双波长紫外线(VUV/UV)对有机污染物强化去除特性与原理
2021-06-23邵婉婷王文龙吴乾元何志明付志敏胡洪营1
邵婉婷, 王文龙, 杜 烨, 吴乾元, 何志明, 付志敏, 胡洪营1,*
1.清华大学环境学院, 环境模拟与污染控制国家重点联合实验室, 北京 100084 2.清华大学深圳国际研究生院, 国家环境保护环境微生物利用与安全控制重点实验室, 广东 深圳 518055 3.清华-伯克利深圳学院深圳环境科学与新能源技术工程实验室, 广东 深圳 518055 4.广东省佛山市柯维光电股份有限公司, 广东 佛山 528518
紫外线广泛用于水处理消毒,可有效灭活病原(指示)微生物,抑制藻类生长,对隐孢子虫和贾第鞭毛虫等氯消毒抗性微生物有很好的灭活效果[1-3]. 紫外线也可活化氧化剂(H2O2、O3、自由氯等)、催化剂(TiO2、ZnO等)生成强氧化性自由基(·OH、ClO·、SO4-·等)[4-7],形成紫外线高级氧化技术,用于去除水中内分泌干扰物、药品和个人护理品等难降解有机污染物[8-10]. 紫外线/氯、紫外线/H2O2等高级氧化技术已应用于美国地下水回灌、直接/间接补充饮用水等污水再生深度处理工程[11].
低压汞蒸气紫外灯(LPUV,254 nm)和中压汞蒸气紫外灯(MPUV,200~400 nm)是应用中最常用的消毒和高级氧化紫外线光源[12]. 但实际应用中面临基质竞争吸收紫外线及消耗自由基、紫外线照射下氧化剂利用效率和自由基产率不佳等难题.
真空紫外线(Vacuum UV,VUV)波长在100~200 nm,以185 nm最为常见,传统低压汞蒸汽发射法可同时发射254和185 nm紫外线,但常规玻璃材料会阻隔185 nm部分. 近年来,随着高透石英管壁、汞齐合金、供电电流技术的发展,可同时发射185 nm (VUV)和254 nm (UV)的紫外线新型光源在水处理领域受到关注. 与LPUV相比,VUV/UV光源的制造成本、运行电耗相近,但VUV光子具有能量高、水分子吸收强、氧化剂活化和自由基生成效率高等优点,有望克服传统紫外线光源的缺点[13]. VUV/UV无需额外添加氧化剂,即可生成丰富的强氧化自由基,是一种绿色的高级氧化技术,在饮用水处理、污水再生深度处理和超纯水制备等领域备受关注.
针对饮用水、污水再生深度处理中难降解、高风险有机污染物的去除需求,该文总结分析了无化学试剂添加下,VUV/UV高级氧化生成强氧化性自由基的主要机制,深入讨论了VUV/UV高级氧化相比UV光解在去除难降解污染物方面的提升效果,阐明了常见水质条件对VUV/UV高级氧化的影响,为VUV/UV的未来研究方向提出了结论与建议.
1 VUV/UV高级氧化原理
1.1 自由基生成过程
VUV/UV光照下,水中生成·OH、O2·-、HO2·等强氧化性自由基和氢原子(H·)、水合电子(eaq-)等强还原性自由基,生成路径及反应方程如图1、表1所示. 其中,·OH是主要强氧化性自由基,由VUV光照下水分子均质裂解〔见式(1)〕和光化学离子化〔见式(2)〕生成,量子产率(Φ)分别为0.33和0.045 mol/mol(产物/光子)[16]. 研究[17]发现,VUV/UV光照下水中·OH浓度为10-10~10-9mol/L. 因此,VUV/UV也被称作VUV/UV高级氧化.

表1 VUV/UV高级氧化过程的主要反应[14-16]
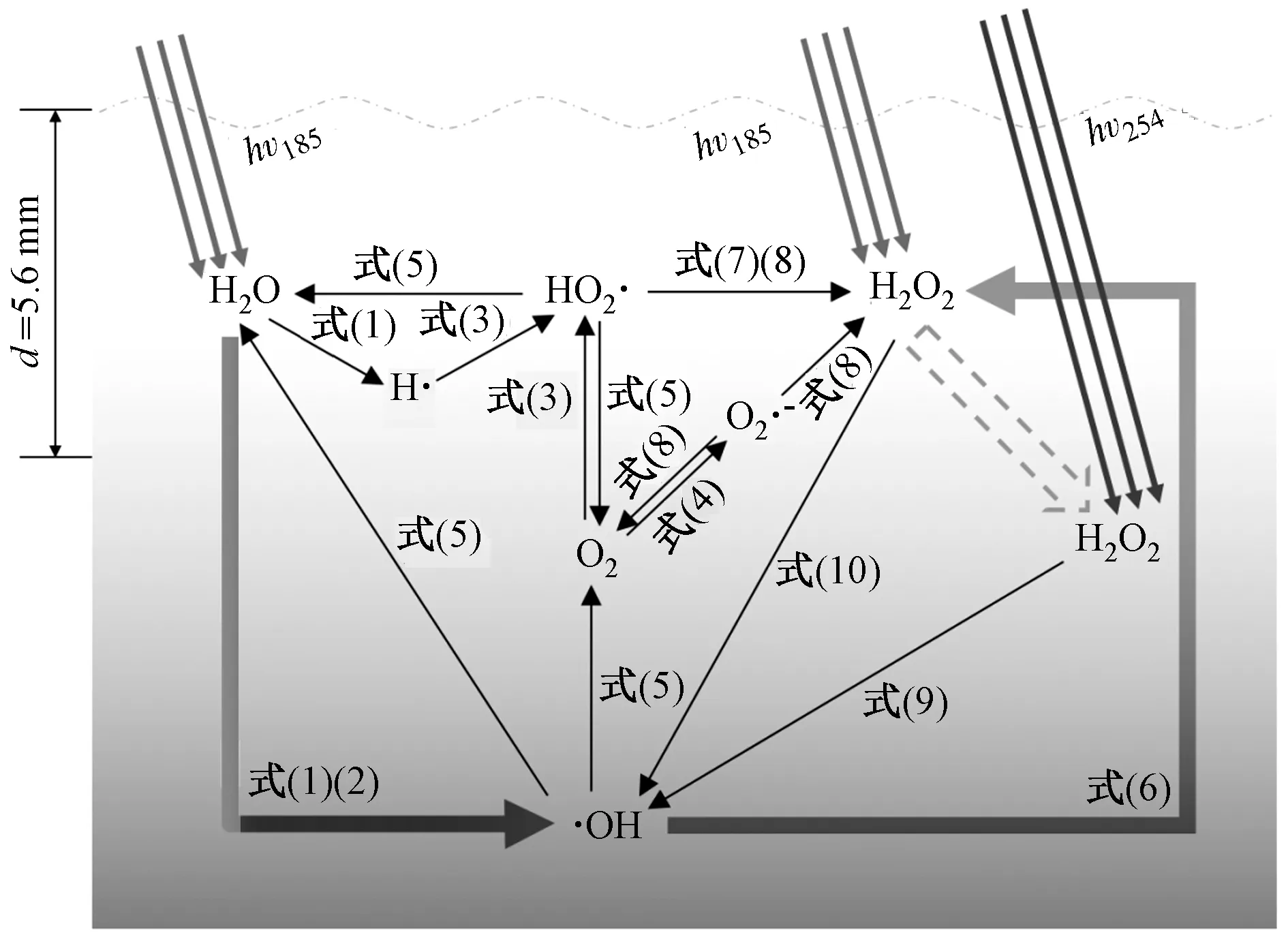
图1 VUV/UV强氧化性自由基生成过程Fig.1 Formation of strong oxidizing radicals in VUV/UV system
VUV/UV可通过光解次生氧化剂H2O2,间接生成强氧化性自由基. VUV可被水分子强烈吸收(吸收系数为1.8 cm-1),5.6 mm水层内衰减率达90%[18]. 因此,VUV/UV灯管附近水层的·OH浓度较高,并与
溶解氧、OH-及其他自由基发生复杂链式反应,生成O2·-、O3·-、H2O2等强氧化性活性物质,共同去除难降解污染物[14]. 此外,H2O2会扩散至距灯管较远水层,形成UV/H2O2高级氧化. 总而言之,作为降解污染物的主要自由基,·OH可由多个氧化途径生成和消耗,包括VUV/H2O、VUV/H2O2、UV/H2O2. 因此,H2O2的生成和光解是影响VUV/UV高级氧化效率的重要因素之一.
