Cardiac papillary fibroelastoma
2021-06-18DominikaZoltowskaEdinSadicKyeeshaBecoatsShreyaGhetiyaAleemAzalAliSrinivasanSattirajuEmilMissov
Dominika M Zoltowska, Edin Sadic, Kyeesha Becoats, Shreya Ghetiya, Aleem Azal Ali,Srinivasan Sattiraju, Emil Missov
Department of Cardiovascular Disease, University of Florida College of Medicine, Jacksonville, FL, USA
ABSTRACT Papillary fibroelastoma (PFE) is a primary, histologically benign endocardial neoplasm. Though PFE has long been reported as the second most common primary cardiac neoplasm, it has since pulled ahead of cardiac myxomas, largely due to evolving cardiac imaging modalities. While PFEs are benign histologically, they have the potential for devastating clinical consequences, transient ischemic attack, stroke, myocardial infarction, syncope, pulmonary, and peripheral embolism. Despite increased detection rate, there remains uncertainty regarding etiology, exact prevalence, and clinical management of PFEs. This paucity of information is reflected by the lack of official guidelines on this matter. In this article, we aim to summarize the current state of understanding regarding PFE and discuss areas of ongoing controversy.
In 1931, valvular tumors were first described by W. M Yater.[1]The term ‘papillary fibroelastoma (PFE)’ was first used in a case report by Cheitlin in 1975.[2]At that time, PFE was thought to be an asymptomatic neoplasm found incidentally at autopsy. Since then, PFE has received more clinical attention due to the fatal and life threaten complications attributed to it. This has spurred more interest on the matter in recent years. It was previously thought that cardiac myxomas were the most common benign cardiac primary neoplasm based on large autopsy case studies. However, with the improvement in echocardiography technology, and with better understanding of the importance of papillary fibroelastoma recent case series studies have found that, in fact, cardiac papillary fibroelastoma may be the most common benign primary cardiac neoplasm. In fact, Tamin,et al.[3]found a rate of 1 PFE per every 1,100 echocardiograms. While PFEs occur more often on cardiac valvular surface, they may be found on non-valvular endocardial surfaces as well. A meta-analysis of 725 histopathologically confirmed cases shows that PFE is found on the aortic valve 44% of the time, followed by the mitral valve 35%, tricuspid valve 15%, and less often on the pulmonary valve 8%. The left ventricle was the most common site of non-valvular occurrence.[4]Just as the site of occurrence can vary, multiple studies showed that the age of occurrence varies widely, while most occur between the 4thand 8thdecade of life, there have been several incidences of neonatal and infant occurrences of PFE as well.
PATHOPHYSIOLOGY
PFE are small (2-70 mm), slow-growing, avascular benign tumors classically characterized by their multiple papillary fronds, which give a sea anemonelike appearance. Histologically, they are comprised of a single layer of endocardial cells covering the papillary surface, matrix consisting of longitudinally oriented collagen with irregular elastic fibers,proteoglycans, and spindle cells that resemble smooth muscle cells or fibroblasts.[5]This layer of elastic fibers is a hallmark of this tumor (Figure 1).
