-hepatitis drugs, may also have antiviral activity against SARS-CoV-2 . Glycyrrhizic acid has a strong affinity for liver steroid metabolism enzymes, and hinders the inactivation of cortisol and aldosterone; it also shows obvious corticosteroid-like effects, such as anti-inflammatory, anti-allergic, and protective film structures, without obvious cortical hormone-like side effects.
Huet al[34 ] retrospectively analyzed the clinical characteristics, susceptible population, and treatment strategies for patients with new coronavirus infection and showed that the mainstay to manage COVID-19 -associated liver injury was to suppress inflammatory response, correct hypoxemia, and provide symptomatic support. Conservative oxygen therapy is preferred, and ventilator-associated pneumonia should be strictly supervised in patients receiving mechanical ventilation[83 ]. Xu et al[83 ] showed that an artificial liver blood purification system could improve the treatment effect in critically ill patients by rapidly removing inflammatory mediators, blocking cytokine storms, and favoring the water–electrolyte balance.Similar findings were reported by Liuet al[84 ] who showed a significant declining trend in the levels of cytokines and inflammatory factors (IL-6 and C-reactive protein)in patients with COVID-19 after a course of artificial liver blood purification. For patients with COVID-19 who are suspected of having liver damage caused by drugs,consideration should be given to stop or reduce the drug dose. Prevention is better than management. Monitoring the liver function and avoiding liver damage play key roles in the treatment of COVID-19 -associated liver injury.
CONCLUSION
In this review, we summarize the latest advances in research on the clinical features,potential mechanisms, exacerbation of underlying hepatic dysfunction in patients with CLD, and treatment strategies for patients with COVID-19 as of January 2021 . Patients with COVID-19 showing liver injury may experience darkening of the skin and hyperpigmentation. To ascertain the presence of liver injury, liver enzymes are the most commonly used markers. Liver biopsy is strongly recommended for those with unexplained acute liver failure. Apparent viral particles have been observed in hepatocytes. However, to date, studies involving liver biopsy in COVID-19 are still limited to case reports. The potential mechanisms of COVID-19 -associated liver injury may include the direct effects of viral infection, inflammatory storm, hypoxemia,endotheliitis, and drugs. The S protein-ACE2 interactor may be the main tunnel for the entry of virus, the activity of which can be regulated by multiple factors, such as hypoxia, fibrosis/cirrhosis, and GM1 . Among patients with CLD, NAFLD was indicated as an independent factor associated with ICU admission and mechanical ventilation after adjusting for comorbidities, such as hypertension, diabetes, and obesity. Patients with cirrhosis, co-infected with SRAS-CoV-2 , experience high mortality, whereas whether hepatitis B and liver transplantation increase the severity of COVID-19 disease remains an open question. The “Four-Anti and Two-Balance”strategy, in which it is necessary to avoid drug abuse that aggravates liver burden and prevention is preferred to management, is recommended to manage COVID-19 -associated liver injury.
杂志排行
World Journal of Gastroenterology的其它文章
- Role of imaging in evaluating the response after neoadjuvant treatment for pancreatic ductal adenocarcinoma
- High fecal calprotectin levels are associated with SARS-CoV-2 intestinal shedding in COVID-19 patients: A proof-of-concept study
- Enhancer of zeste homolog 2 contributes to apoptosis by inactivating janus kinase 2 / signal transducer and activator of transcription signaling in inflammatory bowel disease
- Interplay between nuclear factor erythroid 2 -related factor 2 and inflammatory mediators in COVID-19 -related liver injury
- Helicobacter pylori promotes invasion and metastasis of gastric cancer by enhancing heparanase expression
- Role of bile acids in liver diseases mediated by the gut microbiome