1.2 双氧水生成特性
VUV光照生成的·OH集中于紫外线灯管附近,但·OH半衰期约4×10-9s,传质距离约60 nm[19]. 大部分·OH会发生自淬灭,生成其他氧化活性物质,其中以生成H2O2为主[20]. 在序批式VUV/UV反应器中,生成的H2O2浓度随光照剂量升高而升高,最终浓度稳定在240 μg/L左右[21];在连续流式反应器中,出水H2O2浓度也高达(90±50)μg/L[17].
·OH复合反应〔见式(6)〕、H·还原溶解氧生成HO2·并发生复合反应〔见式(3)(7)〕是生成H2O2的两条主要途径[22]. 基于上述原理,pH、溶解氧被认为是影响水中H2O2稳态浓度的重要因素. 当pH由弱酸性(pH=6.3)升至碱性(pH=10.0)时,H2O2生成浓度由240 μg/L降至50 μg/L;当溶解氧浓度降低时,H2O2生成和分解速率均加快[21]. 生成的H2O2在UV和VUV光照下光解生成·OH〔见式(9)(10)〕,特别是扩散至距灯管较远的H2O2对UV光化学氧化效率具有强化作用,但其对污染物降解的强化效率尚鲜见报道.
2 VUV/UV的强化氧化效果与影响因素
2.1 VUV/UV的强化氧化效果
与传统低压紫外线(UV)相比,VUV/UV可通过直接光解和自由基间接氧化去除污染物,在紫外线吸光率、紫外线利用效率、处理效果及成本费用等方面都具有优势,如表2所示.

表2 VUV/UV与UV的光解特性比较
VUV/UV包括自由基氧化作用和UV紫外线直
接光解作用,对大部分难降解有机污染物去除效果显著优于UV光解,去除速率是UV直接光解的2.0~19.3倍(见图2). 根据污染物结构特征,可被分为卤代烷烃(三卤甲烷、一氯/二氯/三氯乙腈)、全氟化合物(全氟辛酸)、烷基醚类(丁基黄原酸钠、甲基叔丁基醚)、苯环取代物(苯酚、苯胺、对氯苯甲酸、4-叔辛基苯酚)和含苯环多官能团化合物(布洛芬、酮洛芬、萘普生、四环素、磺胺甲恶唑)等.
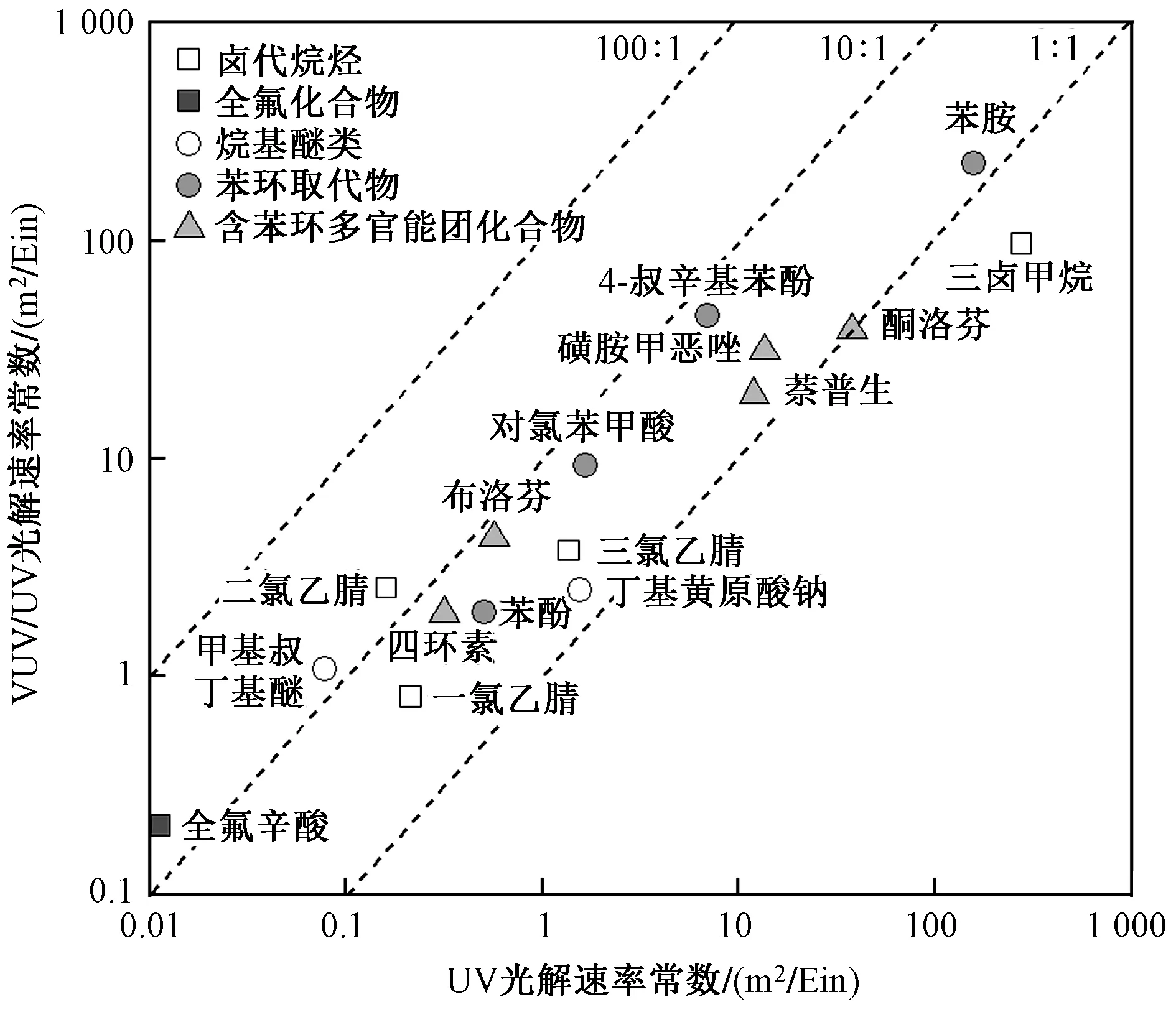
图2 VUV/UV高级氧化与UV光解对典型污染物降解速率比较Fig.2 Comparison of the elimination of representative pollutants between VUV/UV and UV
VUV/UV对卤代烷烃的强化效率为1.8~15.8倍,其中对氯代乙腈的强化效率为2.8~15.8倍,对碘代甲烷的强化效率为1.8倍[30-32]. 一方面,卤代烷烃类污染物对UV254吸收较低、光敏化降解过程较弱,直接光解速率较低;另一方面,其与VUV/UV光照系统中的·OH反应速率较快,其中氯代乙腈与·OH二级反应速率为0.16×109~1.35×109L/(mol·s)[31],三碘代甲烷(CHI3)与·OH反应速率为833 m2/Ein[32]. 因此,VUV/UV通过生成强氧化性自由基,显著提升了UV直接光解速率较低的卤代烷烃类污染物的降解速率.