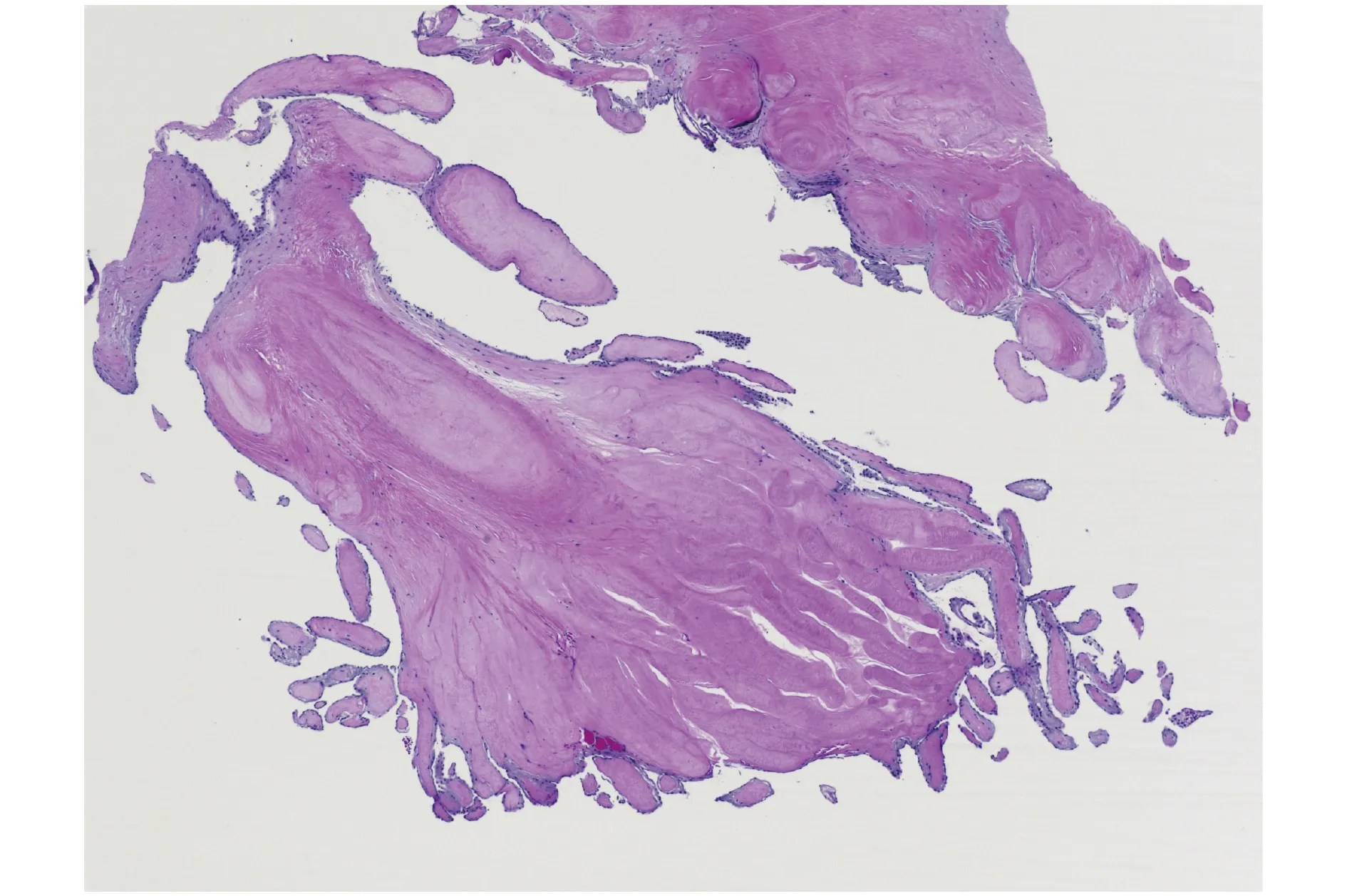
Figure 1 Microscopic picture characteristic for papillary fibroelastoma.
The histogenesis of PFE is unclear. Hypotheses regarding its etiology include neoplasm, harmatomas, organized thrombi and an unusual endocardial response to infection or iatrogenic causes (post radiation, surgery, hemodynamic trauma).[6]The most widely accepted explanation is the microthrombus theory, which suggests that small thrombi coalesce on the coapting margins of valves at the site of minor endothelial damage.[7]This theory is supported by the presence of fibrin, hyaluronic acid and laminated elastic fibers, which are commonly found in routine thrombus formation.
CLINICAL PRESENTATION AND NATURAL COURSE
PFE are predominantly found in men and persons older than 40 years, with the majority being diagnosed at age 60.[8]Nevertheless, there are several case reports which have described PFE in neonates and young children, mostly in those with congenital cardiac abnormalities.[9]The clinical presentation of PFE varies widely, with 54% remaining asymptomatic at time of diagnosis. For this reason, the majority of PFEs are discovered incidentally. Of those that are symptomatic, the clinical presentation is often non-specific and insidious,leading to delayed diagnosis and management.[10]In a review of 54 PFE cases by Cianciulli,et al.,[11]the most common symptom was dyspnea in 37.5% and transient ischemic attack tied at 37.5%, followed by angina in 12.5%, and syncope in 12.5%. Case reports even mention PFE manifested by isolated pyrexia, antiphospholipid syndrome, thyroid dysfunction, and even thrombocytopenia, all of which resolved after incidental discovery and removal of the PFE. Ultimately, the clinical presentation is influenced by many factors, including tumor location,size, growth rate, and tendency for embolization.[4]Owing to the fact that over 95% of PFE present on the left side of the heart, systemic embolism is the most frequent presentation.[4]Embolization to the coronary arteries, lungs, brain, kidneys, retina, spinal cord, mesentery vessels, and lower limbs have all been reported, some with disastrous and permanent damage.[4,11]In the case of right-sided PFE, while embolic phenomena are more scarce, pulmonary emboli have been well-documented-some of which have led to the development of pulmonary hypertension and death.[4]Furthermore, in the presence of a patent foramen ovale (PFO), right-sided PFE can lead to systemic embolization as well.
