Population structure and reproductive biology of the mysid Nanomysis siamensis (Crustacea: Mysida) in Songkhla Lagoon system, southern Thailand*
2021-06-15RofizaYOLANDAVachiraLHEKNIM
Rofiza YOLANDA , Vachira LHEKNIM
Division of Biological Science, Faculty of Science, Prince of Songkla University, Songkhla 90112, Thailand
Abstract Songkhla Lagoon in southern Thailand is a large lagoon with brackish water where the mysid Nanomysis siamensis inhabit. N. siamensis has long been the dominant mysid species in this lagoon.However, nothing is known about the biology and ecology of N. siamensis. Here for the first time, we provide information about its demographic structure and reproductive biology in the Songkhla Lagoon system. We conducted monthly sampling from June 2017 to June 2018 at five stations in Thale Sap and seven stations in Thale Sap Songkhla. Individuals were classified into six categories. The monthly mean density ranged 8.44–807.33 inds./100 m 2 in Thale Sap and 8.81–434.44 inds./100 m 2 in Thale Sap Songkhla. The peak occurrence of this species was in May 2018 in Thale Sap. The population composition was as follows:juvenile 10.13%, immature males 13.45%, immature females 10.54%, mature males 26.19%, mature females 21.63% and ovigerous females 18.05%. The proportion of ovigerous females was statistically significant and brooding females in the nauplioid stage were more dominant than embryonic phase and post-nauplioid stages. Brood size varied from 1 to 17, the embryo diameter ranging from 0.2 to 0.4 mm and the lengths of the larvae ranged from 0.5 to 1.0 mm. There was a positive correlation between brood size and brood length with ovigerous female body length. Overall, mature males were predominant over the five population composition types, and reproduction in N. siamensis was observed every month.
Keyword: brackish water; reproductive trait; Thale Sap; Thale Sap Songkhla
1 INTRODUCTION
Mysids are dominant coastal and estuarine organisms (Mees and Hamerlynck, 1992; Mees et al.,1993; Azeiteiro and Marques, 1999; Granda et al.,2004). They are omnivores that feed on detritus,phytoplankton, and zooplankton (Fockedey and Mees, 1999), and play an important role in the transfer of energy from primary food sources towards secondary consumers at a higher trophic level (Mees and Jones, 1997; Viherluoto, 2001; Stewart et al.,2009). Some of them also serve as a food source for human consumption (Mauchline, 1980; Mantiri et al.,2012).
These small crustaceans have been studied throughout the world. However, biological and ecological studies on them in tropical regions are still lacking compared to the abundant studies in temperate regions, such as from USA (Allen, 1984; Turpen et al., 1994; Pothoven et al., 2000), Norway (Kjellberg et al., 1991), Japan (Ikeda, 1992; Yamada et al., 2007;Chikugo et al., 2013), Netherlands (Mees and Hamerlynck, 1992; Mees et al., 1993, 1994; Rappé et al., 2011), Spain (Baldó et al., 2001; Delgado et al.,2013), Germany (Scharf and Koschel, 2004). Most studies on Southeast Asian mysids have focused on taxonomy, for example from Indonesia (Nouvel,1957; Bacescu, 1993; Hanamura et al., 2008a, 2011;Bamber and Morton, 2012; Hanamura et al., 2014),Malaysia (Hanamura et al., 2008a, 2011; Tan and Azman, 2017, 2018), Philippines (Murano, 1997;Hanamura et al., 2011) and Thailand (Tattersall, 1921;Murano, 1988; Fukuoka and Murano, 2002;Hanamura et al., 2008a, 2011; Moriya et al., 2015),with few studies on seasonal abundance (Hanamura et al., 2008b; Biju et al., 2009, 2010; Biju and Panampunnayil, 2010, 2011; Ramarn et al., 2012).Since the population and life history of shallow water mysids diff er at spatial and temporal scales between tropical and temperate regions (Mauchline, 1980),studies are needed to provide more information about the organisms.

Fig.1 Twelve sampling stations (black dots) in Songkhla Lagoon, southern Thailand
The Songkhla Lagoon system (also known as Songkhla Lake) is one of the largest lagoons in southern Thailand (1 040 km2) and can be characterized by its shallow depth (approximately 1 to 2 m), muddy and sandy substrate and large variation in salinity (0 to 33). Although numerous studies on benthic fauna and small crustaceans have been carried out in this lagoon system, information about mysids is limited. The first data on the mysids in this lagoon were reported by Tattersall in 1921 with two species,NanomysissiamensisandRhopalophthalmusegregius(nowR.orientalis, see Tattersall (1957)) from Thale Sap, and to date (almost 100 years), only one species has been identified as a new species (Yolanda et al.,2019). Other aspects of the biology of the mysids,such as population, distribution, and reproduction have not been recorded from this lagoon, especially forN.siamensisthat is very abundant in this lagoon.Due to its abundance, this mysid is projected to play an important role in this ecosystem, and information about its biology is crucial to the provision of comprehensive data to improve fisheries management and aquaculture in the lagoon. The objective of the present study is thus to provide basic information on the population structure and reproductive biology ofN.siamensisin the waters of Thale Sap and Thale Sap Songkhla, Songkhla lagoon system, southern Thailand.
2 MATERIAL AND METHOD
2.1 Sampling procedure
Diurnal sampling was carried out monthly at five stations in Thale Sap and at seven stations in Thale Sap Songkhla (Fig.1) from June 2017 to June 2018 except December 2017 when extreme weather conditions prevented sampling. Both of the main lagoons have diff erent water salinity characteristics,where Thale Sap is always influenced by freshwater inflow, and Thale Sap Songkhla is influenced by seawater from the Gulf of Thailand. At each station,samples were collected at two sites with three replicates each in the littoral zone approximately 200 m from the shore. The distance between sites at any given station was approximately 100 m, and the environmental conditions were always similar between both sites. Within-site replication was intended to increase sample size and avoid bias due to the likelihood of zero-abundance records in individual samples. Mysids were collected with a modified Riley hand push-net with two nets (mouth area 0.50 m×0.30 m; mesh openings 0.5 and 2 mm) that could reach the bottom of the lagoon. The net was pushed by hand while walking through the shallow zone of the lagoon for 30 m at depths of 50 to 150 cm.Densities of the mysid were calculated as the number ofindividuals per 100 m2(inds./100 m2), which was swept by the net (net width×distance of tow). Mud and debris were cleaned from the samples and the entire contents were then washed into a sample bottle and fixed with 4% lagoon water-buff ered formalin and rose bengal. Environmental variables(temperature, salinity, and pH) were measured at the surface during sampling sessions. Temperature and salinity with a digital AZ instrument (AZ 8371) and pH with an Adwa instrument (AD12).