类似地,VUV/UV对全氟化合物、烷基醚类等紫外线吸收较低、直接光解/光敏化降解较弱的污染物强化效果显著,强化效率为14.0~18.3倍[33-36]. 但VUV/UV对丁基黄原酸钠强化效率较低,仅为1.58倍[35].
VUV/UV对含苯环多官能团化合物的强化效率存在较大差异,如对四环素和布洛芬的强化效率分别为UV的6.2和7.7倍,但对酮洛芬的强化效率基本维持不变,这主要与苯环外其他特征官能团的结构与性质相关[37-38]. 四环素和布洛芬自身结构稳定,吸收UV后直接光解速率较慢,但在VUV产生的·OH作用下会发生开环断键反应. 酮洛芬结构中的羰基在吸收UV后易发生断裂,VUV产生的自由基对其降解促进效果不明显.
VUV/UV对多种难降解污染物都具有较好的去除效果. VUV/UV体系中,大部分含苯环多功能团化合物如萘普生、布洛芬、酮洛芬、磺胺甲恶唑等,15 min内可被100%去除[38-40]. VUV/UV对烷基醚类如甲基叔丁基醚、丁基黄原酸钠可分别实现30 min 97.4%和40 min 95%的去除率[34-35]. 但VUV/UV对卤代烷烃去除效果不佳,三卤甲烷、一氯乙腈、二氯乙腈、三氯乙腈等污染物15 min仅能被去除约20%[30-31]. 具体VUV/UV降解部分有机物的指标如表3所示.
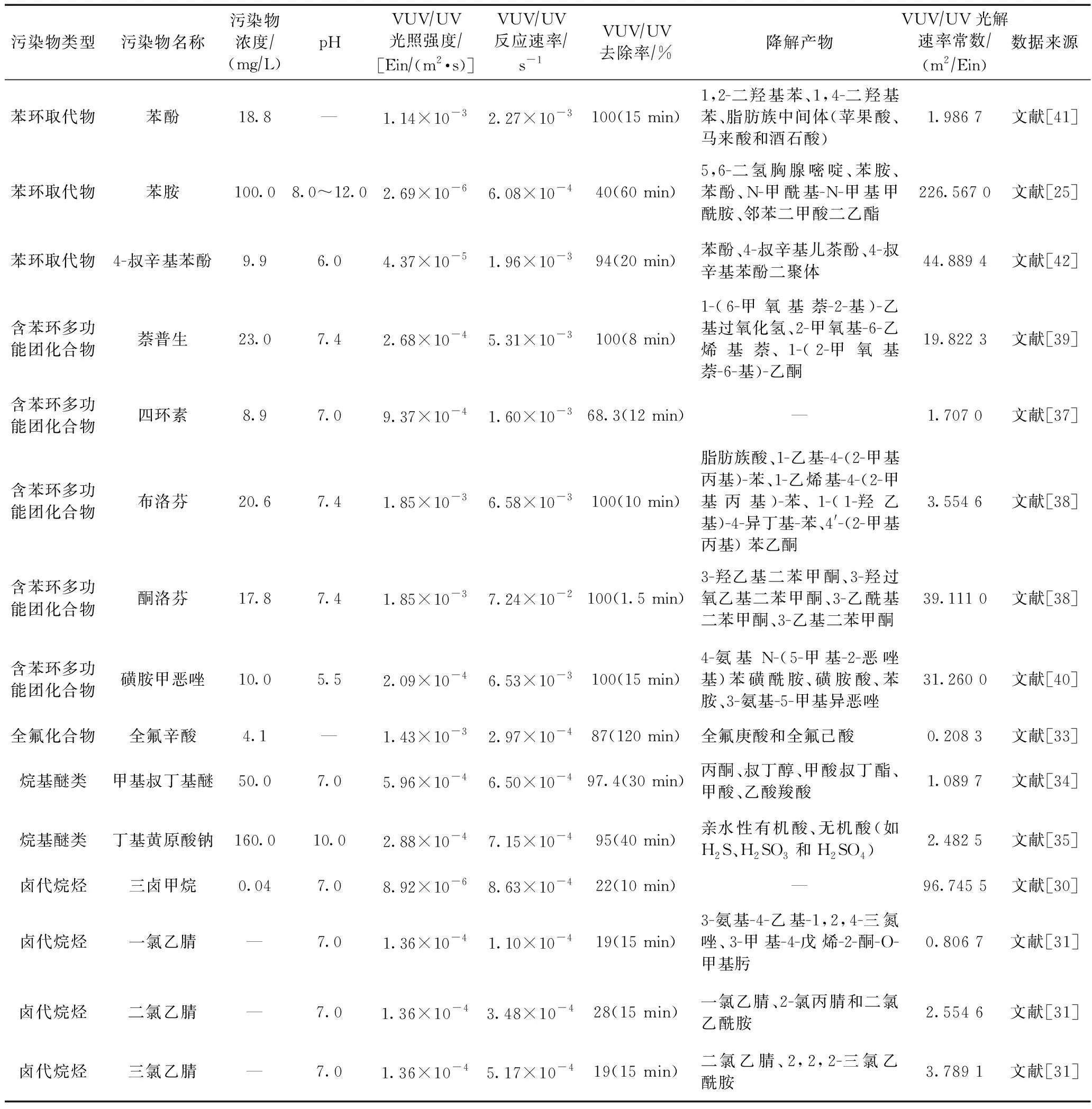
表3 VUV/UV降解部分有机物相关指标
总的来说,VUV/UV对难降解污染物去除的强化效果与污染物直接光解速率、·OH二级反应速率相关[43]. 如图2所示,VUV/UV的强化效率与UV光解速率存在一定关联,UV光解速率小于10 m2/Ein时,强化效果为2~18倍,UV光解速率大于10 m2/Ein时,强化效果仅为0.3~2.3倍. VUV/UV的强化效率与·OH二级反应速率呈正相关. 此外,VUV光照仅能穿透5.6 mm厚水层,当反应器水层过厚时,VUV光化学活性体积占比较小,对难降解污染物强化去除效果较弱.