Much like other cardiac masses, PFEs can have significant hemodynamic effects. PFEs attached to the aortic valve have been documented to cause angina (often exertional) and sudden cardiac death in otherwise young and healthy individuals. This is secondary to obstruction of coronary blood flow,most commonly when the PFE is attached to the right coronary cusp. This is thought to be the case because the right coronary artery ostium is located immediately above the right coronary cusp, whereas,the ostium of the left main coronary artery is found mid-way between the commissures of the left coronary cusp, while the non-coronary cusp is positioned posteriorly.[12]
Additionally, aortic valve PFEs can impair filling of the left ventricle, which can lead to recurrent pulmonary edema and symptoms of right-sided heart failure, and mimicking of valvular stenosis or regurgitation.[13]Depending on the tumor’s size, texture, and overall mobility, temporary or complete obstruction of the orifice of the mitral or tricuspid valve may occur, resulting syncope or sudden death.[4]Further still, the to-and-fro swing of the PFE may even impede the proper coaptation of mitral and tricuspid valve leaflets, resulting in valve insufficiency.[4]On the physical exam, while these effects may manifest as either systolic and/or diastolic murmurs, it is important to remember that the intensity of the murmur(s) could change depending on body position.[4]Furthermore, the classic “diastolic tumor plop”, often associated with atrial myxomas, has not been described with PFE, except for one case of tricuspid PFE.[4,14]Clinical course and astute clinical suspicion, therefore, of utmost importance.
DIAGNOSIS
Definitive diagnosis of PFE requires pathological confirmation. Initial evaluation is accomplished with the use of multi-modality imaging, most often with echocardiography.
While there are no official echocardiographic diagnostic criteria, the diagnostic accuracy of echocardiography in diagnosing PFE is in excess of 85%.[15,16]Classically, PFE presents as a small < 1.5 cm, round, echo-dense, and pedunculated mass with high independent motion. Its characteristic“shimmery” appearance correlates with filiform projections.[4,15,16]As was discussed earlier, PFE can arise from any endocardial structure, though the highest predilection is shown to cardiac valves(> 80%).[4,17]Most findings of PFE are incidental and only rarely associated with overt valvular dysfunction.[15,16]However, when valves are involved, the aortic valve is involved most frequently, followed by mitral, tricuspid, and very rarely pulmonic valve.[4,15]As a general rule, PFE of semilunar valves has no clear preference to the valvular site. In contrary, atrioventricular PFEs tends to occupy the atrial surface of the valve.[15,18]Detection of a smaller PFE, < 5 mm often require higher resolution than offered by transthoracic echocardiogram (TTE). Hence,transesophageal echocardiogram (TEE) should be considered in the case of a negative TTE and high suspicion of cardioembolic phenomenon.[15]Contrast enhanced and 3D echocardiography may provide further enhanced image quality in elusive cases. The Video 1-4 demonstrates all four valvular echocardiographic presentations of PFE.
Cardiac computed tomography and magnetic resonance Imaging are second-line modalities in PFE evaluation.[19]Although their utilization is supported by the appropriate use criteria, their role in relation to the diagnosis of PFE is still not well established (Table 1).[20]The main advantage of cardiac MRI is utilization of tissue dependent signal characterization.[21,22]PFE is reported to demonstrate hypointense signal intensity on cine images, and intermediate signal intensity on both T1 and T2 weighted images, which mimics myocardial tissue.[23,24]Late gadolinium enhancement can be used to further differentiate it from other masses, as late enhancement is commonly absent, reflecting the absence of tumor vascularity.[25]In the case of cardiac CT imaging, PFE shows up as a hypodense mass with irregular borders.[26]The advantage of this modality is its ability to visualize the exact anatomical point of PFE attachment along with simultaneous evaluation of the coronary arteries.[27]This is especially helpful in cases where suspicion for PFE-induced syncope or chest pain is high. While both CT and MRI are ideal in answering specific clinical questions concerning PFE, the small size and high mobility of PFE are major limiting factors in their utilization.[28]For this reason, echocardiography remains the most accurate, reproducible, and reliable modality for assessment of PFE. The advantages and disadvantages of each imaging technique are summarized in Table 2.