2.2 Laboratory analysis
In the laboratory,N.siamensisand other mysids were sorted and counted under a stereomicroscope,and mysid density per station was calculated as inds./100 m2. Body length (BL) was measured from the anterior margin of the rostrum to the posterior end of the telson excluding apical spines (Ramarn et al.,2012) for 200 individuals (when available), with an ocular micrometer mounted on a stereomicroscope.Each individual was then classified into one of six main categories based on their reproductive and sexual characteristics, following Mauchline (1980):(1) juveniles, without secondary sexual characteristics;(2) immature males, with developing secondary sexual characteristics (4thpleopod); (3) immature females, with undeveloped marsupial or brood pouch;(4) mature males, with completely developed secondary sexual characteristics; (5) mature females,with completely developed secondary sexual characteristics and empty marsupia; and (6) ovigerous or brooding females, with embryo or larvae in the marsupium. In the case of brooding females, the brood was classified into one of three categories of development according to San Vicente et al. (2014):embryonic phase, the marsupium contained eggs or embryos; nauplioid stage, embryo developed into larvae but without eyes and post-nauplioid stage,molted larvae presented with stalked eyes and thoracic appendages. Embryos and larvae were also measured.The embryo diameter was measured along the longest axis, and lengths of the larvae were measured from the frontal to the terminal tip of the body.
2.3 Data analysis
A two-way ANOVA test was performed to evaluate the diff erences in the abundance and body size of each category ofN.siamensisamong months and between the two lagoons. Development was also analyzed descriptively. Significant diff erences in the proportion of sex ratio (Thale Sap, Thale Sap Songkhla and combination of the two lagoons) and in brooding females (combination of Thale Sap and Thale Sap Songkhla) were tested with the chi-square tests. Specific diff erences in the brood size (embryo,nauplioid and post-nauplioid stage) and body length of brooding female were tested with one-way ANOVA and post hoc Tukey’s HSD. Multiple linear regressions were used to investigate any correlation between the body length of brooding females with the brood size and length of brood members (embryo, nauplioid, and post-nauplioid stage). The value of the standard error(±SE) is presented after the mean value. All the data were tested for normality and homoscedasticity,failing data were transformed with log (n+1) (Sokal and Rohlf, 1995) and statistical analyses were performed using Statistix 10.0.
3 RESULT
3.1 Environmental variables
Measurements of water temperature, salinity, and pH from June 2017 to June 2018 are presented in Fig.2a–c, with the exception of temperature in June 2017 due to equipment malfunction. At Thale Sap,monthly mean water temperatures fluctuated throughout the study period between 25.81 and 31.41 °C, with an overall mean of 29.40±1.91 °C.Although the water temperature exhibited a slight peak during October 2017, monthly variation was generally minor. In contrast, the mean monthly salinity exhibited a temporal variation, from 0 to 11.01, with an overall mean of 3.49±3.48. The pH ranged from 6.45 to 10.2. At Thale Sap Songkhla, the mean monthly water temperatures ranged from 26.75 to 31.01 °C, with an overall mean of 29.02±1.12 °C;the mean salinity exhibited monthly fluctuations from 0 to 22.06 with an overall mean of 8.71±7.39; and the pH ranged from 6.7 to 9.3.
3.2 Abundance
The monthly mean population densities ofN.siamensisduring this study exhibited significant temporal variation (Fig.3). In Thale Sap, density increased from June to August 2017; however, from September 2017, the trend reversed, reaching the lowest density in November 2017, and then rising again from January 2018 to a peak density in May 2018. Overall, the mean density ranged from 8.44 to 807.33 inds./100 m2. In contrast, in Thale Sap Songkhla, the density showed a downward trend from July to September 2017 with an increase from October 2017, reaching maximal density in November 2017.However, from January 2018 density declined,reaching a minimal value in March 2018. The mean density ranged 8.81–434.44 inds./100 m2. Overall,monthly mean densities ofN.siamensiswere higher in Thale Sap than in Thale Sap Songkhla except in October 2017, November 2017 and June 2018, and there was a significant abundance diff erence among the population from Thale Sap and Thale Sap Songkhla with monthly collection. Detailed results of the statistical analysis are shown in Table 1.