2.2 pH的影响
溶液pH是影响VUV/UV氧化去除难降解污染物的重要水质因素. 一方面,溶液pH会影响VUV光照下·OH生成和利用效率;另一方面,也会改变污染物化学形态和降解速率,并对水质基质产生影响. 因此溶液pH对VUV/UV氧化效率的影响是多种因素共同作用的结果,且影响效果随水中污染物种类而变化(见图3和表4).

表4 pH对VUV/UV降解污染物的影响
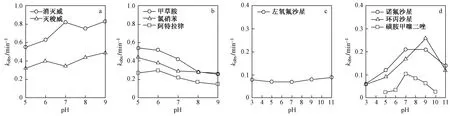
图3 VUV/UV降解污染物速率随pH变化情况[38,44-46]Fig.3 Schematic diagram of pollutants degradation rate changing with pH[38,44-46]
2.2.1pH对·OH生成效率和利用效率的影响
pH会直接影响·OH的生成和利用效率. 一方面,随pH升高,·OH氧化电位下降(由pH=0时的2.59 V降至pH=7时的2.18 V),解离为氧化性较低的O-·(·OHH++O-·,pKa=11.9),氧化能力减弱;另一方面,碱性条件下OH-浓度的升高会消耗更多·OH[47],使作用于微量有机污染物的·OH减少[32]. pH减少一级·OH生成的同时,会减少H2O2及二级自由基的生成.
2.2.2pH对污染物形态和氧化效率的影响
溶液pH会改变污染物的聚集状态和解离形态,
继而影响其氧化降解特性[48]. 酸性条件下,污染物的氨基等结构易质子化形成正电形态;碱性条件下,污染物的羟基、羧基等易解离质子,形成负电形态.
因此诺氟沙星、环丙沙星等氟喹诺酮类抗生素的VUV/UV光解特性受pH显著影响,当pH由3升至9时,诺氟沙星和环丙沙星由正电与质子化形态,转变为易受·OH进攻分解的负电形态,光降解速率常数先显著升高后降低[44].
pH对常见抗生素四环素也有类似作用机制[37],而左氧氟沙星虽然也存在解离形态的变化,但pH对其光降解动力学常数影响并不明显[44]. 此外,杀虫剂涕灭威和灭梭威的氨基甲酸酯基团在碱性条件下发生显著水解,加快了它们在VUV/UV体系中的分解速率[46].
2.2.3pH对水质基质的影响
含碳体系中,pH影响无机碳形态. 当pH呈碱性时,水中HCO3-浓度下降,CO32-浓度升高(HCO3-+OH-H2O+CO32-),而CO32-与·OH的反应速率是HCO3-的45.9倍,即pH通过影响无机碳形态加强其对·OH的淬灭效果[49].
2.3 无机阴离子对VUV/UV氧化的影响
2.3.1硝酸根
硝酸根是水中常见的光化学活性阴离子,且光化学活性会随紫外线光源变化. 中压紫外线光源(300~360 nm)照射下,硝酸根产生·OH,促进卡马西平的降解[50];但在低压紫外线直接光解和高级氧化中,硝酸根会抑制污染物的降解[51].
VUV/UV光照下,硝酸根发生更加复杂的光化学转化(见表5),并抑制VUV/UV对污染物的降解效率. 例如,当硝酸根浓度从0增至500 μmol/L时,VUV/UV降解1,4-二恶烷的一级反应速率降低90%[54].

表5 VUV/UV光照下硝酸根/亚硝酸根相关反应[52-53]
硝酸根的主要抑制机理为竞争吸收紫外线和消耗自由基. 首先,硝酸盐对VUV的摩尔吸光系数为 4 779 L/(mol·cm),远高于对UV的摩尔吸光系数3.51 L/(mol·cm)[55]. VUV/UV体系中,硝酸根和水分子竞争吸收VUV,并生成亚硝酸根和·OH、氧原子,但自由基量子产率低于水分子裂解[23]. 其次,硝酸根及其光解产物亚硝酸根会和污染物竞争消耗·OH、H·、eaq-等[56-57].
以城市污水处理厂二级处理出水为例,硝酸根浓度约为0.5 mmol/L,水分子浓度约为55.5 mol/L. 计算得硝酸根的VUV吸收约是水分子的22%,该结果表明,硝酸根竞争吸收紫外线和消耗自由基均是抑制VUV/UV氧化效果的重要原因[18].
2.3.2碳酸根/碳酸氢根
碳酸根/碳酸氢根被认为是地表水和城市污水厂二级出水中淬灭·OH的主要无机离子〔见图4及表6中式(29)(30)〕,与·OH的二级反应速率分别为3.9×108和8.5×106L/(mol·s),抑制了紫外线高级氧化对难降解有机污染物的去除[49,58]. 在VUV/UV高级氧化中,碳酸根/碳酸氢根还会强烈吸收VUV,摩尔吸光系数分别为630和269 L/(mol·cm)[55],抑制了VUV生成强氧化性自由基及降解污染物的效率[32].