Nevertheless, despite the guidance of multiple imaging modalities, clinical presentation can vary widely and a strong diagnosis can remain elusive.The differential diagnoses of PFE is broad and includes valvular vegetation, Lambl’s excrescences,myxoma, cysts, thrombus, fenestration, fibroma,and artifact. Non-valvular PFE may be even more diagnostically challenging. Tamin,et al[3]. suggests obtaining blood cultures, antiphospholipid antibodies,and screening for systemic lupus erythromatosis in all cases. The clinical background and presentation in conjunction with imaging studies leads to correct diagnosis in most cases.
TREATMENT
There are no clear guidelines regarding the management of PFE. Surgical excision is curative. The surgeon must ensure complete removal of the tumor including the stalk and the underlying endocardium without any fragmentation or emboliza-tion or residual tumor. Inspection of all four chambers should be made for any other co-existing tumor. Special care should be taken while resecting the tumor from valve leaflets. Injury to the valvular structure may require valve repair or replacement.Long term antiplatelet (single or dual agent) and anticoagulation therapy have been recommended in certain patients with PFE. However, these recommendations are not derived from randomized control studies. Most of our conclusions in this review article are derived from the data available from three retrospective observational studies. Sun,et al.[16]is a retrospective and prospective analysis of 162 cases of CPF published in 2001; Gowda,et al.[4]is a retrospective analysis of 725 cases of CPF published in 2003; and Tamin,et al.[3]is the most recent,retrospective analysis involving 511 cases published in 2015.
Table 1 Appropriateness for CCT and CMR use.
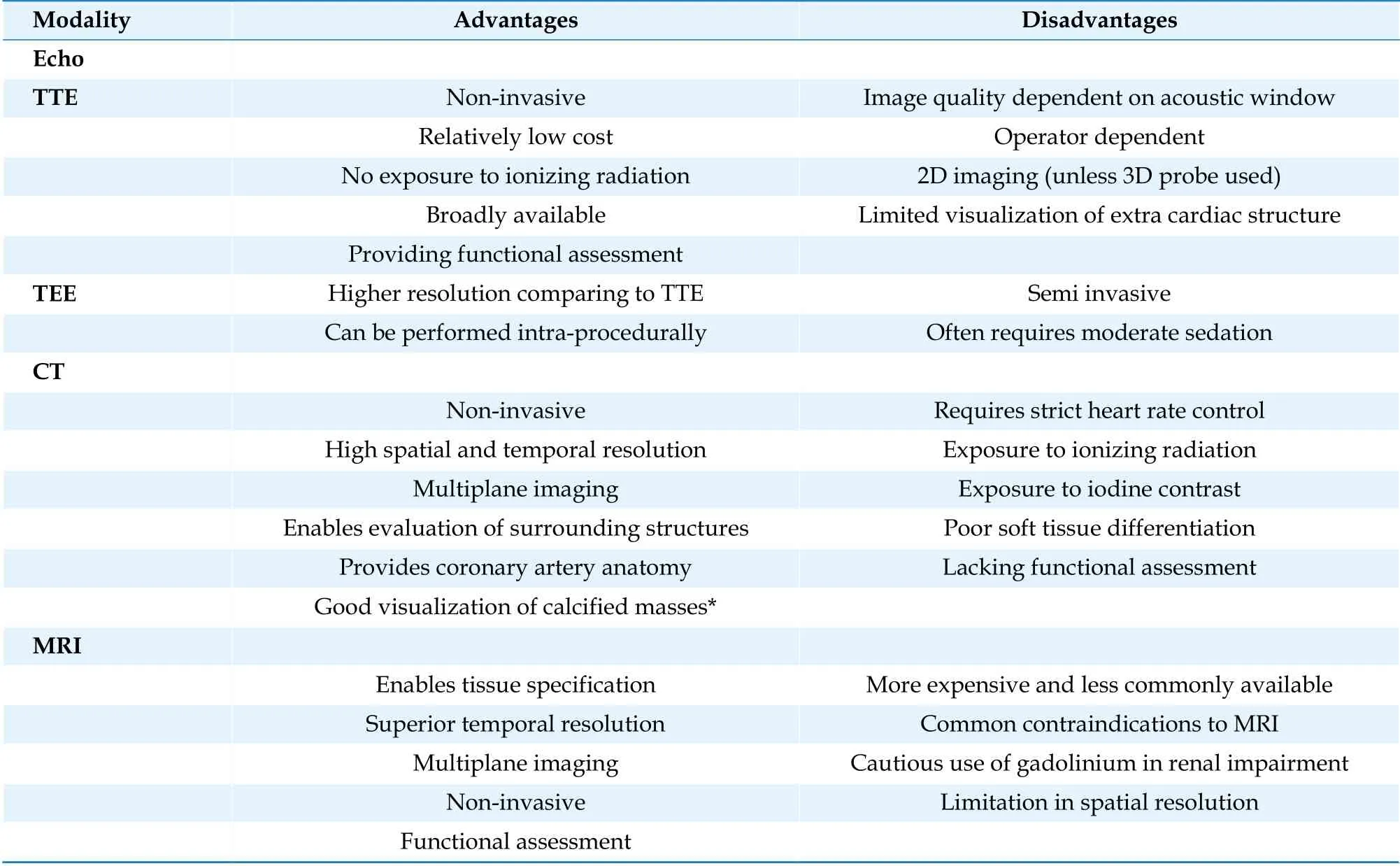
Table 2 Comparison of different imaging modalities.
Surgical resection remains the treatment of choice for all symptomatic patients that are good surgical candidate (Society of Thoracic Surgeon score < 1%).For symptomatic patients who are not surgical candidate, long term antiplatelets or anticoagulation therapy should be initiated. Asymptomatic patients should have surgical resection if the size of the tumor is > 1 cm or has increased mobility, since these are the predictors of nonfatal embolization or death.Asymptomatic patients with small (< 1cm), nonmobile (no stalk) left sided tumor should be clinically observed without intervention