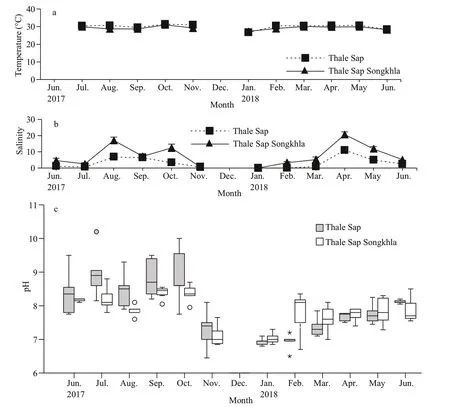
Fig.2 Monthly water variables (June 2017–June 2018) at Thale Sap and Thale Sap Songkhla, Songkhla lagoon, southern Thailand

Fig.3 Monthly mean (±SE) abundance of N. siamensis W. Tattersall, 1921 from Thale Sap and Thale Sap Songkhla, Songkhla Lagoon, southern Thailand (June 2017–June 2018)
Measurements ofN.siamensisbody size showed variation in each life stage of this mysid (Table 2).The juvenile stage was distinguished by the absence of genitalia and genital appendages, and the size ranged from 1.2 to 2.4 mm in Thale Sap and Thale Sap Songkhla. Immatures were diff erentiated by the development of the fourth pleopod into immature male, at 2.2 to 3.3 mm in Thale Sap and 2.4 to 3.3 mm in Thale Sap Songkla, and reached maturity with the elongation of the fourth pleopod, which in mature specimens (3.1 to 5.0 mm in Thale Sap and 3.1 to 5.3 mm in Thale Sap Songkla) was elongated as far as the sixth abdominal somite and even the statocyst. In females, oostegites appeared in individuals measuring 2.3 to 3.3 mm in Thale Sap and 2.4 to 3.3 mm in Thale Sap Songkla, and formed a large brood pouch on the ventral side of the thorax in adults. Mature females were collected that measured 3.0 mm and the largest was 5.4 mm in Thale Sap and 6.1 mm in Thale Sap Songkhla. The brood pouch developed into the marsupium and brooding females were found at 2.9 mm in Thale Sap and 3.1 mm in Thale Sap Songkhla, with body lengths up to 5.7 mm in Thale Sap and 5.6 mm in Thale Sap Songkhla. Statisticalanalysis also showed that there was a significant body length diff erence among each life stage category (two way ANOVAF5,16387=15 486.62,P=0.000), between the two lagoons (two way ANOVAF1,16387=211.88,P=0.000) and also an interaction occurred between the lagoon and each category (two way ANOVAF5,16387=26.84,P=0.000) (Fig.4).
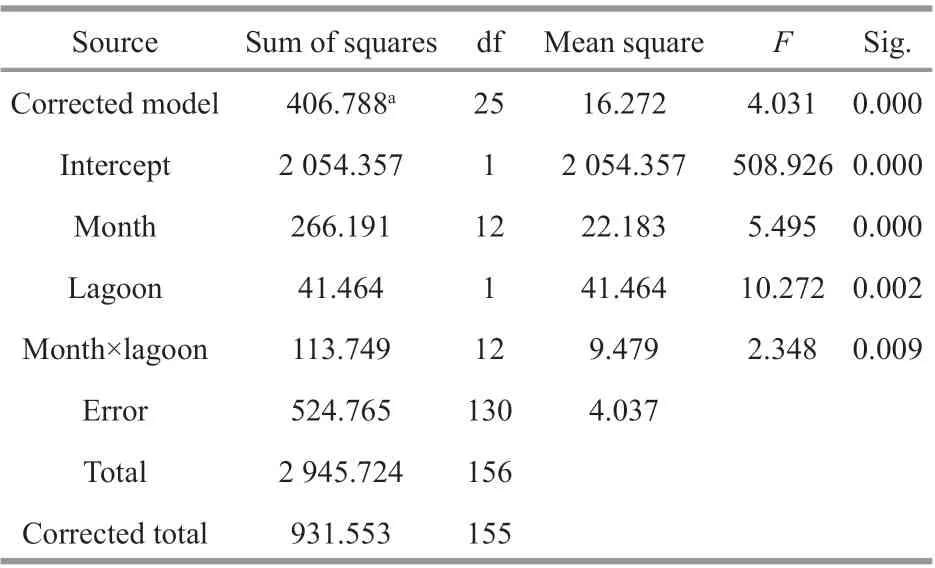
Table 1 Results of two-way ANOVA test on the abundance of N. siamensis with monthly collection and the two lagoons (Thale Sap and Thale Sap Songkhla)
3.3 Population structure and sex ratio
The populations ofN.siamensisin both Thale Sap and Thale Sap Songkhla showed similar trends, in which the mature males were predominant among the six categories (Fig.5a–b). At Thale Sap, mature males accounted for the largest proportion of the samples with, on average, 26.26% of the whole population;mature females with 21.17%; juveniles, immature males and immature females formed about 9.84%,13.61%, and 10.61% of the populations, respectively,and ovigerous females contributed approximately 20.19% (Fig.5a). The highest proportion of mature males was found during January 2018, while the lowest was observed in September 2017. Conversely,the largest proportion of mature females was found in October 2017, with the lowest proportion in April 2018. Juveniles and immature males were most abundant in September 2017 and at their lowest levelin October 2017; immature females were equally most abundant in September 2017, but lowest in June 2017. Ovigerous females peaked in May 2018 and were at their lowest abundance in July 2017. There was a significant diff erence in the sex ratio between males and females at Thale Sap monthly (χ2=205.523,df=11,P=0.00), and females were more predominant than males.