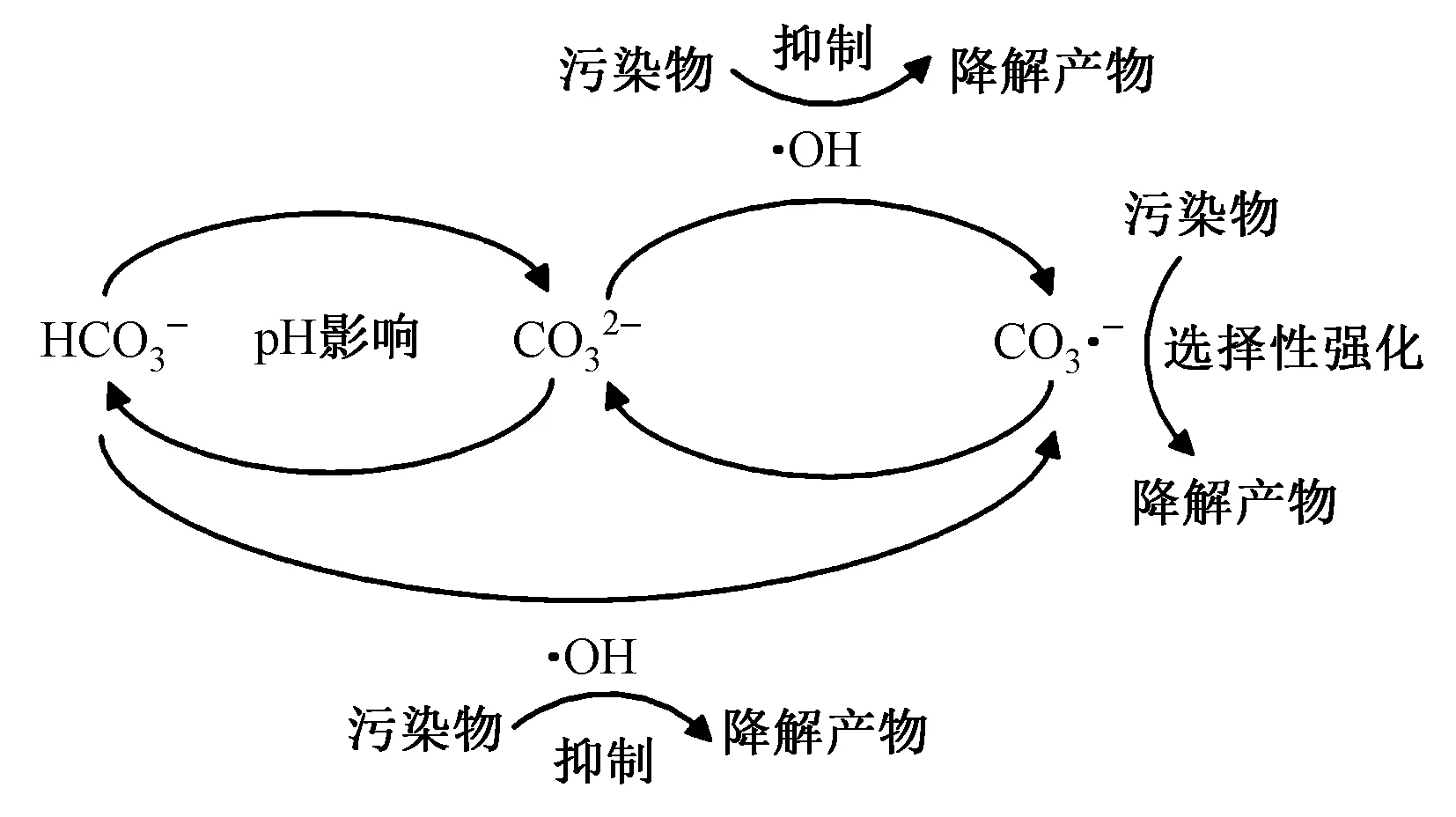
图4 VUV/UV光照下碳酸根/碳酸氢根转化示意Fig.4 Schematic diagram of carbonate / bicarbonate conversion under VUV/UV irradiation

表6 VUV光照下碳酸根/碳酸氢根相关反应[53]
当碳酸氢根/碳酸根浓度由0.5 mmol/L升至5 mmol/L时,VUV/UV对亚甲基蓝的降解速率常数降低15.6%[59]. 另有研究[60]显示,当碳酸氢根/碳酸根浓度由6.39×10-3mmol/L升至4.9 mmol/L时,双氯西林降解速率常数约下降90%. 污染物浓度和碳酸根/碳酸氢根浓度比值变化试验表明,自由基淬灭过程是其主要抑制原理. 例如,当罗丹明b与碳酸根/碳酸氢根的浓度比由0.05升至0.5时,VUV/UV对罗丹明b的降解速率常数升高了3.3倍[49].
2.3.3卤素阴离子
卤素离子是常见的光化学活性阴离子,会竞争吸收VUV/UV光子,抑制其对污染物的降解效率. 如溴离子在VUV/UV体系中会生成溴酸盐,不仅竞争吸收VUV,也会消耗VUV间接光解产生的H2O2(HO2-+HOBr→Br-+O2+H2O),削弱VUV/UV的氧化降解能力(见图5)[61-63].
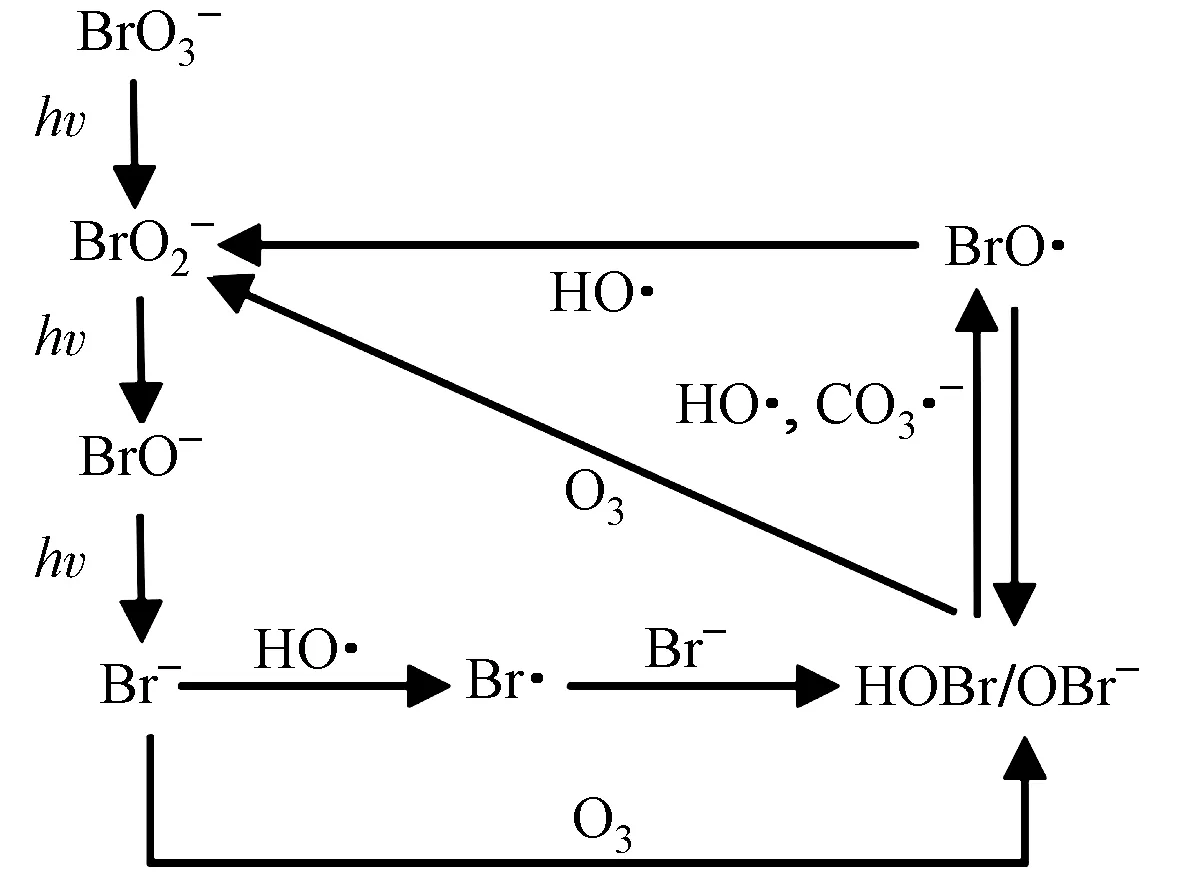
图5 VUV/UV光照中溴离子转化示意[61-63]Fig.5 Diagram of bromine conversion under VUV/UV irradiation[61-63]
此外,部分卤素离子在VUV/UV体系中会生成有毒有害副产物. 60 min VUV照射下,1 mg/L的碘离子约被转化83%,且大部分生成物为高毒性碘酸根离子[64]. 但一些卤素离子如氯离子在VUV照射下转化率不超过0.5%,对VUV/UV去除难降解污染物影响不明显[65-66].