Tamin,et al.[3]suggest surgical resection of all left sided tumors regardless of size, mobility or symptoms, for patients that are good surgical candidates.Their observation shows that the incidence of recurrent neurological events significantly decreases in those patients who had surgical resection. For patients that are not surgical candidates, medical therapy with aspirin and anti-platelet should be offered in the absence of any contraindications. However,the efficacy of medical therapy alone remains to be undetermined.
There is not sufficient data to recommend surgical resection of right sided tumors. Surgery should be reserved for patients with a very large, mobile, pedunculated tumor causing hemodynamically significant obstruction to the flow and/or embolization especially in the presence of patent foramen ovale and significant right to left shunt. Minimally invasive robotic surgery can safely remove tumors from the aortic valve or the mitral valve via transaortic or transpetal approach respectively.[29]We believe that this approach will significantly improve the outcomes and surgical morbility and mortality. This approach sparing the valvular anatomy and avoids left ventriculotomy.
CONCLUSION
Despite its benign histology, PFE are associated with significant morbidity and mortality. Echocardiography remains the most commonly utilized diagnostic modality. Familiarity with the echocardiographic appearance of the tumor is important.Once the diagnosis is made, further treatment should be discussed in a multidisciplinary team approach. The cardio-embolic risk is high in left sided lesions. Surgical treatment with valve sparing procedure should always be considered, even in asymptomatic patients. A minimally invasive surgical approach is associated with excellent outcomes.There is not enough evidence supporting the routine use of antithrombotic therapy. Antithrombotic therapy should be considered in non-surgical candidates.
杂志排行
Journal of Geriatric Cardiology的其它文章
- Atrial fibrillation in patients with systolic heart failure:pathophysiology mechanisms and management
- Beta-blocker treatment in heart failure patients with atrial fibrillation: challenges and perspectives
- A case series of precipitous cardiac tamponade from suspected perimyocarditis in COVID-19 patients
- A valve-in-valve approach to manage severe bioprosthetic tricuspid valve stenosis
- Coronary artery disease presenting as intractable hiccups: an unclear mechanism
- Anticoagulation for atrial fibrillation in heart failure patients:balancing between Scylla and Charybdis