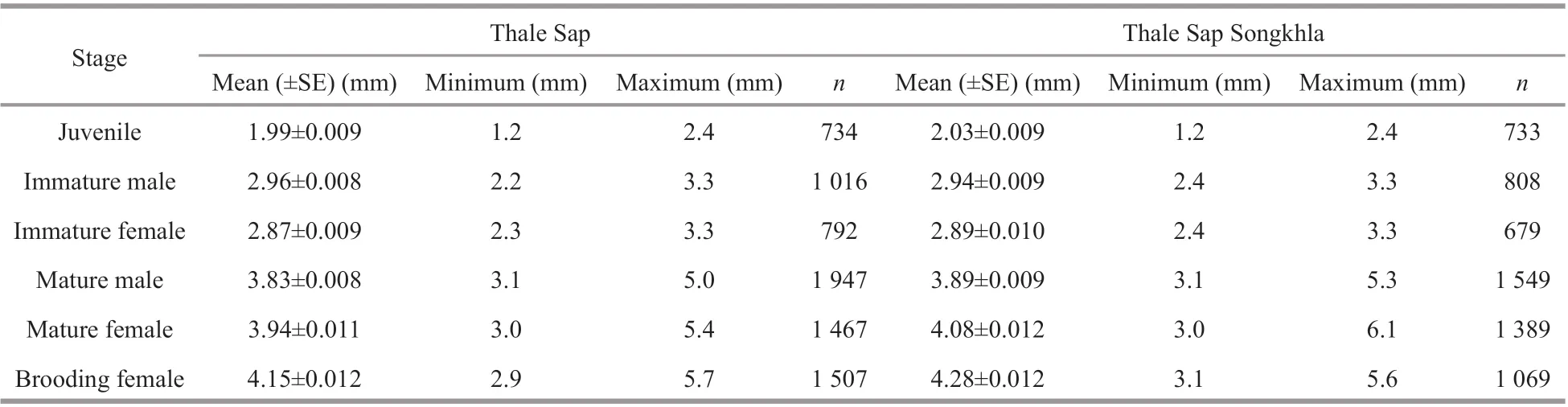
Table 2 Body length of the mysid N. siamensis W. Tattersall, 1921, from Thale Sap and Thale Sap Songkhla, Songkhla Lagoon, southern Thailand (June 2017–June 2018)
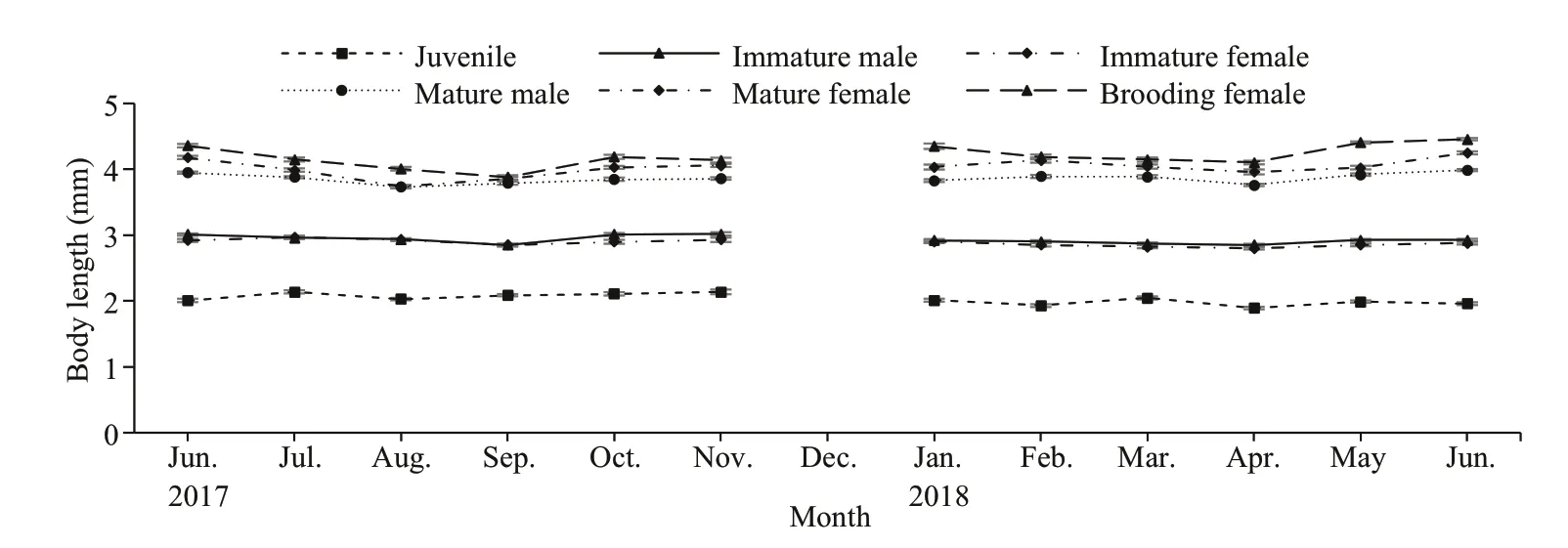
Fig.4 Monthly mean (±SE) variation in body length for each category of N. siamensis W. Tattersall, 1921, from Songkhla Lagoon, southern Thailand (June 2017–June 2018), combined data from Thale Sap and Thale Sap Songkhla

Fig.5 Population structure of N. siamensis W. Tattersall,1921 from Thale Sap (a) and Thale Sap Songkhla (b),Songkhla Lagoon, southern Thailand (June 2017–June 2018)
At Thale Sap Songkhla, mature males and mature females formed 24.87% and 22.30% of the whole population, respectively. Juveniles, immature males and immature females were presented at similar levels(11.77%, 12.97%, and 10.90%, respectively). At the same time, ovigerous females formed 17.18% of the population (Fig.5b). The largest group of mature males was observed in November 2017 and the smallest group was observed in March 2018. The prevalence of mature females was highest and lowest in November 2017 and August 2017, respectively; for juveniles these levels occurred in January 2018 and November 2017, and for immature males and immature females, these level occurred in March 2018 and July 2017, respectively. The largest population of ovigerous females was observed in May 2018 and the smallest was observed in January 2018. There was a significant diff erence in the sex ratio between males and females at Thale Sap Songkhla monthly (χ2=42.711, df=11,P=0.00), and females were predominant. Overall, the monthly sex ratio between males and females combined from Thale Sap and Thale Sap Songkhla, was 0.77, which was significantly diff erent from 1 (χ2=192.65, df=11,P=0.00), and females were more abundant.