2.3.4DOM的影响
DOM成分复杂,对VUV/UV体系的影响远大于传统UV体系,主要通过降低污染物吸收VUV的效率,消耗包括·OH、H·、eaq-在内的强氧化性自由基,从而削弱VUV/UV去除目标污染物的优势[52,67].
部分DOM、中间体及降解副产物会淬灭强氧化性自由基(DOM+·OH→CO2+H2O+无机酸),与·OH反应速率高达3×108L/(mol·s)[68-69]. 因此DOM会显著抑制VUV/UV体系对难降解有机污染物的去除效率. 如VUV/UV对二级出水中丙咪嗪的降解效率仅为超纯水体系的50%[70];当DOM浓度从1 mg/L升至2 mg/L,从2 mg/L升至4 mg/L时,VUV/UV对1,4-二甲烷的去除率分别降低13%和22%[15].
3 结论与建议
a) VUV/UV光源可在不额外添加氧化剂条件下,生成·OH、O2·-等强氧化性自由基,实现难降解污染物快速去除,且具有自由基产率高、污染物降解速率快、抗水质干扰能力强等优点,具有较好的水处理应用前景,值得开展后续研究.
b) VUV/UV生成强氧化性自由基的途径主要包括VUV直接裂解水分子和VUV/UV光解次生氧化剂H2O2,但不同来源自由基对污染物的降解贡献率还不清楚. 此外,VUV/UV的直接光解和自由基氧化降解会受到pH、无机阴离子及DOM等水质条件影响,但各水质因素对水分子裂解和次生氧化剂H2O2光解等不同来源自由基氧化的影响研究还未开展.
c) VUV/UV受水层厚度影响显著,而现有研究多以平行光照系统和紫外线剂量当量的实验室研究为主,实际连续流式运行和水力参数对水层扰动及氧化效果的影响还有待进一步研究,未来从实验室规模向实际规模的转变必不可少.
参考文献(References):
[1] HIJNEN W A M,BEERENDONK E F,MEDEMA G J.Inactivation credit of UV radiation for viruses,bacteria and protozoan (oo)cysts in water:a review[J].Water Research,2006,40(1):3-22.
[2] 郭美婷,胡洪营,陈健,等,紫外线对铜绿微囊藻的抑制效果及特性研究[J].环境科学,2011,32(6):1608-1613.
GUO Meiting,HU Hongying,CHEN Jian,etal.Inhibitory effects of ultraviolet irradiation on the growth of microcystis aeruginosa[J].Environmental Science,2011,32(6):1608-1613.
[3] ANTONOPOULOU M,EVGENIDOU E,LAMBROPOULOU D,etal.A review on advanced oxidation processes for the removal of taste and odor compounds from aqueous media[J].Water Research,2014,53:215-234.
[4] LIU Kai,RODDICK-FELICITY A,FAN Linhua.Impact of salinity and pH on the UVC/H2O2treatment of reverse osmosis concentrate produced from municipal wastewater reclamation[J].Water Research,2012,46(10):3229-3239.
[5] ALEXANDER J C,RAMIREZ-CORTINA C R.A comparative study:degradation of 2,5-dichlorophenol in wastewater and distilled water by ozone and ozone-UV[J].Ozone-Science & Engineering,2016,38(3):181-193.
[6] YANG Haiyan,LI Yi,CHEN Yiihua,etal.Comparison of ciprofloxacin degradation in reclaimed water by UV/chlorine and UV/persulfate advanced oxidation processes[J].Water Environment Research,2019,91(12):1576-1588.
[7] KANDAVELU V,KASTIEN H,THAMPI K R.Photocatalytic degradation of isothiazolin-3-ones in water and emulsion paints containing nanocrystalline TiO2and ZnO catalysts[J].Applied Catalysis B:Environmental,2004,48(2):101-111.
[8] THOMSON J,RODDICK F,DRIKAS M,etal.Natural organic matter removal by enhanced photooxidation using low pressure mercury vapour lamps[J].Water Science & Technology Water Supply,2002,2(5/6):435-443.
[9] IMOBERDORF G,MOHSENI M.Degradation of natural organic matter in surface water using vacuum-UV irradiation[J].Journal of Hazardous Materials,2011,186(1):240-246.
[10] DUBOWSKI Y,ALFIYA Y,GILBOA Y,etal.Removal of organic micropollutants from biologically treated greywater using continuous-flow vacuum-UV/UVC photo-reactor[J].Environmental Science and Pollution Research,2020,27(7):7578-7587.
[11] MARISA T,RYAN A,ROBERT B.2017 Potable reuse compendium[M].Washington DC:US EPA,2017.
[12] AFIFI M Z,BLATCHLEY E R.Effects of of UV-based treatment on volatile disinfection byproducts in a chlorinated,indoor swimming pool[J].Water Research,2016,105:167-177.
[13] LI Mengkai,QIANG Zhimin,HOU Pin,etal.VUV/UV/Chlorine as an enhanced advanced oxidation process for organic pollutant removal from water:assessment with a novel mini-fluidic VUV/UV photoreaction system (MVPS)[J].Environmental Science & Technology,2016,50(11):5849-5856.
[14] BAGHERI M,MOHSENI M.A study of enhanced performance of VUV/UV process for the degradation of micropollutants from contaminated water[J].Journal of Hazardous Materials,2015,294:1-8.
[15] BAGHERI M,MOHSENI M.Pilot-scale treatment of 1,4-dioxane contaminated waters using 185 nm radiation:experimental and CFD modeling[J].Journal of Water Process Engineering,2017,19:185-192.