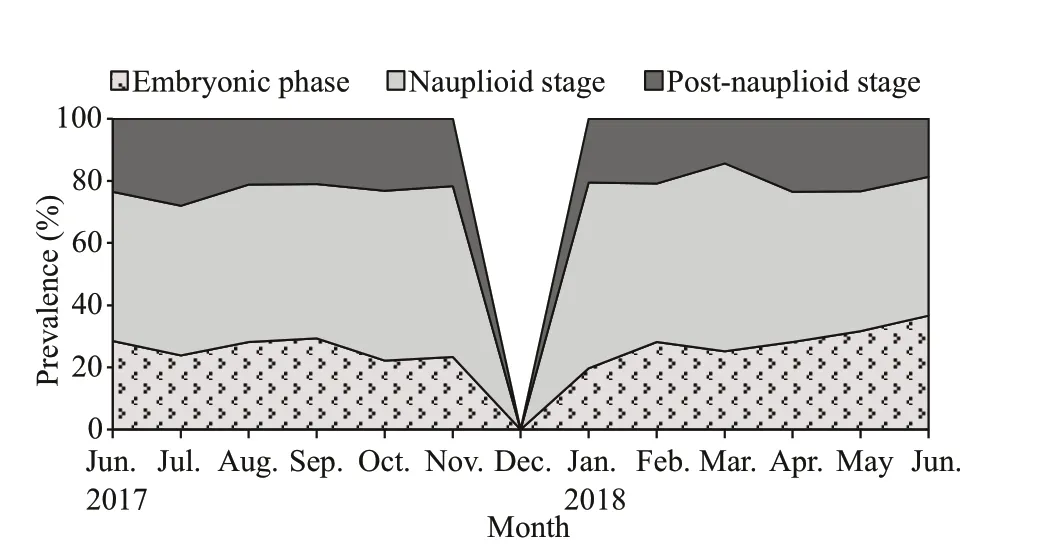
Fig.6 The proportion of brooding females N. siamensis W. Tattersall, 1921 from Songkhla Lagoon, southern Thailand (June 2017–June 2018), combined data from Thale Sap and Thale Sap Songkhla
3.4 Brooding female, brood size and length
Of the brooding females, those in the nauplioid stage accounted for the largest proportion at 50% of the population, while those in the embryonic phase and post-nauplioid stage accounted for 29% and 21%of the population, respectively (Fig.6). The proportions of these groups were significantly diff erent among months (χ2=39.393, df=22,P=0.013). The mean body length of the brooding females was significantly diff erent between brooding stages (ANOVAF2,33=4.613,P=0.017); brooding females with postnauplioid stage were the largest, and those in the nauplioid stage were larger than embryonic phase as follows: embryonic phase: 4.03±0.05 mm, nauplioid stage: 4.19±0.05 mm, and post-nauplioid stage:4.26±0.05 mm. There was no significant size diff erence between brooding females with nauplioid and post-nauplioid stages (Tukey’s HSD,P=0.632),but there was a significant size diff erence between brooding females in post-nauplioid stage and embryonic phase (Tukey’s HSD,P=0.015). The maximum number of embryos, nauplioids and postnauplioids per female were 17 (5.59±0.42), 15(4.87±0.49), and 15 (4.62±0.43), respectively(Table 3), although these numbers were not statistically significant (ANOVAF2,33=3.154,P=0.056).
As expected there was a significant correlation between brood size in the diff erent stages and the body length of the ovigerous females (Fig.7a) and the correlations can be expressed in the following equations:
embryonic phase: number of embryos=-4.566+2.601BL,
nauplioid stage: number oflarvae=-5.807+2.601BL,
post-nauplioid stage: number of larvae=-6.189+2.601BL,

Fig.7 Relationship between brood size (a) and brood length (b) with the body length of brooding females N. siamensis W.Tattersall, 1921 from Songkhla Lagoon, southern Thailand, June 2017–June 2018
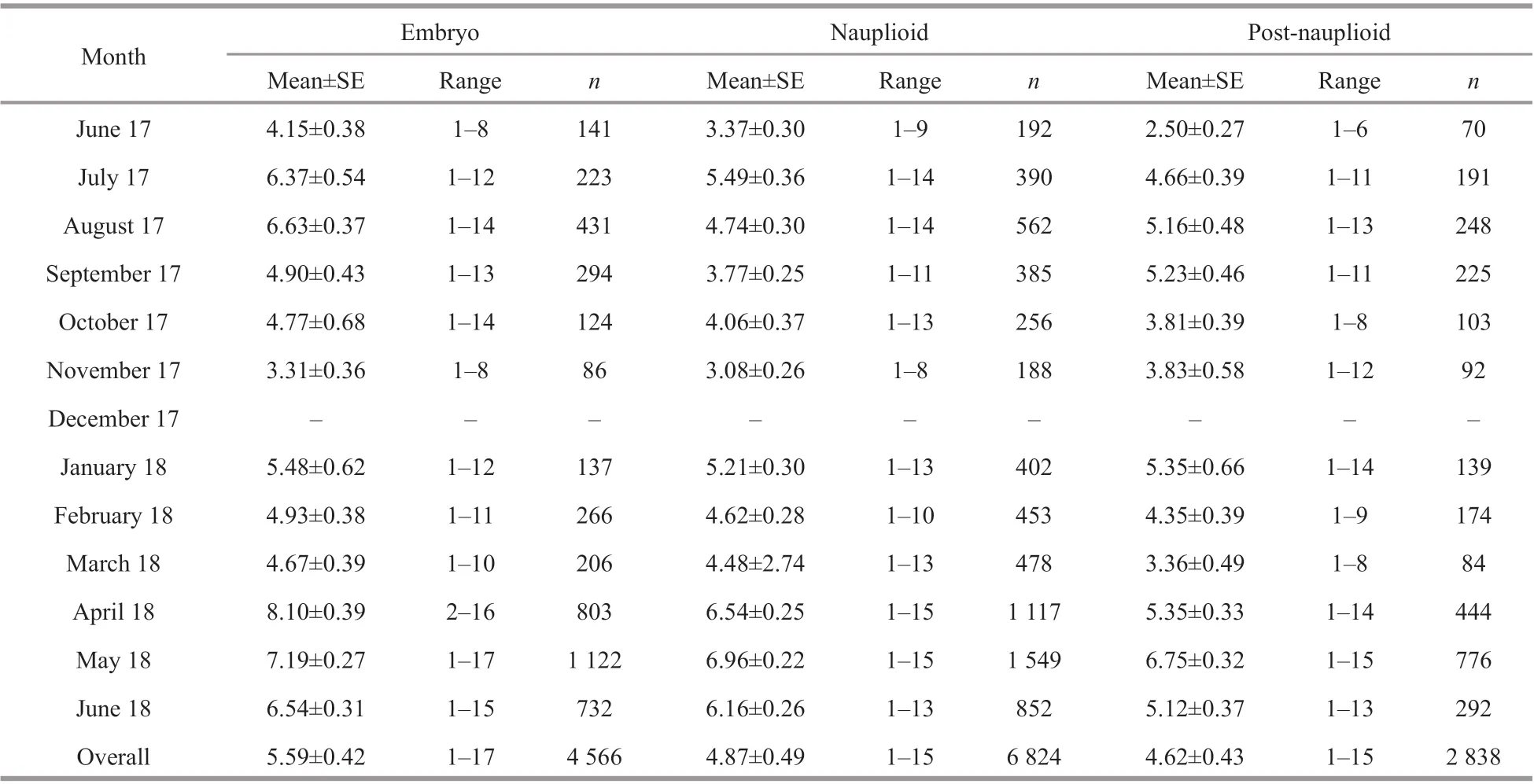
Table 3 Number of broods of N. siamensis W. Tattersall, 1921, from Songkhla Lagoon, southern Thailand, June 2017–June 2018 (combined data from Thale Sap and Thale Sap Songkhla)
(R2=0.165,n=2 481, ANOVAF5,2475=162.95,P<0.000 1).
On the other hand, a significant correlation between brood member length and ovigerous female body length in each brooding stage showed a diff erent increasing trend (Fig.7b), as expressed in the following equations:
embryonic phase: length of embryos=0.179+0.024BL1,
nauplioid stage: length oflarvae=0.327+0.064BL2,
post-nauplioid stage: length of larvae=0.584+0.043BL3,
(R2=0.931,n=13 632, ANOVAF5,13626=36 924.12,P<0.05).
From our observations, the morphology and length of the brood varied within each stage (Fig.8). Embryo or egg diameter ranged from 0.2 to 0.4 mm(0.28±0.04 mm,n=4 566), the length of nauplioid stage ranged from 0.5 to 0.7 mm, (0.59±0.06 mm,n=6 824) and the length of post-nauplioid stage ranged from 0.5 to 1.0 mm (0.78±0.07 mm,n=2 838).The monthly variation in the brood size is presented in Fig.9.
4 DISCUSSION
The population density of the mysidNanomysissiamensisshowed a wide range of fluctuations in the Songkhla Lagoon system and the density was higher in Thale Sap than in Thale Sap Songkhla. The diff erence might be related to geographical condition of the two lagoons. The mouth of the lagoon in Thale Sap Songkhla is connected directly with seawater from the Gulf of Thailand, while Thale Sap is located in the upper part of Thale Sap Songkhla. The water condition of Thale Sap Songkhla might fluctuate directly due to the tidal current, which would aff ect the mysids in this area. Several researchers have noted that the tidal current of the water could influence the distribution of zooplankton and mysids(Wooldridge and Bailey, 1982; Hill, 1991; Hough and Naylor, 1991, 1992; Moff at and Jones, 1993).The total average density ofN.siamensisin this study (3.6 inds./m2) is low compared to other mysid species from tropical regions (in Malaysia) such asAcanthomysisthailandica(405 inds./m2) (e.g.,Ramarn et al., 2012) andMesopodopsisorientalis(49 inds./m2and 709.2 inds./m2) (e.g., Hanamura et al., 2008b, 2009). The variability in the occurrence and abundance of some species of mysids might be related to weather pattern, monsoons, or seasonal behavior and migration (Nair, 1939; George, 1958;Mauchline, 1980; Biju and Panampunnayil, 2011).Overall, the sampling ofN.siamensisin the littoral zone using Riley hand push net was eff ective in representing its occurrence and abundance in the Songkhla Lagoon system.
In the present study, the measured environmental variables showed that variation in water temperature and pH was minor, although a large variation in salinity occurred (Fig.2a–c). Wangkulangkul (2018)noted that the great variation in salinity at this location may be caused by the rainy season in southern Thailand and that during the rainy season in Songkhla Lagoon there is an increase in the freshwater inflow from Thale Sap to Thale Sap Songkhla. Tropical mysids can be found in water temperatures from approximately 25 °C until 35 °C and a pH from 6 to 8.Suitable salinity conditions for mysids are freshwater(Gupta and Gupta, 1984) and brackish water to seawater (Hanamura et al., 2008b. 2009; Biju et al.,2009; Biju and Panampunnayil, 2011; Ramarn et al.,2012). Overall, there was no statistically significant correlation between the abundance ofN.siamensisand water conditions.