[16] GONZALEZ M G,OLIVEROS E,WORNER M,etal.Vacuum-ultraviolet photolysis of aqueous reaction systems[J].Journal of Photochemistry and Photobiology C-Photochemistry Reviews,2004,5(3):225-246.
[17] BAGHERI M,MOHSENI M.Computational fluid dynamics (CFD) modeling of VUV/UV photoreactors for water treatment[J].Chemical Engineering Journal,2014,256:51-60.
[18] MORA A S,MOHSENI M.Temperature dependence of the absorbance of 185 nm photons by water and commonly occurring solutes and its influence on the VUV advanced oxidation process[J].Environmental Science-Water Research & Technology,2018,4(9):1303-1309.
[19] RUTH R,SHIGEFUMI OKADA.Estimation of life times and diffusion distances of radicals involved in X-ray-induced DNA strand breaks or killing of mammalian cells[J].Radiation Research Society,1975,64(2):306-320.
[20] YANG Laxiang,LI Mengkai,LI Wentao,etal.A green method to determine VUV (185 nm) fluence rate based on hydrogen peroxide production in aqueous solution[J].Photochemistry and Photobiology,2018,94(4):821-824.
[21] ZHANG Qi,WANG Lei,CHEN Baiyang,etal.Understanding and modeling the formation and transformation of hydrogen peroxide in water irradiated by 254 nm ultraviolet (UV) and 185 nm vacuum UV (VUV):effects of pH and oxygen[J].Chemosphere,2020.doi:https://doi.org/10.1016/j.chemosphere.2019.125483.
[22] IMOBERDORF G,MOHSENI M.Modeling and experimental evaluation of vacuum-UV photoreactors for water treatment[J].Chemical Engineering Science,2011,66(6):1159-1167.
[23] ZOSCHKE K,BOERNICK H,WORCH E.Vacuum-UV radiation at 185 nm in water treatment:a review[J].Water Research,2014,52:131-145.
[24] MASON J D,CONE M T,FRY E S.Ultraviolet (250-550 nm) absorption spectrum of pure water[J].Applied Optics,2016,55(25):7163-7172.
[25] FU Pingfeng,MA Yanhong,LEI Bolan,etal.Decomposition of refractory aniline aerofloat collector in aqueous solution by an ozone/vacuum-UV (O3/VUV) process[J].Environmental Technology,2019.doi:10.1080/09593330.2019.1642389.
[26] HUANG Haibao,LEUNG D Y C,KWONG P C W,etal.Enhanced photocatalytic degradation of methylene blue under vacuum ultraviolet irradiation[J].Catalysis Today,2013,201:189-194.
[27] PUSPITA P,RODDICK F A,PORTER N A.Decolourisation of secondary effluent by UV-mediated processes[J].Chemical Engineering Journal,2011,171(2):464-473.
[28] LI Mengkai,LI Wentao,BOLTON-JAMES R,etal.Organic pollutant degradation in water by the vacuum-ultraviolet/ultraviolet/H2O2process:inhibition and enhancement roles of H2O2[J].Environmental Science & Technology,2019,53(2):912-918.
[29] BOLTON-JAMES R,BIRCHER-KEITH G,TUMAS-WILLIA M,etal.Figures-of-merit for the technical development and application of advanced oxidation technologies for both electric- and solar-driven systems (IUPAC Technical Report) [J].Journal of Advanced Oxidation Technologies,2001,1(4):627-637.
[30] HIRUN-UTOK C,PHATTARAPATTAMAWONG S.Degradation and transformation of natural organic matter accountable for disinfection byproduct formations by UV photolysis and UV/chlor(am)ine[J].Water Science and Technology,2019,79(5):929-937.
[31] KIATTISAKSIRI P,KHAN E,PUNYAPALAKUL P,etal.Photodegradation of haloacetonitriles in water by vacuum ultraviolet irradiation:mechanisms and intermediate formation[J].Water Research,2016,98:160-167.
[32] HU Jun,WANG Chen,YE Zhen,etal.Degradation of iodinated disinfection byproducts by VUV/UV process based on a mini-fluidic VUV/UV photoreaction system[J].Water Research,2019,158:417-423.
[33] CAO Menghua,WANG Beibei,YU Hongsheng,etal.Photochemical decomposition of perfluorooctanoic acid in aqueous periodate with VUV and UV light irradiation[J].Journal of Hazardous Materials,2010,179(1/2/3):1143-1146.
[34] AMANOLLAHI H,MOUSSAVI G,GIANNAKIS S.VUV/Fe(Ⅱ)/H2O2as a novel integrated process for advanced oxidation of methyl tert-butyl ether (MTBE) in water at neutral pH:process intensification and mechanistic aspects[J].Water Research,2019,166.
[35] FU Pingfeng,FENG Jie,YANG Huifen,etal.Degradation of sodium n-butyl xanthate by vacuum UV-ozone (VUV/O3) in comparison with ozone and VUV photolysis[J].Process Safety and Environmental Protection,2016,102:64-70.
[36] GIRI R R,OZAKI H,GUO X,etal.Oxidative-reductive photodecomposition of perfluorooctanoic acid in water[J].International Journal of Environmental Science and Technology,2014,11(5):1277-1284.
[37] YAO Hong,PEI Jin,WANG Hui,etal.Effect of Fe(Ⅱ/Ⅲ) on tetracycline degradation under UV/VUV irradiation[J].Chemical Engineering Journal,2017,308:193-201.
[38] SZABO R K,MEGYERI C,ILLES E,etal.Phototransformation of ibuprofen and ketoprofen in aqueous solutions[J].Chemosphere,2011,84(11):1658-1663.
[39] ARANY E,SZABO R K,APATI L,etal.Degradation of naproxen by UV,VUV photolysis and their combination[J].Journal of Hazardous Materials,2013,262:151-157.
[40] MOUAMFON M V N,WENZHEN L I,SHUGUANG L U,etal.Photodegradation of sulfamethoxazole applying UV- and VUV-based processes[J].Water Air and Soil Pollution,2011,218(1/2/3/4):265-274.
[41] ALAPI T,GAJDA-SCHRANTZ K,ILISZ I,etal.Comparison of UV- and UV/VUV-induced photolytic and heterogeneous photocatalytic degradation of phenol,with particular emphasis on the intermediates[J].Journal of Advanced Oxidation Technologies,2008,11(3):519-528.
[42] HUANG Li,JING Hengye,CHENG Zhonghong,etal.Different photodegradation behavior of 4-tert-octylphenol under UV and VUV irradiation in aqueous solution[J].Journal of Photochemistry and Photobiology A:Chemistry,2013,251:69-77.
[43] 吴彦霖,余焱,袁海霞,等,水溶液中对叔辛基酚的紫外光降解研究[J].中国环境科学,2010,30(10):1333-1337.
WU Yanlin,YU Yan,YUAN Haixia,etal.UV photodegradation of p-tert-octyl phenol(4-OP) in water[J].China Environmental Science,2010,30(10):1333-1337.
[44] GENG Cong,LIANG Zhijie,CUI Fuyi,etal.Energy-saving photo-degradation of three fluoroquinolone antibiotics under VUV/UV irradiation:kinetics,mechanism,and antibacterial activity reduction[J].Chemical Engineering Journal,2020,383:123145.
[45] KUTSCHERA K,BOERNICK H,WORCH E.Photoinitiated oxidation of geosmin and 2-methylisoborneol by irradiation with 254 nm and 185 nm UV light[J].Water Research,2009,43(8):2224-2232.
[46] YANG Laxiang,LI Mengkai,LI Wentao,etal.Bench- and pilot-scale studies on the removal of pesticides from water by VUV/UV process[J].Chemical Engineering Journal,2018,342:155-162.
[47] RATPUKDI T,SIRIPATTANAKUL S,KHAN E.Mineralization and biodegradability enhancement of natural organic matter by ozone-VUV in comparison with ozone,VUV,ozone-UV,and UV:effects of pH and ozone dose[J].Water Research,2010,44(11):3531-3543.
[48] 杨毅,杨霞霞,马新培,等,pH对城市污水二级出水中溶解性有机物的荷电、聚集与光谱特性的影响[J].环境化学,2015,34(10):1804-1808.
YANG Yi,YANG Xiaxia,MA Xinpei,etal.Effect of pH on the charge aggregation and spectral characteristics of DOM in secondary effluent of municipal wastewater[J].Environmental Chemistry,2015,34(10):1804-1808.
[49] MEROUANI S,HAMDAOUI O,SAOUDI F,etal.Influence of bicarbonate and carbonate ions on sonochemical degradation of Rhodamine B in aqueous phase[J].Journal of Hazardous Materials,2010,175(1/2/3):593-599.
[50] LI Boqiang,MA Xiaoyan,LI Qingsong,etal.Factor affecting the role of radicals contribution at different wavelengths,degradation pathways and toxicity during UV-LED/chlorine process[J].Chemical Engineering Journal,2020.doi:10.1016/j.cej.2020.124552.
[51] WANG Wenlong,WU Qianyuan,HUANG Nan,etal.Synergistic effect between UV and chlorine (UV/chlorine) on the degradation of carbamazepine:influence factors and radical species[J].Water Research,2016,98:190-198.
[52] BUCHANAN W,RODDICK F,PORTER N.Formation of hazardous by-products resulting from the irradiation of natural organic matter:comparison between UV and VUV irradiation[J].Chemosphere,2006,63(7):1130-1141.
[53] GONZALEZ M C,BRAUN A M.VUV photolysis of aqueous-solutions of nitrate and nitrite[J].Research on Chemical Intermediates,1995,21(8/9):837-859.
[54] MATSUSHITA T,SUGITA W,ISHIKAWA T,etal.Prediction of 1,4-dioxane decomposition during VUV treatment by model simulation taking into account effects of coexisting inorganic ions[J].Water Research,2019.doi:10.1016/j.watres.2019.114918.
[55] WANG Lei,ZHANG Qi,CHEN Baiyang,etal.Some issues limiting photo(cata)lysis application in water pollutant control:a critical review from chemistry perspectives[J].Water Research,2020.doi:10.1016/j.watres.2020.115605.
[56] ZOSCHKE K,DIETRICH N,BOERNICK H,etal.UV-based advanced oxidation processes for the treatment of odour compounds:efficiency and by-product formation[J].Water Research,2012,46(16):5365-5373.
[57] MACK J,BOLTON J R.Photochemistry of nitrite and nitrate in aqueous solution:a review[J].Journal of Photochemistry and Photobiology A:Chemistry,1999,128(1/2/3):1-13.
[58] BUXTON G V,GREENSTOCK C L,HELMAN W P,etal.Critical review of rate constants for reactions of hydrated electrons,hydrogen atoms and hydroxyl radicals (·OH/·O-) in aqueous solution[J].Journal of Physical and Chemical Reference Data,1988,17(2):513-886.
[59] WEN Dong,LI Wentao,LV Jinrong,etal.Methylene blue degradation by the VUV/UV/persulfate process:effect of pH on the roles of photolysis and oxidation[J].Journal of Hazardous Materials,2020.doi:10.1016/j.jhazmat.2019.121855.
[60] VILLEGAS-GUZMAN P,SILVA-AGREDO J,FLOREZ O,etal.Selecting the best AOP for isoxazolyl penicillins degradation as a function of water characteristics:effects of pH,chemical nature of additives and pollutant concentration[J].Journal of Environmental Management,2017,190:72-79.
[61] RATPUKDI T,CASEY F,DESUTTER T,etal.Bromate formation by ozone-VUV in comparison with ozone and ozone-UV:effects of pH,ozone dose,and VUV power[J].Journal of Environmental Engineering,2011,137(3):187-195.
[62] LIANG S,PALENCIA L S,YATES R S,etal.Oxidation of MTBE by ozone and peroxone processes[J].Journal American Water Works Association,1999,91(6):104-114.
[63] VON GUNTEN U.Ozonation of drinking water.Part Ⅱ:disinfection and by-product formation in presence of bromide,iodide or chlorine[J].Water Research,2003,37(7):1469-1487.
[64] BU Yinan,SONG Mingrui,HAN Jiarui,etal.A facile and green pretreatment method for nonionic total organic halogen (NTOX) analysis in water-Step Ⅱ.using photolysis to convert NTOX completely into halides[J].Water Research,2018,145:579-587.
[65] ALEGRE M L,GERONES M,ROSSO J A,etal.Kinetic study of the reactions of chlorine atoms and Cl-2(center dot-) radical anions in aqueous solutions.1. reaction with benzene[J].Journal of Physical Chemistry A,2000,104(14):3117-3125.
[66] COSTA C R,OLIVI P.Effect of chloride concentration on the electrochemical treatment of a synthetic tannery wastewater[J].Electrochimica Acta,2009,54(7):2046-2052.
[67] IMOBERDORF G,MOHSENI M.Kinetic study and modeling of the vacuum-UV photoinduced degradation of 2,4-D[J].Chemical Engineering Journal,2012,187:114-122.
[68] MCKAY G,DONG M M,KLEINMAN J L,etal.Temperature dependence of the reaction between the hydroxyl radical and organic matter[J].Environmental Science & Technology,2011,45(16):6932-6937.
[69] XIE Pengchao,YUE Siyang,DING Jiaqi,etal.Degradation of organic pollutants by Vacuum-Ultraviolet (VUV):kinetic model and efficiency[J].Water Research,2018,133:69-78.
[70] XIE Pengchao,ZOU Yujia,JIANG Shan,etal.Degradation of imipramine by vacuum ultraviolet (VUV) system:influencing parameters,mechanisms,and variation of acute toxicity[J].Chemosphere,2019,233:282-291.