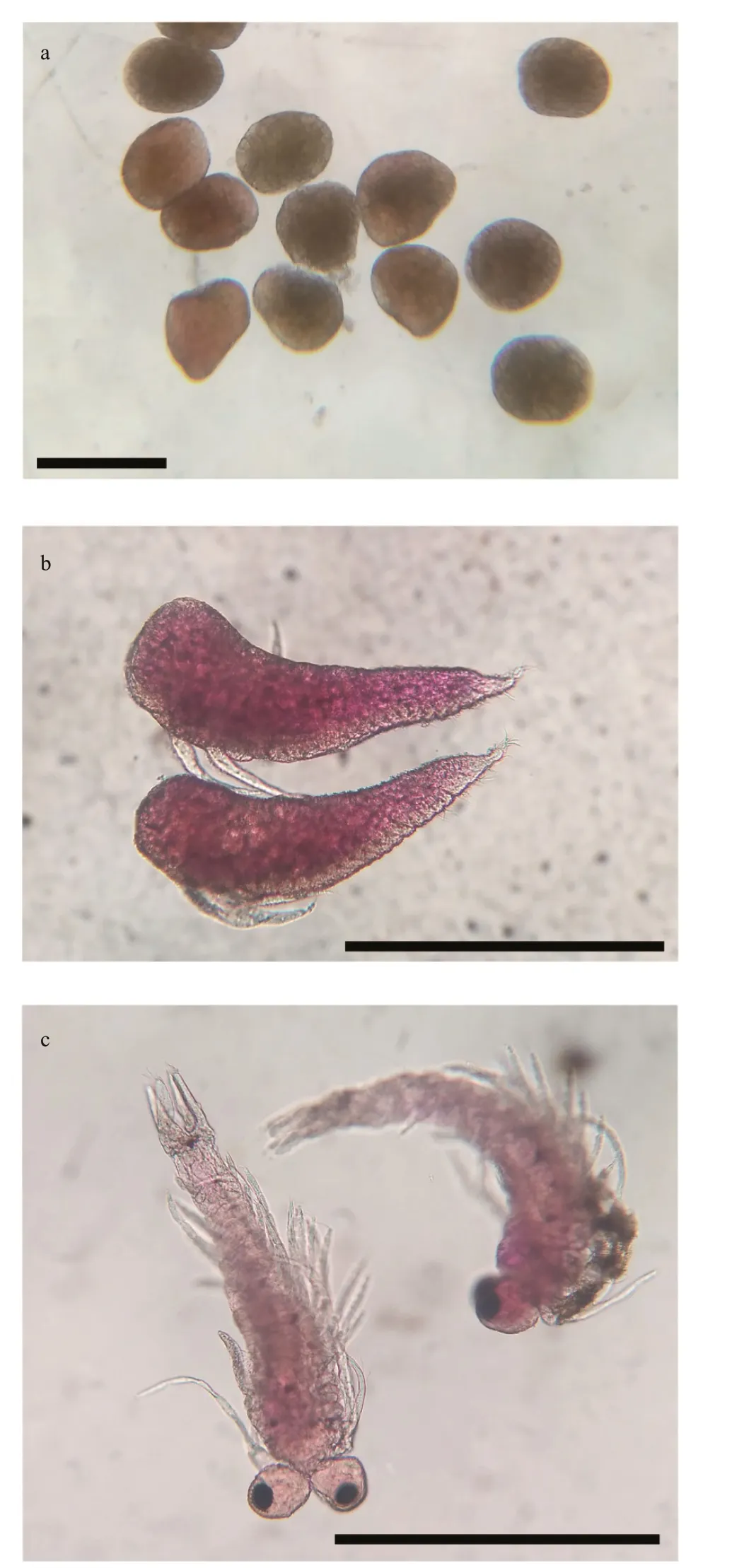
Fig.8 Successive phases of development of N. siamensis from Songkhla Lagoon (the embryonic phase and the larval stage with two larval stages)
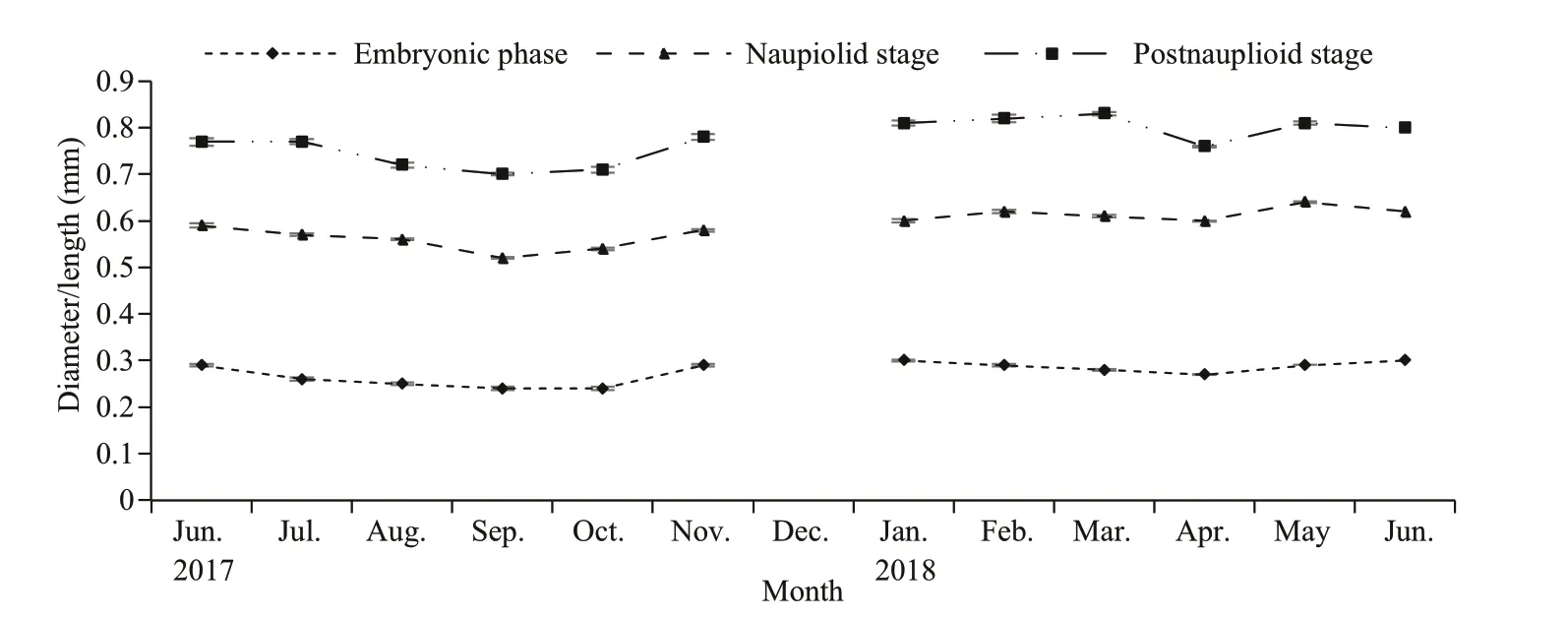
Fig.9 Monthly mean (±SE) variation in the size of the embryonic phase, nauplioid stage and post-nauplioid stage of N.siamensis W. Tattersall, 1921 from Songkhla Lagoon, southern Thailand, June 2017–June 2018
A further biotic factor that might influence the occurrence and abundance of mysids is food availability (Hanamura et al., 2009). Phytoplankton and zooplankton are the main food sources of mysids(Mauchline, 1980; Viherluoto, 2001), and in Songkhla, phytoplankton and copepod communities are found throughout the year with peak occurrence during the northeast monsoon and southwest monsoon, although heavy rain causes some decreases in the communities (Angsupanich and Rakkheaw,1997; Angsupanich et al., 1997). A further investigation is needed to elucidate the diet composition of the species. Predation by fishes also determines the density of the mysids (Hostens and Mees, 1999; Boscarino et al., 2009; Castro et al.,2013). However, to date, there has been no scientific report about the predation of mysids by fishes or gelatinous plankton in Songkhla lagoon.
The current study ofN. siamensisshows that in comparison to males, females reach reproductive maturity at a smaller size than male, while mature females are also larger than mature males (Table 2).Our findings thus contrast to those of Tattersall (1921)who reported that the largest mature male was 5 mm and the largest mature female was 4 mm, as opposed to 5.3 mm and 6.1 mm, respectively, found in this study. Variations in size at sexual maturity of mysids not only exist within populations of a species during diff erent seasons but also between populations in diff erent areas during the same season (Mauchline,1980). For example, in Malaysia, in comparison adult females, adult malesM. orientalisin a mangrove zone reach maturity at a smaller size (Hanamura et al.,2008b), while in a coastal area, adult females matured at a smaller size than adult males (Hanamura et al.,2009). Similarly, adult femalesAcanthomysis thailandicamatured at a smaller size than the adult males (Ramarn et al., 2012). In general, female mysids grow larger than males due to more frequent molts in males and slower growth rates (Mauchline,1980).
The diff erence in the sex ratio may be influenced by migration, minimum size variation when secondary sexual characteristics emerge (Mauchline, 1980),species life span, or the variation of male and female mortality rates (Hakala, 1978). In this study, the adult female (combination of mature/empty female and ovigerous female)N.siamensiswas significantly more predominant than the adult male (1.53:1)annually (χ2=215.854, df=11,P=0.00), with the highest variation found in October 2017. Mauchline(1980) explained that sex ratios within mysid populations are variable, but the number of females is frequently higher than the number of males. The higher proportion of female mysids compared to male mysids has also been reported by other researchers forA.thailandica(Ramarn et al., 2012),Hemimysislamornaemediterrania(Delgado et al., 2013),M.orientalis(Hanamura et al., 2008b, 2009) andSiriellajaltensis(Delgado et al., 2018).
The continuous reproduction ofN.siamensissuggest that spawning occurs throughout the year(Fig.6). Other species, includingA.thailandica,M.orientalisandM.zeylanica, also show year-round reproduction (Hanamura et al., 2008b, 2009; Biju et al., 2009; Biju and Panampunnayil, 2011; Ramarn et al., 2012). Goodbody (1965) also reported that tropical mysids reproduced continuously. However,temperate mysids only produced 2 or 3 short-lived generations a year (Mauchline, 1980). The predominance of the brooding femaleN.siamensisin the nauplioid stage is due to the duration oflarval development in the marsupium. The development of the nauplioid stage takes longer than the development of embryonic phase and post-nauplioid stage is the shortest development stage (Delgado et al., 2013).The brood size and brood length ofN.siamensisin this study were positively correlated with the body length of the brooding females (Fig.7a–b). This indicates that a brooding female with a large body could produce and carry a relatively larger brood in the marsupium (Saltzman, 1996). The present results also showed variation in the brood size of each stage,as the embryo ranged from 1 to 17, while the nauplioid and post-nauplioid stages ranged from 1 to 15 (Table 3). These findings suggest mortality during development in each stage in the brood pouch;however, there was no statistically significant diff erence. According to Mauchline (1980), the number of young per brood varies greatly between species and sometimes between closely related species. Furthermore, mortality is possible during marsupial development (Mauchline, 1973) due to parasitism (Daly and Damkaer, 1986; Ohtsuka et al.,2011) and cannibalism (Wittmann, 1984). During this study, no evidence of parasitic or cannibalistic activity was found to account for the observed variation in brood size ofN.siamensis, although this should be investigated by more detailed studies.
5 CONCLUSION
The population structure, reproductive traits ofN.siamensisand water conditions in Songkhla Lagoon system were assessed from June 2017 to June 2018. Mean temperature and pH showed a minor variation but mean salinity showed a great variation from 0 to 11.01 in Thale Sap and 22.06 in Thale Sap Songkhla. The density ofNanomysissiamensiswas greater in Thale Sap (8.44–807.33 inds./100 m2) than Thale Sap Songkhla (8.81–434.44 inds./100 m2) and the peak occurrence was observed in May 2018. The sex ratio showed that adult females were dominant than adult males and reproduction occurred yearround, similar to other tropical mysid species. The species also exhibited a correlation of brood size and brood length with brooding female body length. The continuation of mysid population research in the Songkhla Lagoon system is necessary to be done due to their role in the food chain of the lagoon.
6 DATA AVAILABILITY STATEMENT
All datasets generated and/or analyzed during this study are available from the corresponding author on reasonable request.
7 ACKNOWLEDGMENT
We would like to thank Mr. Naratip Tubtimtong and Mr. Sompong Pachonchit for helping during the field work and we also thank Dr. Sammy De Grave(Oxford University Museum of Natural History) for the correction on this manuscript. Finally, we greatly appreciate the editor and reviewers for the valuable comments to improve the manuscript.
杂志排行
Journal of Oceanology and Limnology的其它文章
- Steady increase in water clarity in Jiaozhou Bay in the Yellow Sea from 2000 to 2018: Observations from MODIS*
- Phylogenetic diversity and bioactivity of culturable deepsea-derived fungi from Okinawa Trough*
- Allelopathic eff ects of mixotrophic dinoflagellate Akashiwo sanguinea on co-occurring phytoplankton: the significance of nutritional ecology*
- Investigation of the decline of Ulva prolifera in the Subei Shoal and Qingdao based on physiological changes*
- Effi ciency of phosphorus accumulation by plankton,periphyton developed on submerged artificial substrata and metaphyton: in-situ observation in two shallow ponds*
- Petroleum exploitation enriches the sulfonamide resistance gene sul2 in off shore sediments
